Abstract
In this study it was aimed to investigate the treatability of cefoperazone with new generation Sb-doped SnO2-Ni anodes. For this purpose, it was studied with Sn/Sb/Ni: 500/8/1 anodes for the oxidation of aqueous solution containing cefoperazone antibiotic by addition of different types of electrolyte. Potassium chloride was found as the best electrolyte type affecting the electrochemical reactions positively even at lower concentrations (750 mg/L−1). At pH 8 the best results were obtained, which is the neutral pH value of the aqueous solution. 50 mA/cm2 was found as the best value for current density parameter, providing full mineralization just after 60 min of reaction. The removal efficiencies increased generally with the increase of current density, because active oxidants occur increasingly at higher current values. According to the results of the study it was seen that, electrochemical oxidation processes with Sn/Sb/Ni–Ti anodes could be carried out efficiently without need adding extra electrolyte (salt) and pH adjustment step for real wastewaters containing antibiotics. Thus, it was found an easy and economic way to perform electrochemical oxidation with Sn/Sb/Ni–Ti anodes for the wastewaters containing cefoperazone antibiotics.
Similar content being viewed by others
Introduction
Antibiotics are used for many years to treat diseases related to bacterial infections for human and animal health, for fish farm practices, and support the growth of animals1,2. However, they cause to the adverse effects on the environment directly or indirectly through contamination and consequently, they may cause to bacterial resistance in the environment at long term. Thus, they could be considered as the most dangerous pollutant types due to have toxicologic properties causing to the microorganism resistance in the environment3,4. Bacteria with antibiotic resistance genes can spread easily in the environment, threatening human health potentially5. However, they are also known for having endocrine disrupting properties.
Endocrine disrupting compounds (EDCs) are known to disrupt endocrine systems in the human body. The presence of these compounds in the environment causes great deterioration in human and environmental health6. Recent studies show that, the presence of endocrine disrupting pharmaceuticals (PhPs) in the aquatic environment may cause adverse effects, such as feminization in fish population7 and crocodiles8 and may also affect the behavior and migration movements of the salmons. Due to have low biodegradability, conventional treatment processes are not efficient enough for the removal of these compounds and lead to contamination in the receiving environment by bioaccumulation9. However, advanced oxidation processes (AOPs) are considered to be a promising methods for the solution of this problem.
AOPs are the oxidation methods based on the formation of reactive radical species (·OH-hydroxyl radical), which play an important role in the elimination of toxic organic compounds10. Advanced Oxidation Processes are used in wastewater treatment due to the formation of ·OH with high oxidation capacity. However, there are many variables affecting the processes; such as reaction time, pH, temperature, amount of catalyst and reagents11. Electrochemical processes, which place among the AOPs are based on production of hydroxyl radicals, which is the strongest oxidant type after the fluorine, and has at least as much reduction potential (E°: 2.8 V), that completely reacts selectively to organic contaminants by hydroxylation/dehydrogenation12,13.
Several anodic materials were used by the researchers for anodic oxidations, such as; PbO2, platinum (Pt), gold (Au), lead (Pb), SnO2, boron doped diamond (BDD) and carbon anodes. While some of the anode types selectively oxidize pollutants, those pollutants do not completely degrade (only the nature of the pollutant phase changes), the main disadvantage of other anode types is the rapid loss of effect due to surface contamination14. However, those anodes require high voltages to operate. The disadvantages of PbO2 anodes are that they have a shorter lifespan than other type of anodes15. Although BDD anodes have shown satisfying results for antibiotic removal, they have high capital cost and are not practical as Sn/Sb/Ni–Ti anodes16. In contrast to BDD anodes, Sn/Sb/Ni–Ti anodes are cost-effective. Additionally, 37% current efficiency could be reached at room temperature with Sn/Sb/Ni–Ti anodes17. Thus, Sb-doped SnO2 anodes are advantageous with their cost efficiencies and practical application. However, most of the other materials are not suitable due to their toxicity, instability and causing to the high costs. In contrast to these materials, even though Antimony (Sb) and Nickel (Ni) are known as toxic metals, because of the high stability and durability of Sn/Sb/Ni anodesachieve those problems sucessfully. Additionally, Sn/Sb/Ni anodes show very promising results in ozone production and electrochemical oxidation18,19,20,21,22. The most important advantage of these anodes in ozone production is their operation at relatively low voltage. On the other hand, they are likely to produce ozone in liquid and gaseous phases and do not need any input such as dry, moist air/pure oxygen and anodic gas source21. Also, the electrodes must be stable and remain stable at a voltage of 1.51 V (the ozone formation voltage) in electrochemical ozone generation with these anodes. Since the other anodes are not very stable, they are prevented by the formation of oxygen at a voltage of 1.23 V after a while, or a stability of 1.51 V can only be achieved at low temperatures23. Thus, in this study, it is investigated electrochemical oxidation of 50 mg L−1 cefoperazone, which place among the cephalosporin group of antibiotics, from aqueous solution with using novel Sn/Sb/Ni (Sb-doped SnO2-Ni) anodes. Goncalves et al. (2012) investigated catalytic ozonation of 50 mg L−1 sulfamethoxazole from aqueous solution24. Trovo et al. (2011)25 prepared simulated wastewater by adding amoxicillin antibiotic with a concentration of 50 mg L−1 for the Photo-Fenton process.
Cephalosporins have a great importance among the other antibiotic groups, because of having widespread usage in Turkey and worldwide (especially in northern european countries)26. These group of antibiotics are of the series of broad-spectrum semisynthetic antibiotics27. Among the cephalosporins, cefoperazone (CFP) is the third-generation type of semisynthetic antibiotic28. It is used against bacteria which are responsible for the infections of respiratory, urinary, skin, and female genital tracts having a broad spectrum of activity. First, cefoperazone was patented in 1974 and now, clinical use of cefoperazone in the U.S. is prohibited while, some of the European countries still use it (under the product name of Sulperazon)29.
However, most of the studies focuse on fluoroquinolone, trimethoprim, sulfonamide and macrolide antibiotics for the removal of them from water and wastewaters, while, just a little of them are made for cephalosporins and there are only a few studies about cefoperazone in literature. Furthermore, there is no such study on treatment of cefoperazone with these new generation Sb-doped SnO2-Ni anodes that make this study unique. Thus, this show that our study have a very important role in terms of filling this great gap in literature.
Materials and methods
Chemicals, anode preparation and reactor configuration
The simulated wastewater was prepared by adding sodium chloride (NaCl) and potassium chloride (KCl) (Merck, Darmstadt, Germany) as the electrolytes and cefoperazon antibiotic (from a local pharmacy, Bursa, Turkey) with a concentration of 50 mg L−1. Millipore Milli-Q ultrapure water (18 MΩ cm) was used for the preparation of simulated wastewater.
Cathodes were prepared in the size of 5 × 5 cm from platinized titanium material which was bought from NRK Electrochem Company, Cornwall, UK. Titanium (Ti) materials were purchased from Dexmet Company, USA (3Ti7-077FA) and then they were prepared by cutting the dimensions as 2.5 cm × 2.5 cm to prepare anodes. To remove all the impurities from Ti materials, they were dipped into the boiling oxalic acid solution (from Merck Company, Darmstadt, Germany). And then, theywere sonicated in ultrasonic bath for 10 min, three times. After drying process at room temperature, Ti materials were dipped into the pyrolysis solution having a molar rate: Sn/Sb/Ni: 500/8/1 for 2 min for dip coating process. And thus, the final product was obtained as a SnO2-based Ni/Sb anode material. SnCl4.5H2O (Tin (IV) Chloride Pentahydrate) and NiO (Nickel(II) oxide) were purchased from Alfa Aeser, MA, USA and Sb2O3 (Antimony(III) oxide) was bought from Merck Company, Darmstadt, Germany, which were used for dip coating process. The oven temperature for drying process was set at 105 °C for 15 min. Then, the oven temperature was set at 520 °C for 15 min time for the annealing process. This coating process was repeated for 19 times. However, the final process (20th) was performed for 75 min30. All of the chemical agents were at the purity of ≥ 99%.
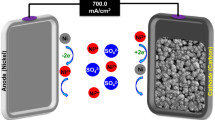
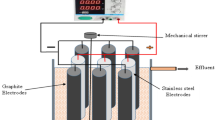
To design of reactor configuration for the electrochemical processes, 250 mL beakers were used. The electrodes (anode and cathode) were placed into the beakers mutually. The experiment conditions were 25 °C temperature and atmospheric pressure. The electrical current was created with DC power supply (Extech Instruments, 382280). In Fig. 1 it was stated the electrochemical reactor configuration including Sn/Sb/Ni–Ti anode.
Analytical studies
A pH meter was used (Cyberscan 10) to measure of the pH values of the water samples. Chemical oxygen demand (COD) and total organic carbon (TOC) measurements were made according to the APHA Standard Methods (1995)31. TOC-L analyser (Shimadzu Company, Japan) was used for TOC analysis. Ultra performanced liquid chromatography (UPLC) with photodiode array (PDA) detector (Thermo scientific, US) was used to measure of CFP amount in water samples. The detector wavelengths were set at 254 and 270 nm. The column chosen for the UPLC measurements was C-18 type: 50 × 2.1 mm; 1.9 um (Hypersil Gold, Thermo scientific, US). The temperature of the column was 35 °C and the flow rate was 0.2 mL min−1. The mobile phase solvent was consisted of MeOH : H2O (0.1% formic asid): 40 : 60 (v:v). All of the measurements were repeated at least in triplicate. EDS (Energy Dispersive Spectroscopy) (EDAX, USA) and SEM (Scanning electron microscope) (Philips XL 30 SFEG, Netherlands), hpAFM (High Performance Atomic Force Microscope) (Nanomagnetic Instruments, Oxford, UK) and XRD (X-ray Diffraction) analysis were made for imaging and to perform qualitative and quantitative analyzes of the anode and to identify phases, crystallinity, and structures of the final products.
Results
The optimization of anode configuration
The pyrolysis solution containing Sn:Sb:Ni was prepared in different molar ratios according to the prescription of the University of Hong Kong32. The effect of the number of coatings on the current efficiency was evaluated during electrolysis in 1 M HClO433. It was observed that the current efficiency increases when the nickel ratio increases. At the same time, it is clearly seen that the current efficiency increases with the increase of number of coatings. Thus, it was decided to study with 500/8/1:S/Sb/Ni ratio. In Fig. 2 it is seen the effect of the number of coatings on the current efficiency in Nickel-plated (Sn/Sb/Ni) anodes.
Determination of electrolyte type
At Fig. 3a, b it is seen the effect of electrolyte type on chemical oxygen demand (COD) removal from the water samples with Sn:Sb:Ni:500:8:1 anode. It was evaluated the effect of electrolyte type (NaCl and KCl) on electrochemical oxidation process with Sn:Sb:Ni:500:8:1 anode at various electrolyte concentrations. While the electrolyte type (NaCl, KCl) and concentration affected the removal efficiencies positively by increasing the conductivity and occuring chlorine gas and hypochlorid acid as efficient oxidants, addition of extra electrolyte may increase the process cost34.
The COD concentrations decreased to the zero after 60 min of anodic oxidation with 2000 mg L−1 NaCl addition, at 50 mA cm−2 current density and pH 8. However, COD concentrations decreased to the zero just after 60 min with 750 mg L−1 KCl.. Although high efficiencies were obtained in a short time for 2000 mg L−1 and 2500 mg L−1 NaCl conc., 750 mg L−1 KCl was regarded as the best electrolyte type and concentration due to the excess use of salt may increase the cost and chemical consumption. Yonar et al. (2019) investigated electrochemical color removal from organized industrial district (OID) wastewater treatment plants using new generation Sn/Sb/Ni–Ti:500/8/0.5 anodes and they studied with NaCl as the electrolyte at the range of 250–1000 mg/L. However, they found that, there was no need to addition of salt due to the study with real wastewater samples containing domestic wastewater35. Buyukada (2018) investigated TiO2 assisted photocatalytic ozonation of leather effluent and it was reported that increase in both of ozone and catalyst doses caused to increase in removals of chemical oxygen demand and turbidity36. Duan et al. (2020) reported electro-oxidation of ceftazidime antibiotic in real municipal wastewaters with PbO2-Ce and SnO2-Sb anodes. NaCl addition significantly enhanced the oxidation rate with 99% removal efficiency within 5 min of reaction with Ti/SnO2–Sb anode, while humic acid conc. inhibited electrochemical catalyzing37. Wang et al. (2022), added 20 mg/L NaCl, NaHCO3, Na3PO4, and HA (humic acid) as the electrolyte into the wastewater containing 4-chlorophenol (4-CP) antibiotic for the electrochemical oxidation of 4-CP by titanium suboxide anode with peroxymonosulfate. 4-CP removal efficiency was decreased at the rate of 97.4% and the corresponding kinetic rate was also decreased from 0.149 to 0.147 min−1 with the addition of Cl− ions, which provide the best conditions38.
Effect of potassium chloride concentration
At Fig. 4 it is shown the effect of potassium chloride on total organic carbon (TOC) and residual CFP removal with Sn:Sb:Ni: 500:8:1 anode. The effect of KCl (potassium chloride) concentration (mg L−1) on TOC and CFP were evaluated between 250 and 1000 mg L−1 KCl doses, at pH 8 and 50 mA cm−2 current density.
TOC was completely mineralized within 60 min reaction with 750 mg L−1 KCl as it was stated in a graph in Fig. 3. In the same way, residual CFP was decayed more efficiently just in 5 min with 750 mg L−1 KCl. Furthermore, after 30 min oxidation with 1000 mg L−1 KCl, the complete mineralization was achieved. However, the excess use of salt may increase the cost and chemical consumption. Thus, 750 mg L−1 was determined as the best dose for the electrochemical oxidation of cefoperazone with Sn:Sb:Ni: 500:8:1 anode. Sivrioğlu and Yonar (2016) investigated the removal of COD and color from textile wastewater with using Sn/Sb/Ni–Ti anode. Although the better efficiency was obtained at high NaCl concentrations, 1 g/L NaCl concentration was chosen as the best salt concentrationto prevent the high cost and environmental problems. Yao et al. (2019) investigated electrochemical oxidation of ammonia in dyeing wastewater with a Ti/PbO2 anode and Ti cathode and they obtained that, ammonia removal increased with increase in NaCl dose and thus, the removal efficiency reached to 100% with 1000 mg L−1 NaCl addition39. Hai et al. (2020) investigated the electrochemical oxidation of sulfamethoxazole (SMX) antibiotic with BDD (boron doped diamond) anode and complete removal of SMX and 65.6% COD removal, 40.1% current efficiency and 72 kWh kg/COD energy consumption rates were obtained with 0.1 M Na2SO4 after 3 h reaction at 30 mA/cm2 current density and pH 740. Qian et al. (2019) investigated the effect of electrolyte addition on the electro-oxidation of tetracyclines (tetracycline, oxytetracycline, chlortetracycline) with Ti/SnO2-Sb2O3/PbO2 anode. While all the antibiotic compounds were completely removed with NH3·H2O-NH4Cl within 2 h, the removal efficiency was found below 80% with the addition of Na2HPO4-NaH2PO4, and after a 2 h of reaction time with Na2SO4, 82.4%, 83.6% and 88.4% removal rates were obtained for tetracycline, oxytetracycline and chlortetracycline, respectively41.
Effect of pH
At Fig. 5 it is shown the effect of pH on chemical oxygen demand (COD), total organic carbon (TOC) and residual cefoperazone (CFP) decay. At pH 8, the best removal efficiencies were obtained. Just after 60 min of reaction, COD and TOC was consumed completely, and the residual CFP was consumed just in 5 min at pH 8. Thus, according to the graphs in Fig. 4, pH 8 was found as the best pH value for the electrochemical oxidation of cefoperazone. According to these results, it was assumed that, it is possible to save costs by working at neutral pH value (pH 8) of water containing cefoperazone antibiotic, because the process doesn’t require extra labor and chemical costs to adjust the pH. Yonar et al. (2019) investigated electrochemical color removal from industrial wastewater with using new generation Sn/Sb/Ni–Ti: 500/8/0.5 anodes between pH 3 and 9. After 30 min of electrochemical reaction, COD and color removal efficiencies reached up to 98% and 99%, respectively at pH 8.2 (T: 25 °C) for colored industrial wastewater35. In a study made by Zakaria and Christensen (2014), it was reported that 100% removal efficiency was achieved within 5 min for Reactiv Blue 50 dye at a concentration of 1000 mg L−1 at pH 4.1, with using Sn/Sb/Ni anode and platinized titanium cathode fed to the membrane electrochemical electrode reactor system42. Abbasi, Soleymani, and Parsa (2015) performed an ozonation process on Rhodamine B molecules in aqueous solution using a reversible electrochemical ozone generator system with a titanium-based anode coated with nanocomposite Sn/Sb/Ni and they saw that the degradation efficiency could reach up to 99.5% at pH 3.7 for a 8 mg/L dye solution after 30 min43.
In the experimental studies of Sivrioğlu and Yonar44, COD and color removal was found as 98% and 99%, respectively as a result of electrochemical oxidation of dyeing wastewater at pH 3. However, although the natural pH value (pH 7.2) of the wastewater showed relatively lower efficiency (3%) compared to the acidic conditions, pH 7.2 was identified as the best in order to avoid extra pH adjustment step and chemical cost. At alkaline pH values, the potential of chlorine gas and hypochlorite ion formation could support the removal of organic compounds45,46. Yao et al. saw that, the increase of initial pH had a great effect on ammonia removal from dyeing wastewater with Ti/PbO2 anode and the best pH value was found as 8.339. In a study of Kaur et al. (2018), with a Ti/RuO2 anode, removal of ofloxacin (OFL) occurred rapidly in the first 15 min of the reaction when the initial pH of the synthetic wastewater was between 2 and 9, and after 30 min, there was no any significant change. It was found that higher removal efficiencies were observed with lower pH values and after only 30 min of electro-oxidation 88.6% OFL degradation was observed at pH 2, while 68.6% OFL degradation was obtained within 30 min at pH 947. Hai et al. (2020) investigated the removal of sulfamethoxazole (SMX) with boron-doped diamond anode at pH 3, pH 7 and pH 11 at a current density of 30 mA cm−2. It was observed that higher removal efficiencies were obtained at neutral pH (pH 7) compared to those at pH 3 and pH 1140.
Effect of current density
The current density has an active role in reaction kinetics and thus, affects the electrochemical reactions significantly46. The best current density value was found as 50 mA cm−2 due to obtainhigher removal efficiencies in shorter times. In the aqueous solutions, active oxidants occur increasingly at higher current density values thus, the removal efficiencies increased generally with the increase of current density in this study. Yonar et al. (2019) investigated electrochemical color removal from industrial wastewater using new generation Sn/Sb/Ni–Ti: 500/8/0.5 anodes, between 10 and 100 mA/cm2 current density values. The best current density value was found as 50 mA cm−2 with 4.05 kWh gCOD−1 energy consumption and the current density was observed as the most effective parameter for COD and color removal35. Duan et al. (2020) reported electro-oxidation of ceftazidime antibiotic in real municipal wastewaters with PbO2-Ce and SnO2-Sb anodes. While 99.37% of ceftazidime degradation and 95.52% COD removal was achieved with Ti/SnO2–Sb anode, 75.15% ceftazidime degradation and 83.54% COD removal was obtained with Ti/PbO2–Ce anode, under 4 mA cm−1 current. Wen et al. (2019) investigated mineralization of cefoperazone (50–300 mg L−1) in acidic medium with photoelectro-Fenton with microwave discharge electrodeless lamp irradiated by using RuO2/Ti and boron doped diamond (BDD) anode. BDD microwave discharge electrodeless lamp photoelectro-Fenton gave 88% mineralization under the best conditions of 0.36 A48. Wang et al. (2022) studied electrochemical oxidation of 4-chlorophenol (4-CP) by titanium suboxide anode with peroxymonosulfate and they saw that the degradation efficiency was obviously increased with the increase of current density from 1 to 10 mA/cm238. Zeng et al. (2022) investigated electrochemical oxidation of sulfamethoxazole (SMX) in natural water and wastewater with TiO2 nanotube array based electrocatalytic membrane. The removal rate of SMX was 86.1% and the energy consumption was 0.55 kWh/m3 with one dimensional nanostructure and the degradation efficiency of SMX was strongly affected by the current density. Both hydroxyl radicals and direct electron transfer affected the sulfamethoxazole degradation, while the sulfate radicals could be ignored49. Yao et al. (2019) reported that ammonia removal increased significantly with increase of applied current density from dyeing wastewater with Ti/PbO2 anode and Ti cathode and the best current density value was found as 20 mA cm−239. Hai et al. (2020) investigated the removal of sulfamethoxazole with BDD anode and according to the results of the study, SMX decomposed completely after 1 h of electrochemical reaction at a current density of 45 mA/cm2, while it decomposed after 3 h at the current density values of 15 mA/cm2 and 30 mA/cm240. Qian et al. (2019) investigated the effect of the current density parameter on the electro-oxidation of tetracyclines with SnO2-Sb2O3 and PbO2 doped Ti anodes. They reported that the degradation of antibiotics within a reaction of 2 h without power supply was less than 2% indicating that the adsorption of antibiotics on the anode surface could be negligibly low. With the increase of current density, the removal rates of tetracyclines gradually increased and the removal rates were 98.1%, 97.6% and 99.5% for tetracycline (TC), oxytetracycline (OTC) and chlortetracycline (CTC), respectively at a current density of 15 mA/cm2 for 2 h. However, the removal rates of tetracycline, oxytetracycline and chlortetracycline were found to be 79.5%, 82.0% and 90.3%, respectively, at a current density of 5 mA/cm241. In Fig. 6 it is shown the effect of current density (10–50 mA cm−2) on total organic carbon (TOC) and residual CFP removal with Sn:Sb:Ni: 500:8:1 anode (pH 8 and KCl conc.: 750 mg L−1).
Evaluation of total intermediate product formation
Total intermediate product formations are shown in Fig. 7 according to the effect of KCl concentration, pH and current density.According to the graphs in Fig. 7, much lower intermediate products were occurred with KCl addition and the organic content was almost completely mineralized just in 60 min with 750 mg L−1 KCl. The lowest occurances of total intermediate products were obtained at pH 4 and pH 8 with the current density value of 50 mA/cm2.
Yahya et al. (2016) investigated the ability of Electro-Fenton process with carbon-felt cathode and Pt anode for degradation and mineralization of levofloxacin (LEV) in aqueous solution. 400 mA current value was observed to be optimum. Chemical oxygen demand and mineralization degree reached to > 91% at the end of 6 h of reaction. A number of intermediate products were identified by using HPLC and LC–MS. N atoms in LEV were released as NH+4 and NO−3 ions. Nitrogen atoms mainly transformed into NH+4 rather than NO−3. The concentration of NH+4 reached to 0.28 mM after 300 min, while that of NO−3 reached to the zero after 300 min. The nitrogen loss could be explained by the formation of volatile nitrogen compounds and the presence of oxamic acid that is hardly oxidizable by hydroxil radicals50. The biggest advantage of the electrochemical oxidation processes is that, pollutants are completely oxidized ideally. However, it is known that, organic compounds with high permanence are more concentrated with phase change instead of oxidation completely in classical processes.Thus, electrochemical oxidation processes are highly promising26.
SEM–EDS, XRD and HpAFM analyzes
To image and to perform qualitative and quantitative analyzes of the anode as a final product and to identify phases, crystallinity, and structures of it SEM–EDS, hpAFM and XRD analyzes were made out. In Fig. 8a, b it is seen typical SEM images (× 150, bar = 200 μm) of Sn/Sb/Ni: 500/8/1 anodes (clean and used anodes, respectively). The developments in nanocomposite material applications in engineering (mechanical, optical, electrical and magnetic applications) are promising51. Thus, electrochemical oxidation processes with nanocomposites are promising with their easy applicability and low energy requirements52,53.
The weight and atomic percentages and the peak intensities in Fig. 9a, b were given in Table 1 for the anode characterization. The peaks of the elements (Sn, Ni, Sb and Ti) were identified by the analysis of anode material. Coating on the anodes (Ni/Sb-SnO2) was thin to enough detection of Ti underlying. Typical SEM micrographs of the anode intersections (assuming thicker coating than strands)30 at × 150 magnification are shown in Fig. 8 for used (contaminated) and unused (clean) anode, respectively. It was observed that the anode materials showed a cracked morphology because of the coating process for clean anodes as stated in other studies54 that was occurred by thermal shocking which is seen generally while cooling suddenly after taking of the anodes from oven. Cracked morphology is seen in thicker anodes mostly with large splits having three dimensional (3D) view55. However in contaminated (used) anodes, a smoother surface was observed that resulting from the coating of surface area with ions (carbon) and salts (Fe3(PO4)2(OH)2) passing from the solution.
Christensen et al. (2013) investigated the effect of Ni and Sb oxide precursors and composition of the anode in ozone production with 1.0 M HClO430. Typical SEM images of the anodes (93.3, 6.0 and 0.7% Sn, Sb and Ni respectively) which were taken from the intersection (× 5000 magnification) showed cracked morphology (sources from thermal shock during the cooling suddenly after withdrawing of anodes from oven), while the coating on strands showed a smoother morphology assuming have thinner coating than the intersection. With EDS spectra of the anode it was seen that, the peak with 4.52 eV may be sourced from Ti underlying that could be derived from thinner catalyst coating than the strands. Christensen et al. (2012) studied the effect of Ni/Sb-SnO2 loading on electrocatalyst and they observed with the SEM images that the electrode was thicker and had very little pores having typical “cracked morphology” with deep crevices55. Moreover, it was observed that Ni/Sb-SnO2 coating was thin sufficiently to able to detect Ti element underlying. Zhi et al. (2017) investigated the degradation of tetracycline antibiotics with Ti/SnO2-Sb anodes and used the sol–gel technique to coat the anode56. Although there were some cracks ranging in size from 1 to 10 μm, SEM images revealed that the anode surface was generally solid and smooth. In addition, they concluded that the formation of these cracks could result in gradual inhibition of the anode during the electrochemical process. Qian et al. (2019) reported that the Ti substrate surface pretreated with Ti/SnO2-Sb2O3/PbO2 anode is irregular and crusted, which is presumed to be formed as a result of oxalic acid application41. Thus, they stated that it may be beneficial to add SnO2-Sb2O3 and PbO2 as interlayers and active layers, respectively.
In addition, Figs. 10 and 11 show AFM images of the anodes showing the topographical height change during the electrochemical oxidation process. It has been observed that there is a very high difference between contaminated and clean anode in terms of topographic height. While topographic height differences tend to increase in parallel for upper cross and bottom cross section for the clean anode; irregularities were observed for contaminated anode, which is thought to be due to ion transfer from the aqueous solution. However, it is known that the physicochemical properties of the anodes are directly related to the preparation methods. Composition ratios, particle size, surface structure, specific surface area and bonding force directly affect the performance of the anode57. Figure 12 shows the XRD results of clean and contaminated Sn/Sb/Ni–Ti anodes. Consequently, XRD results confirmed other SEM–EDS findings. It was observed that the surface of the used (contaminated) anode was filled with other ions (carbon) and salts (Fe3(PO4)2(OH)2) may source from the solution. It was worked a total of 300 h with the anodes, and thus, it was obtained that the anode material is not corroded significantly.
Conclusions
In this study, electrochemical oxidation of aqueous solution containing antibiotics with using new generation Sn/Sb/Ni: 500/8/1 anodes were carried out. pH, electrolyte type and conc. and current density parameter effects and total intermediate product formation was evaluated.
KCl was found as the best electrolyte type affecting the elctrochemical reactions positively the most even in lower concenrations. Thus, according to the results of the study, it could be possible to obtain higher removal efficiencies with real water/wastewater samples (assuming include KCl ions, mostly) without additon extra chemicals as the electrolyte. pH 8 is the neutral value of the aqueous solution containing antibiotics was obtained as the best due to provide higher removal efficiencies. Therefore, it could be possible to operate process easier and more economically by working at neutral pH values, due to there is no need to additional chemical cost for extra pH arrangement step. The removal efficiencies increased generally with the increase of current density, because of the occuring of active oxidants increasingly at higher values. 50 mA/cm2 was found as the best for current density, having full mineralization after 60 min. In our study, it was obtained more efficient results at lower current densities (50 mA/cm2) with Sn/Sb/Ni anode, compared to the most of the researches on electrochemical treatment of antibiotics. According to the results of the study, it is thought that the electrochemical oxidation processes could be carried out in real wastewaters without need to adding extra salt and pH arrangement step. So, it is easy and economical way to perform electrochemical treatment processes with Sn/Sb/Ni–Ti anodes with very high removal percentages for wastewaters containing antibiotics. Also, it needs less reaction time than the conventional treatment methods. At this respect, working with these anodes is promising for the future studies. In contrast to the other materials used for anode production, Sb-doped SnO2-Ni anodes don’t have much more toxicity and instability causing to the high costs. Additionally, these new generation anodes show very promising results in ozone production. However, most of the studies have focused on fluoroquinolone, trimethoprim, sulfonamide and macrolide for the removal of them from aquatic environments, while, just a little of them have been made for cefoperazone antibiotic. There are just only a few studies made about cefoperazone. The fact that there is no such studies ontreatment of these antibiotics with these new generation anodes has made this study unique.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Bennett, J. W. & Chung, K.-T. Alexander Fleming and the discovery of penicillin. Adv. Appl. Microbiol. 49, 163–184 (2001).
Manzetti, S. & Ghisi, R. The environmental release and fate of antibiotics. Mar. Pollut. Bull. 79, 7–15 (2014).
Petrović, M., Hernando, M. D., Díaz-Cruz, M. S. & Barceló, D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 1067, 1–14 (2005).
Blackwell, P. A. et al. Fast and robust simultaneous determination of three veterinary antibiotics in groundwater and surface water using a tandem solid-phase extraction with high-performance liquid chromatography–UV detection. J. Chromatogr. A 1045, 111–117 (2004).
Oberlé, K., Capdeville, M.-J., Berthe, T., Budzinski, H. & Petit, F. Evidence for a complex relationship between antibiotics and antibiotic-resistant Escherichia coli: From medical center patients to a receiving environment. Environ. Sci. Technol. 46, 1859–1868 (2012).
Anderson, P. D. Technical Brief: Endocrine Disrupting Compounds and Implications for Wastewater Treatment (Water Environment Research Foundation, 2005).
Orlando, E. F. et al. Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ. Health Perspect. 112, 353–358 (2004).
Guillette, L. J. Jr. et al. Alligators and endocrine disrupting contaminants: A current perspective. Am. Zool. 40, 438–452 (2000).
Kümmerer, K. Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks (Springer, 2008).
Klavarioti, M., Mantzavinos, D. & Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 35, 402–417 (2009).
Altikat, A., Ceylan, Z. & Gulbe, A. Forecasting of chlorophenols removing with advanced oxidation processes: An artificial neural networks application. Environ. Eng. Manag. J. (EEMJ) 19, 1275–1287 (2020).
Sirés, I. & Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 40, 212–229. https://doi.org/10.1016/j.envint.2011.07.012 (2012).
Yao, J., Mei, Y., Jiang, J., Xia, G. & Chen, J. Process optimization of electrochemical treatment of COD and total nitrogen containing wastewater. Int. J. Environ. Res. Public Health 19, 850 (2022).
Polcaro, A., Palmas, S., Renoldi, F. & Mascia, M. On the performance of Ti/SnO2 and Ti/PbO2 anodesin electrochemical degradation of 2-chlorophenolfor wastewater treatment. J. Appl. Electrochem. 29, 147–151 (1999).
Correa-Lozano, B., Comninellis, C. & De Battisti, A. Service life of Ti/SnO2–Sb2O5 anodes. J. Appl. Electrochem. 27, 970–974 (1997).
Wirzal, M. D. H., Yusoff, A. R. M., Zima, J. & Barek, J. Degradation of ampicillin and penicillin G using anodic oxidation. Int. J. Electrochem. Sci 8, 8978–8988 (2013).
Abbasi, M., Soleymani, A. R. & Parssa, J. B. Operation simulation of a recycled electrochemical ozone generator using artificial neural network. Chem. Eng. Res. Des. 92, 2618–2625. https://doi.org/10.1016/j.cherd.2014.02.027 (2014).
Haenni, W. et al. Diamond-sensing microdevices for environmental control and analytical applications. Diam. Relat. Mater. 7, 569–574 (1998).
Foord, J. S., Holt, K. B., Compton, R. G., Marken, F. & Kim, D.-H. Mechanistic aspects of the sonoelectrochemical degradation of the reactive dye Procion Blue at boron-doped diamond electrodes. Diam. Relat. Mater. 10, 662–666 (2001).
Choi, K. J., Kim, S. G., Kim, C. W. & Kim, S. H. Effects of activated carbon types and service life on removal of endocrine disrupting chemicals: Amitrol, nonylphenol, and bisphenol-A. Chemosphere 58, 1535–1545 (2005).
Christensen, P. et al. Room temperature, electrochemical generation of ozone with 50% current efficiency in 0.5 M sulfuric acid at cell voltages < 3V. Ozone Sci. Eng. 31, 287–293 (2009).
Yang, X., Zou, R., Huo, F., Cai, D. & Xiao, D. Preparation and characterization of Ti/SnO2–Sb2O3–Nb2O5/PbO2 thin film as electrode material for the degradation of phenol. J. Hazard. Mater. 164, 367–373. https://doi.org/10.1016/j.jhazmat.2008.08.010 (2009).
Cheng, S.-A. & Chan, K.-Y. Electrolytic generation of ozone on an antimony-doped tin dioxide coated electrode. Electrochem. Solid State Lett. 7, D4 (2004).
Gonçalves, A. G., Órfão, J. J. & Pereira, M. F. R. Catalytic ozonation of sulphamethoxazole in the presence of carbon materials: Catalytic performance and reaction pathways. J. Hazard. Mater. 239, 167–174 (2012).
Trovo, A. G., Nogueira, R. F. P., Agüera, A., Fernandez-Alba, A. R. & Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process—Chemical and toxicological assessment. Water Res. 45, 1394–1402 (2011).
Yonar, T. & Kurt, A. Treatability studies of hospital wastewaters with AOPs by Taguchi’s experimental design. Glob. Nest J. 19, 505–510 (2017).
Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 75, 417–434 (2009).
Liang, D., Wang, Y. & Wang, Y. A practical synthesis of deuterium-labeled cefuroxime. Mendeleev Commun. 4, 252–253 (2015).
EMBL-EBI. Cefoperazone (CHEBI:3493) www.ebi.ac.uk (2020).
Christensen, P. A., Zakaria, K., Christensen, H. & Yonar, T. The effect of Ni and Sb oxide precursors, and of Ni composition, synthesis conditions and operating parameters on the activity, selectivity and durability of Sb-doped SnO2 anodes modified with Ni. J. Electrochem. Soc. 160, H405–H413 (2013).
Federation, W. E. & Association, A. P. H. Standard Methods for the Examination of Water and Wastewater (American Public Health Association (APHA), 2005).
Wang, Y. H. Electrochemical Generation of Ozone on Antimony and Nickel Doped Tin Oxide (The University of Hong Kong, 2006).
Sivrioğlu, Ö. Yeni Elektrokatalistlerle Elektrokimyasal Ozon Üretimi Ve Uygulamalarının Araştırılması (Bursa Uludag University, 2016).
Pillai, I. M. S. & Gupta, A. K. Anodic oxidation of coke oven wastewater: Multiparameter optimization for simultaneous removal of cyanide, COD and phenol. J. Environ. Manag. 176, 45–53 (2016).
Yonar, T., Shakir, F. & Kurt, A. Investigation of electrochemical color removal from organized industrial district (OID) wastewater treatment plants using new generation Sn/Sb/Ni-Ti anodes. Glob. Nest J. 21, 106–112 (2019).
Buyukada, M. Turbidity and COD removal from leather effluents using TiO2–assisted photocatalytic-ozonation by response surface methodology. Environ. Res. Technol. 1, 1–10 (2018).
Duan, P., Jia, X., Lin, J. & Xia, R. Electro-oxidation of ceftazidime in real municipal wastewater using PbO2–Ce and SnO2–Sb electrodes: Influence of electrolyte and degradation pathway. J. Appl. Electrochem. 51, 183–195 (2020).
Wang, S.-D., He, L.-X., Zhou, L., Xian, S.-D. & Liu, J.-H. Electrochemical activation of peroxymonosulfate with titanium suboxide anode for 4-chlorophenol degradation: Influencing factors, kinetics, and degradation mechanism. Sep. Purif. Technol. 291, 120964 (2022).
Yao, J. et al. Process optimization of electrochemical oxidation of ammonia to nitrogen for actual dyeing wastewater treatment. Int. J. Environ. Res. Public Health 16, 2931 (2019).
Hai, H., Xing, X., Li, S., Xia, S. & Xia, J. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Sci. Total Environ. 738, 139909. https://doi.org/10.1016/j.scitotenv.2020.139909 (2020).
Qian, S. et al. Electrochemical degradation of tetracycline antibiotics using a Ti/SnO2-Sb2O3/PbO2 anode: Kinetics, pathways, and biotoxicity change. J. Electrochem. Soc. 166, E192 (2019).
Zakaria, K. & Christensen, P. A. The use of Ni/Sb–SnO2-based membrane electrode assembly for electrochemical generation of ozone and the decolourisation of Reactive Blue 50 dye solutions. Electrochim. Acta 135, 11–18 (2014).
Abbasi, M., Soleymani, A. R. & Parsa, J. B. Degradation of Rhodamine B by an electrochemical ozone generating system consist of a Ti anode coated with nanocomposite of Sn–Sb–Ni oxide. Process Saf. Environ. Prot. 94, 140–148 (2015).
Sivrioglu, O. & Yonar, T. 48-52 (Institute of Research Engineers and Doctors).
Li, X.-M., Wang, M., Jiao, Z. & Chen, Z. Study on electrolytic oxidation for landfill leachate treatment. China Water Wastewater 17, 14–17 (2001).
Deng, Y. & Englehardt, J. D. Electrochemical oxidation for landfill leachate treatment. Waste Manag. 27, 380–388 (2007).
Kaur, R., Kushwaha, J. P. & Singh, N. Electro-oxidation of Ofloxacin antibiotic by dimensionally stable Ti/RuO2 anode: Evaluation and mechanistic approach. Chemosphere 193, 685–694 (2018).
Wen, Z. et al. Mineralization of cefoperazone in acid medium by the microwave discharge electrodeless lamp irradiated photoelectro-Fenton using a RuO2/Ti or boron-doped diamond anode. J. Hazard. Mater. 374, 186–194. https://doi.org/10.1016/j.jhazmat.2019.03.124 (2019).
Zeng, W. et al. Efficient electrochemical oxidation of sulfamethoxazole by a novel reduced TiO2 nanotube arrays-based flow-through electrocatalytic membrane. Sep. Purif. Technol. 289, 120720 (2022).
Yahya, M. S., El Karbane, M., Oturan, N., El Kacemi, K. & Oturan, M. A. Mineralization of the antibiotic levofloxacin in aqueous medium by electro-Fenton process: Kinetics and intermediate products analysis. Environ. Technol. 37, 1276–1287 (2016).
Letti, C. J. et al. Synthesis, morphology and electrochemical applications of iron oxide based nanocomposites. Adv. Nano Res. 5, 215. https://doi.org/10.12989/anr.2017.5.3.215 (2017).
Isarain-Chávez, E. et al. Comparative electrochemical oxidation of methyl orange azo dye using Ti/Ir-Pb, Ti/Ir-Sn, Ti/Ru-Pb, Ti/Pt-Pd and Ti/RuO2 anodes. Electrochim. Acta 244, 199–208 (2017).
Souza, F. et al. Applicability of electrochemical oxidation using diamond anodes to the treatment of a sulfonylurea herbicide. Catal. Today 280, 192–198. https://doi.org/10.1016/j.cattod.2016.04.030 (2017).
Kurt, A. & Yonar, T. Endokrin Bozucu Antibiyotik Bileşiklerinin UV/H2O2 Prosesi ile Taguchi Deneysel Dizaynına Göre Arıtılabilirliği. Afyon Kocatepe Üniversitesi Fen Ve Mühendislik Bilimleri Dergisi 17, 854–860. https://doi.org/10.5578/fmbd.57594 (2016).
Christensen, P., Zakaria, K. & Curtis, T. Structure and activity of Ni-and Sb–doped SnO2 ozone anodes. Ozone Sci. Eng. 34, 49–56. https://doi.org/10.1080/01919512.2012.639687 (2012).
Zhi, D., Qin, J., Zhou, H., Wang, J. & Yang, S. Removal of tetracycline by electrochemical oxidation using a Ti/SnO2–Sb anode: Characterization, kinetics, and degradation pathway. J. Appl. Electrochem. 47, 1313–1322 (2017).
Shmychkova, O., Luk’yanenko, T., Dmitrikova, L. & Velichenko, A. Modified lead dioxide for organic wastewater treatment: Physicochemical properties and electrocatalytic activity. J. Serb. Chem. Soc. 84, 187–198 (2019).
Funding
The authors acknowledge the support of the Bursa Uludag University Research Projects Department for this study. The Project No. OUAP(MH)-2018/8.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.K. and T.Y.; funding acquisition, T.Y.; investigation, A.K. and T.Y.; methodology, A.K. and T.Y.; project administration, A.K. and T.Y.; resources, T.Y.; supervision, T.Y.; visualization, A.K.; writing—original draft, A.K.; writing—review and editing A.K. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurt, A., Yonar, T. The evaluation of parameter effects on cefoperazone treatability with new generation anodes. Sci Rep 12, 14096 (2022). https://doi.org/10.1038/s41598-022-18486-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18486-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.