Abstract
Microbial fuel cells (MFCs) are a self-sustaining and environmentally friendly system for the simultaneous was tewater treatment and bioelectricity generation. The type and material of the electrode are critical factors that can influence the efficiency of this treatment process. In this study, graphite plates and carbon felt were modified through the electrodeposition of nickel followed by the formation of a biofilm, resulting in conductive bio-anode thin film electrodes with enhanced power generation capacity. The structural and morphological properties of the electrode surfaces were characterized using X-ray diffraction, energy-dispersive X-ray spectroscopy, elemental mapping, and field-emission scanning electron microscopy techniques. Maximum voltage, current density, and power generation were investigated using a dual-chamber MFC equipped with a Nafion 117 membrane and bio-nickel-doped carbon felt (bio-Ni@CF) and bio-nickel-doped graphite plate (bio-Ni@GP) electrodes under constant temperature conditions. The polarization and power curves obtained using different anode electrodes revealed that the maximum voltage, power and current density achieved with the bio-Ni@CF electrode were 468.0 mV, 130.72 mW/m2 and 760.0 mA/m2 respectively. Moreover, the modified electrodes demonstrated appropriate stability and resistance during successful runs. These results suggest that nickel-doped carbon-based electrodes can serve as suitable and stable supported catalysts and conductors for improving efficiency and increasing power generation in MFCs.
Similar content being viewed by others
Introduction
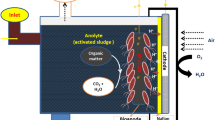
Microbial fuel cells (MFCs) represent a novel and eco-friendly technology for the simultaneous treatment of wastewater and generation of bioelectricity1,2. The configuration of MFCs can be single-chambered or dual-chambered designs, depending on the objective of the study, dual-chambered mfc is frequently employed3. In Dual-Chamber MFCs (DC-MFC), the cathode anode are completely separated by a Proton Exchange Membrane (PEM), facilitating the maintenance of distinct conditions within each chamber. Moreover, the ability to integrate MFCs with other treatment methods allows for the cathode chamber to be utilized for the removal of various organic and pharmaceutical pollutants4,5. In the anaerobic anodic chamber, electrogenic microbes serve as active biocatalysts for the oxidation of organic materials, producing protons, electrons, and carbon dioxide as final products6 (Eq. 1). Electrons and protons are transferred from the anodic chamber to the cathodic chamber via the external circuit and membrane, generating an electric current through electrochemical reactions with electron acceptors6,7 (Eq. 2).
The generation of bioelectricity in MFCs is subject to variation under disparate conditions8. For example, the application of nanomaterials for the modification of anode and cathode electrode surfaces, the utilization of diverse substrates such as acetate and glucose at varying concentrations, the configuration of the cell, the type of ion exchange membrane employed, temperature, anolyte pH, internal and external cell resistance, as well as electrode distance can all exert significant influence on the electricity generation process8. Recent investigations have concentrated on enhancing output power and optimizing performance with in MFC systems. However, poor power generation and high cost are drawbacks that impede the widespread commercialization and practical application of MFCs8. To address these issues, researchers are focusing on the modification and construction of new materials for anodes and cathodes as an effective approach to improving MFC performance9,10. Ideal electrode materials should possess properties such as high electronic conductivity, high surface area, environmental friendliness, suitable chemical and mechanical stability, and economic affordability10. Carbon fibers, carbon cloth, carbon paper, carbon brushes, carbon felt, graphite, and graphene are some of the materials that have been utilized as electrodes in MFCs11. It is noteworthy that altering the physical and chemical characteristics of bare electrodes can increase MFC efficiency by improving electron transfer and microbial attachment12. In recent years, doping and modification of bare electrodes with various nanoparticles such as gold13 manganese14, platinum15, and its alloys16 have been commonly used as supported catalysts to increase current density and improve electron transfer in MFCs. However, their high cost and limited availability present obstacles to large-scale applications17. In recent investigations, nickel nanoparticles have been scrutinized as a coating for cathode Ni/C18, Ti/Ni19, rGO/Ni20, Fe/Ni21 and other doping methods for the anode electrode such as Ni@Fe2O3/MXene-cf22, owing to their cost- effectiveness, high conductivity, low resistance, and high biocompatibility. As a result, scientists have focused on the use of inexpensive and readily available catalysts with simple and eco-friendly modification techniques23,24. Anodic and cathodic electrodeposition25,26,27,28,29, paired electrodeposition30, electrochemically assisted self-assembly31, electro-grafting32 and electro-polymerization33,34,35 are green, versatile, and eco-friendly procedures for the fabrication and modification of electrodes with different electroactive thin films for targeted applications. Electroplating is a general procedure for preparing metallic thin layer films on inert substrates through the cathodic electrodeposition of dissolved or anodically released metal cations under a direct electric current density36,37,38. Black nickel and hard nickel are two different types of nickel electrodeposition. In this study, the hard nickel method was chosen due to its tensile strength and increased resistance to abrasion and corrosion36,37,38. The aim of this study was to use electroplating or cathodic electrodeposition as a simple, one-step, green, and in-situ method for modifying carbon felt and graphite plate with a nickel thin film, resulting in a low-cost, highly conductive, and highly resistant electrode. Subsequently, the nickel-doped electrodes were post-modified with microorganisms to create novel and efficient bio-anode materials through the growth of biofilms. To the best of our knowledge, no previous studies have reported on the cathodic electrodeposition of bio-nickel doped carbon felt (bio-Ni@CF) or bio-nickel doped graphite plate (bio-Ni@GP) electrodes for use as bio-anodes in an MFC system. In this comprehensive and comparative study, various parameters such as maximum voltage, current density, power generation, and electrode stability were investigated using a dual-chamber microbial fuel cell equipped with bio-Ni@CF and bio-Ni@GP electrodes under constant temperature conditions for bioelectricity generation.
Experimental section
Chemical and materials
Potassium dihydrogen phosphate (KH2PO4), dipotassium hydrogen phosphate (K2HPO4), ammonium chloride (NH4Cl), glucose (C6H12O6, H2O), calcium chloride (CaCl2), magnesium sulfate (MgSO4), potassium chloride (KCl), sodium chloride (NaCl), sulfuric acid (H2SO4 95.0–97.0% purity), hydrochloric acid (HCl 37.0%), sodium hydroxide (NaOH), Nafion 117 (Sigma-Aldrich), peptone, yeast, anhydrous sodium sulfate (Na2SO4), boric acid (H3BO3), were purchased from Merck Company and used without further purification. All aqueous solutions were prepared daily using distilled water at room temperature. All chemicals used were of analytical grade.
Preparation of modified electrodes
The electroplating or cathodic electrodeposition method was employed to coat the surfaces of carbon felt and graphite electrodes with a nickel metallic thin film. Initially, the CF and GP electrodes were pretreated by soaking in acetone solution for 20 min, boiling in 0.1 M HCl for 15 min, and then washing with water to remove any oils and foreign particles from their surfaces39. The electroplating process was carried out in an undivided homemade reactor consisting of a metallic nickel plate as the anode, graphite plate and carbon felt as the cathode, and nickel sulfate (NiSO4·6H2O) as the supporting electrolyte in an aqueous solution of boric acid (H3BO3) at room temperature (Table 1). By applying a suitable current density for a specific period of time, the metallic anode begins to oxidize and releases the desired metallic cations (Ni2+) into the solution. Simultaneously, the dissolved metallic cations undergo cathodic electrodeposition onto the graphite plate and carbon felt electrodes. Finally, the nickel-coated graphite plate and carbon-felt electrodes are removed from the solution and rinsed with water (Figs. 1 and 2).
Microbial fuel cell set-up and operation
This study was conducted using a dual-chambered plexiglass homemade (100 × 100 × 30 mm) Microbial Fuel Cell (DC-MFC) reactor with anodic and cathodic chambers, each with a volume of 350.0 mL40,41. The anodic and cathodic chambers were separated by a proton exchange membrane (Nafion 117, Sigma-Aldrich, USA) to facilitate the migration of H+ ions from the anodic to the cathodic chamber42. The Nafion membrane was pre-treated in five steps to remove any foreign contaminants and improve proton transportability. The membrane was washed sequentially with deionized water, H2O2 (3.0%), deionized water, 1.0 M HCl, and finally deionized water, with each step lasting for one hour at a temperature of 80 °C43. Carbon felt was used as the cathode in all reactors, while the anodes were made of bare graphite plate (GP), bare carbon felt (CF), nickel- coated carbon felt (Ni@CF), and nickel-coated graphite plate (Ni@GP), each with a surface area of 12.0 cm2 (40.0 mm * 30.0 mm * 3.0). The MFC reactor operated in batch mode at a constant temperature of 30 ± 1 °C and atmospheric pressure. Anaerobic sludge procured from the wastewater treatment plant in Urmia city was utilized as the source of requisite microorganisms for the anode chamber. To foster the growth and proliferation of these microorganisms and to prepare the inoculum, they were introduced into a culture medium (g/L) composed of 0.5 ammonium chloride, 5 glucose, 3 yeast extract, and 1 peptone. This mixture was subsequently incubated under anaerobic conditions for a duration of 24 h at a temperature of 30 °C. A v/v ratio of 10% of the prepared inoculum was introduced into the anode chamber6. Additionally, glucose at a concentration of 0.5 g per liter was employed as a carbon substrate and synthetic wastewater containing K2HPO4 (1.4), KH2PO4 (0.25), NH4Cl (0.31), MgSO4 (0.1), KCl (0.13), NaCl (0.1), and 1 mL/L of a mineral solution (g/L) encompassing FeCl3.6H2O (1.5), H3BO3 (0.15), CuSO4.5H2O (0.03), KI (0.03), MnCl2.4H2O(0.12), NaMoO4.2H2O (0.06), ZnSO4.H2O (0.12), and CoCl2.6H2O (0.15) was utilized in the anode chamber39,44. The anolyte was adjusted to a pH of 7.2 by phosphate buffer for microbial activity in the seed sludge39. Prior to the start of each run and the addition of inoculum, the anolyte was purged with N2 gas for 5 min to create an anaerobic environment and remove any oxygen6. In addition, in the cathode chamber, a buffer of 100 mM was employed, and dissolved oxygen was consistently supplied via the utilization of an air pump45 (Fig. 3).
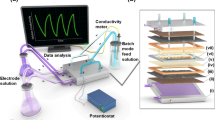
A data logger device (DGHS-EL 2501, Danesh Gostar Hamgam Ba Sanat Company, Iran) for data recording was interfaced with a computer system. This system, equipped with MINITAB and MATLAB software, possesses the capability for online recording of open circuit voltage (OCV). Within the reactor setup, to capture OCV without the application of resistance, the generated voltage was recorded online at 30-min intervals. Upon establishing stable conditions and generating a constant voltage, the maximum power density and current density were ascertained by applying variable resistances ranging from 0.01 to 100.0 kΩ. Subsequently, the maximum power density (mW/m2) and current (mA/m2) were normalized in accordance with Eqs. (3) and (4), and voltage and power density curves were plotted against the current46.
where I, R, P, and V indicate the produced current, electrical resistance, power density, and cell voltage, respectively.
Instrumental section
Field-emission scanning electron microscopy (FE-SEM; JEOL JSM-840A, Japan) was employed to investigate the surface morphology of the bio-anodes. The presence of nickel (Ni) on the bio-anodes was confirmed using energy-dispersive X-ray spectroscopy mapping (EDX; Bruker XFlash6L10) and elemental mapping. The crystal patterns of the deposited Ni thin film were analyzed using X-ray diffraction (XRD; Ultima IV, Rigaku). The voltage produced by the MFC was recorded using a data logger system (DGHS-EL2501, Danesh Gostar Hamgam Ba Sanat Company, Iran).
Results and discussions
Characterization of modified electrodes
In this study, the electroplating or cathodic electrodeposition method was utilized to coat graphite plate (GP) and carbon felt (CF) electrode surfaces with a nickel metallic thin film. The modification and various characteristics of the nickel-coated GP (Ni@GP) and nickel-coated CF (Ni@CF) electrodes were confirmed and characterized using X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDS), elemental mapping, and field-emission scanning electron microscopy (FE-SEM) techniques. The crystal structure of Ni@GP and Ni@CF was investigated using XRD (Fig. 4). The well-defined crystal structure of the coated nickel thin film was evidenced by the wide-angle XRD pattern, as reported in a previous publication47. The observed distinct peaks at 2Ɵ = 44.22°, 51.9° and 77.0° were attributed to the diffraction planes of [100], [40] and [20], respectively, confirming the synthesis of Ni nanoparticles on graphite plate and carbon felt based on the referenced JCPDS Cards Number of Ni-3600-r1.
Figures 5 and 6 depict the energy-dispersive X-ray (EDX) spectrum and elemental mapping images of the Ni@GP and Ni@CF electrode surfaces, respectively. The elemental analysis of the Ni@CF and Ni@GP electrodes reveals distinct peaks corresponding to nickel (deposited ions) and carbon (underlying substrate), confirming the accuracy of the electroplating procedure (Figs. 5-Up and 6-Up). Furthermore, the homogeneous and uniform distribution of nickel on the carbon plate and graphite felt is evidenced by the presence of nickel in the structure (Figs. 5-Down and 6-Down).
Figure 7 presents the field-emission scanning electron microscopy (FE-SEM) images of the bare and nickel-coated carbon felt (CF) and graphite plate (GP) electrodes. As can be seen from the large and close-up views of the recorded images, nickel nanoparticles have been continuously deposited onto the CF and GP electrode surfaces, in contrast to the bare electrodes. The observed morphological images reveal that the size and dimensions of the prepared and deposited nickel nanoparticles on the CF are smaller than those on the GP, due to the larger surface area of the stranded CF compared to the flat GP under constant applied current density for a fixed duration.
In the bio-anode synthesis procedure, the prepared nickel-coated carbon felt (Ni@CF) and nickel-coated graphite plate (Ni@GP) electrodes in a microorganism solution for one month during several loading were immersed in a microorganism solution to facilitate the growth and stabilization of a biofilm on the Ni-coated electrodes, resulting in the fabrication of bio-Ni@CF and bio-Ni@GP electrodes. Figure 8 depicts the presence of microorganisms as a biofilm on the Ni-coated GP and CF electrodes. As mentioned before, To supply the microbial communities and form a biofilm layer, mixed active microorganisms from anaerobic sludge were used. Recognized species such as Klebsiella Pneumonia, Bacillus Subtilis, Shewanella, Enterobacter, Geobacter, Pseudomonas, Aeromonas. These electrogenic bacteria are capable of forming a biofilm layer and performing direct and mediated extracellular electron transfer(EET)48. A comparison of the images of the bio-Ni@CF and bio-Ni@GP electrodes, as shown in Fig. 8, reveals that, the characteristics of the anode surface have a significant impact on the colonization of exoelectrogenic bacteria. CF has a larger and more suitable surface area than GP for the penetration and attachment of microorganisms due to its high porosity49. GP may exhibit poor microbial attachment due to its uniform and smooth surface50. In addition the use of Nanomaterials as a catalyst with high biocompatibility can enhance electron transfer, reduce internal resistance, increase energy generation, and improve the efficiency of microbial fuel cells due to increased interaction between the electrode surface and microorganisms51. A similar study conducted by Zhong et al. found that modification of the CF surface affected the microbial community and increased the formation of biofilm, leading to an increase in voltage, current density, and power output18. Additionally, the results of a study by Ouis et al. confirmed that modification of the graphite electrode with conductive substances increased electron transfer and power density, which was attributed to the high adhesion of bacterial species on the anode and the formation of a more stable biofilm52.
Furthermore, Fig. 9a–f illustrates real photographic images of the bare GP and CF, Ni@GP and Ni@CF, bio-Ni@GP and bio-Ni@CF electrodes, respectively.
Electrochemical performance of MFC
The open circuit voltage (OCV) of the MFC was automatically recorded using a data logger connected to a computer system systemwithout applying resistance every 30 min for 80 h. The voltage increased rapidly with each loading due to the addition of new substrate, then reached a steady state before decreasing due to substrate depletion and reloading. Monitoring the OCV data for four loadings of the MFC with different anodes indicated the suitable resistance and stability of the Ni film over time, as well as the development and growth of the biofilm.
According to the obtained OCV data, the highest average voltage output was observed using modified bio-Ni@CF (468.0 mV) and bio-Ni@GP (422.0 mV) electrodes, compared to bare CF(382.0 mV) and GP (301.0 mV) electrodes. Polarization and power density curves were obtained for different electrodes after several loading steps and biofilm formation, as well as ensuring a steady state of MFC conditions in terms of voltage generation by applying variable resistances (0.01–100.0 kΩ). As shown in Fig. 10b, the average maximum voltage output increased from 216.0 mV to 368.0, 382.0, and 394.0 mV for bio-Ni@CF, bio-Ni@GP, bio-CF, and bio-GP electrodes, respectively.
As shown in Fig. 10c, the type of electrode used had an effect on increasing power density output in the MFC system. The maximum power density and current density using the bio-Ni@CF electrode were 130.72 mW/m2 and 760.0 mA/m2, respectively. On the other hand, the maximum power density using bio-Ni@GP, bio-CF, and bio-GP reached 106.58, 55.04, and 27.01 mW/m2, respectively, which was 1.22, 2.37, and 4.83 times lower than that of the bio-Ni@CF electrode. Furthermore, the maximum current density for bio-Ni@GP, bio-CF, and bio-GP electrodes was 185.0, 430.0, and 730.0 mA/m2.
Therefore, as shown in Fig. 10, the bio-Ni@CF electrode with maximum voltage, power density, and current density was determined to be the optimal electrode and most suitable alternative material for improving MFC performance and electricity generation.
This study found that the efficiency of the modified carbon felt electrode was higher than that of the modified graphite electrode. Carbon felt has a porous structure due to its dispersed carbon fibers, as shown in the corresponding field-emission scanning electron microscopy (FE-SEM) images, which allows for greater attachment of electrogenic microorganisms to its surface and increased electron transfer rates compared to the smooth surface of graphite. A study by Rashidi et al. compared the performance of carbon felt and graphite electrodes in a two-chamber fuel cell and found that the maximum voltage, power density, and current density achieved using carbon felt electrodes were 1.38, 3.97, and 1.87 times higher, respectively, than those achieved using graphite electrodes.
The increase in power production observed with carbon felt was attributed to its highly active surface and structure compared to the smooth surface of graphite45.
Modifying the anodic electrode with nanomaterials to enhance bacterial growth on the anode and strengthen microbial attachment leads to improved performance and increased efficiency of microbial fuel cells (MFCs). As shown in Fig. 10, the bio-Ni@GP and bio-Ni@CF electrodes exhibit the highest energy generation efficiency compared to the bare GP and CF electrodes. In other words, nickel acts as a catalyst to increase conductivity and improve the electroactive properties of the electrodes. The investigation into the maximum power and current output using nickel foam as an anode, by Karthikeyan et al.53 also emphasized that the emproved of anode structural significantly effective in inhansing the generation power and current density. In another study Tahir et.al observed that the maximum power and current density in modified anodes significantly surpassed those of Carbon Felt (CF). In fact, the maximum power and current output using NiFe2O4-MXene@CF were 5.6 and 6.6 times higher than CF, respectively22. The power generated in the two mentioned studies showed a significant increase compared to our study, and this difference can be attributed to the fact that nickel particles were used in the present study, while in the mentioned studies, the synergistic effect of different compounds led to an increase Conductivity and more load transfer, also the use of different substrates in the anode chamber can be one of the factors influencing the production power. Yang et al. reported that the maximum power and current output obtained by the modified CF/Bio-Feox electrode were 71.64% and 59.21% respectively, higher than that of CF due to the high conductivity of metal oxide and high catalytic activity of the modified anode, leading to increased electron transfer, reduced internal resistance, and ultimately increased power output54. Similarly, Zhang et al. reported that the power density of the graphite/polyaniline-tourmaline electrode as a bio-cathode was 492.6% and 192.8% higher than that of unmodified cathodes and graphite/aniline cathode, respectively55. Table 2 shows the comparison of the present paper with previously reviews on the application of nanomaterial in MFCs.
The results obtained in this study, as well as previous studies, have demonstrated that Ni can be used as a stable catalyst compatible with the conditions of microorganisms in the bio-anode. One of the factors affecting the stability of doped nanoparticles on the electrode is the doping method19. In previous reports, Ni was used as a catalyst in the cathode to modify activated carbon, which led to a reduction in electrode conductivity and electrical performance due to the use of polytetrafluoroethylene (PTFE)56. However, in the present study, the direct and in-situ electroplating or cathodic electrodeposition process was identified as an applicable method for the resistant and stable doping of nanoparticles on the electrode. As shown in Fig. 10a, the voltage output of four loadings using Ni@CF and Ni@GP electrodes did not change in the bio-anode. It should be noted that the results obtained in this study are consistent with those reported by Jia Liu et al. on the use of Ni, Fe, and Ni/Fe as cathode catalysts. Their study indicated that the use of Fe and Ni nanoparticles stimulated and increased bacterial growth on the cathode. However, over time, there was a reduction due to the destruction of the Fe catalyst. In contrast, Ni had the greatest effect compared to Fe and Ni/Fe on bacterial growth due to the relative stability of Ni nanoparticles compared to other catalysts57.
Given that the concentration of the coated catalyst can be an important parameter for improving the performance of microbial fuel cells (MFCs), the maximum voltage, power density, and current density output at different concentrations of nickel (Ni) on the carbon felt electrode were investigated to determine the optimal electrode (Fig. 11). Therefore, optimizing the concentration of the catalyst is crucial for improving and modifying the surface of anode electrodes. According to Table 3, the maximum voltage, power density, and current density output were obtained in the presence of bare carbon felt and modified carbon felt at concentrations of 0.2, 0.4, and 0.6 mg/cm2 of Ni. The results shown in Fig. 11a,b indicate an increase in maximum voltage, power density, and current density with increasing concentration of the utilized catalyst in the anodic chamber. The lowest voltage, current density, and power density were attributed to the bare carbon felt electrode, while the highest voltage, current density, and power density were obtained for the modified carbon felt electrodes with a concentration of 0.6 mg/cm2, indicating increases of 25.97%, 43.42%, and 57.89%, respectively. Increasing the concentration of employed catalyst in the structure of the anodic electrode can be effective in increasing power density and improving the performance of MFCs due to the increase in surface area and high conductivity of nanomaterials57. However, the results of the present and previous studies have shown that using higher concentrations above threshold limits can have a negative impact on microbial growth and activity due to increased toxicity and reduced biocompatibility, leading to microbial inactivation. On the other hand, increasing the concentration of the catalyst beyond threshold limits may lead to saturation of the electrode surface and reduced attachment of microorganisms on the electrode58.
This survey demonstrated that a Ni-coated carbon felt electrode, produced using the electroplating method, can be utilized as a high-efficiency and stable bio-anode material for bio-electrogeneration and wastewater treatment processes. Our team’s research on the use of bio-Ni@CF electrodes in microbial fuel cell (MFC) systems for electrogeneration and removal of various pollutants from water environments is ongoing.
Conclusion
The results of this study demonstrated that nickel, as a catalyst with high biocompatibility, increases conductivity and improves the electrical properties of carbon felt and graphite electrodes as bio-anodes, thereby enhancing the performance and efficiency of microbial fuel cells (MFCs). The maximum voltage, power density, and current density produced using carbon felt as an optimal electrode were significantly higher than those of other electrodes, at 468.0 mV, 130.72 mW/m2, and 760.0 mA/m2, respectively. However, increasing the concentration of the employed catalyst in the bio-anode led to a decrease in microbial growth due to increased toxicity and electrode surface saturation. As such, a concentration of 0.5 mg/cm2 of nickel was determined to be optimal. Additionally, the electrochemical deposition method can be used as a green, versatile, and applicable method for the resistant and stable deposition of nanoparticles on bio-anode electrodes. Finally, the fabrication of bio-Ni@CF modified electrodes using electroplating techniques can be employed as a novel, efficient, and versatile bio-anode in different MFC systems for increasing conductivity, improving electron transfer kinetics, promoting higher growth and stabilization of microorganisms, enhancing power density, and improving wastewater treatment.
Data availability
The authors declare that the all data generated or analyzed during this study are included in this published article.
References
Hemdan, B. A., El-Taweel, G. E., Naha, S. & Goswami, P. Bacterial community structure of electrogenic biofilm developed on modified graphite anode in microbial fuel cell. Sci. Rep. 13, 1255 (2023).
Yang, X. et al. Microbial fuel cell cathode with dendrimer encapsulated Pt nanoparticles as catalyst. J. Power Sour. 196, 10611–10615 (2011).
Bose, D., Santra, M., Sanka, R. V. S. P. & Krishnakumar, B. Bioremediation analysis of sediment-microbial fuel cells for energy recovery from microbial activity in soil. Int. J. Energy Res. 45, 6436–6445 (2021).
Saravanan, N. & Karthikeyan, M. Study of single chamber and double chamber efficiency and losses of wastewater treatment. Int. Res. J. Eng. Technol. 5, 1225–1230 (2018).
Soltani, F., Navidjouy, N., Khorsandi, H., Alizadeh, S. & Rahimnejad, M. Tetracycline removal from wastewater and electricity generation in microbial electro-Fenton system in different electrical circuit conditions. J. Mazandaran Univ. Med. Sci. 32, 123–134 (2022).
Rahmani, A. R. et al. Application of the eco-friendly bio-anode for ammonium removal and power generation from wastewater in bio-electrochemical systems. J. Clean. Prod. 243, 118589 (2020).
Koffi, N. D. J. & Okabe, S. High voltage generation from wastewater by microbial fuel cells equipped with a newly designed low voltage booster multiplier (LVBM). Sci. Rep. 10, 18985 (2020).
Zhang, X., Li, X., Zhao, X. & Li, Y. Factors affecting the efficiency of a bioelectrochemical system: A review. RSC Adv. 9, 19748–19761 (2019).
Hindatu, Y., Annuar, M. & Gumel, A. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 73, 236–248 (2017).
Zhong, D., Liao, X., Liu, Y., Zhong, N. & Xu, Y. Enhanced electricity generation performance and dye wastewater degradation of microbial fuel cell by using a petaline NiO@ polyaniline-carbon felt anode. Bioresour. Technol. 258, 125–134 (2018).
Dessie, Y. & Tadesse, S. Nanocomposites as efficient anode modifier catalyst for microbial fuel cell performance improvement. J. Chem. Rev. 3, 320–344 (2021).
Mashkour, M., Rahimnejad, M., Raouf, F. & Navidjouy, N. A review on the application of nanomaterials in improving microbial fuel cells. Biofuel Res. J. 8, 1400–1416 (2021).
Wu, X. et al. Anode modification by biogenic gold nanoparticles for the improved performance of microbial fuel cells and microbial community shift. Bioresour. Technol. 270, 11–19 (2018).
Qiu, S., Guo, Z., Naz, F., Yang, Z. & Yu, C. An overview in the development of cathode materials for the improvement in power generation of microbial fuel cells. Bioelectrochemistry 141, 107834 (2021).
Zhao, S. et al. Three-dimensional graphene/Pt nanoparticle composites as freestanding anode for enhancing performance of microbial fuel cells. Sci. Adv. 1, e1500372 (2015).
Quan, X., Mei, Y., Xu, H., Sun, B. & Zhang, X. Optimization of Pt-Pd alloy catalyst and supporting materials for oxygen reduction in air-cathode microbial fuel cells. Electrochim. Acta 165, 72–77 (2015).
Cetinkaya, A. Y., Ozdemir, O. K., Koroglu, E. O., Hasimoglu, A. & Ozkaya, B. The development of catalytic performance by coating Pt–Ni on CMI7000 membrane as a cathode of a microbial fuel cell. Bioresour. Technol. 195, 188–193 (2015).
Choi, Y.-J. et al. Electrophoretically fabricated nickel/nickel oxides as cost effective nanocatalysts for the oxygen reduction reaction in air-cathode microbial fuel cell. Int. J. Hydrogen Energy 45, 5960–5970 (2020).
Satar, I. et al. Performance of titanium–nickel (Ti/Ni) and graphite felt-nickel (GF/Ni) electrodeposited by Ni as alternative cathodes for microbial fuel cells. J. Taiwan Inst. Chem. Eng. 89, 67–76 (2018).
Pattanayak, P., Papiya, F., Pramanik, N. & Kundu, P. P. Deposition of Ni–NiO nanoparticles on the reduced graphene oxide filled polypyrrole: Evaluation as cathode catalyst in microbial fuel cells. Sustain. Energy Fuels 3, 1808–1826 (2019).
Liu, J. & Vipulanandan, C. Effects of Fe, Ni, and Fe/Ni metallic nanoparticles on power production and biosurfactant production from used vegetable oil in the anode chamber of a microbial fuel cell. Waste Manag. 66, 169–177 (2017).
Tahir, K. et al. Nickel ferrite/MXene-coated carbon felt anodes for enhanced microbial fuel cell performance. Chemosphere 268, 128784 (2021).
Luo, S. & He, Z. Ni-coated carbon fiber as an alternative cathode electrode material to improve cost efficiency of microbial fuel cells. Electrochim. Acta 222, 338–346 (2016).
Mashkour, M. et al. Application of wet nanostructured bacterial cellulose as a novel hydrogel bioanode for microbial fuel cells. ChemElectroChem 4, 648–654 (2017).
Soury, S., Bahrami, A., Alizadeh, S., Shahna, F. G. & Nematollahi, D. Development of a needle trap device packed with zinc based metal-organic framework sorbent for the sampling and analysis of polycyclic aromatic hydrocarbons in the air. Microchem. J. 148, 346–354 (2019).
Naseri, A. M. et al. Synthesis and application of [Zr-UiO-66-PDC-SO3H] Cl MOFs to the preparation of dicyanomethylene pyridines via chemical and electrochemical methods. Sci. Rep. 11, 16817 (2021).
Kalhor, S. et al. Anodic electrosynthesis of MIL-53 (Al)-N (CH2PO3H2)2 as a mesoporous catalyst for synthesis of novel (N-methyl-pyrrol)-pyrazolo [3, 4-b] pyridines via a cooperative vinylogous anomeric based oxidation. Sci. Rep. 11, 19370 (2021).
Souri, Z., Mazloum-Ardakani, M., Alizadeh, S. & Nematollahi, D. Template-free electrodeposition of sponge-like porous polymer interwoven with the bi-metallic metal-organic framework and reduced graphene oxide and application in energy storage device. J. Energy Storage 55, 105381 (2022).
Rahimpoor, R., Firoozichahak, A., Alizadeh, S. & Nematollahi, D. Urinary bio-monitoring of amphetamine derivatives by needle trap device packed with the zirconium-based metal–organic framework. Sci. Rep. 12, 13702 (2022).
Alizadeh, S. & Nematollahi, D. Convergent and divergent paired electrodeposition of metal-organic framework thin films. Sci. Rep. 9, 14325 (2019).
Alizadeh, S. & Nematollahi, D. Electrochemically assisted self-assembly technique for the fabrication of mesoporous metal–organic framework thin films: composition of 3D hexagonally packed crystals with 2D honeycomb-like mesopores. J. Am. Chem. Soc. 139, 4753–4761 (2017).
Salehzadeh, H., Nematollahi, D. & Alizadeh, S. Electrografting of 4-tert-butylcatechol on GC electrode. Selective electrochemical determination of homocysteine. Electroanalysis 27, 2738–2744 (2015).
Youseflouei, N., Alizadeh, S., Masoudi-Khoram, M., Nematollahi, D. & Alizadeh, H. A comprehensive electrochemical study of 2-mercaptobenzoheterocyclic derivatives. Air-assisted electrochemical synthesis of new sulfonamide derivatives. Electrochim. Acta 353, 136451 (2020).
Pourghobadi, R., Nematollahi, D., Baezzat, M. R., Alizadeh, S. & Goljani, H. Electropolymerization of catechol on wireless graphite electrode. Unusual cathodic polycatechol formation. J. Electroanal. Chem. 866, 114180 (2020).
Souri, Z., Alizadeh, S., Nematollahi, D., Mazloum-Ardakani, M. & Karami, A. A green and template-free electropolymerization of imipramine. The decoration of sponge-like polymer film with gold nanoparticles. J. Electroanal. Chem. 894, 115340 (2021).
Schlesinger, M. & Paunovic, M. Modern Electroplating Vol. 52 (John Wiley & Sons, 2014).
Dubpernell, G. The Story of Nickel Plating (M. & T. Chemicals, Incorporated, 1959).
Nebiolo, W. P. & Chemicals, R. The history of electroplating and a historical review of the evolution of NASF. History 86, 1–14 (2022).
Rahmani, A. R. et al. Effect of different concentrations of substrate in microbial fuel cells toward bioenergy recovery and simultaneous wastewater treatment. Environ. Technol. 43, 1–9 (2022).
Bose, D., Mukherjee, A. & Mitra, G. Energy recovery prospects of fuel cell technologies: Sustainability and bioremediation. Aust. J. Mech. Eng. 20, 736–748 (2022).
Bhattacharya, R. et al. Bioremediation and bioelectricity from Himalayan rock soil in sediment-microbial fuel cell using carbon rich substrates. Fuel 341, 127019 (2023).
Bhattacharya, R. et al. Overview of the advances in plant Microbial fuel cell technology for sustainable energy recovery from rhizodeposition. Biotechnol. Bioeng. 120, 1455–1464 (2023).
Yavari, Z. et al. www. SID. ir.
Khorsandi, H., Movahedyan, H., Bina, B. & Farrokhzadeh, H. Innovative anaerobic upflow sludge blanket filtration combined bioreactor for nitrogen removal from municipal wastewater. Int. J. Environ. Sci. Technol. 8, 417–424 (2011).
Rashadi, F., Navidjouy, N., Aghapour, A. A. & Rahimnejad, M. Application of dual chamber microbial fuel cell with aeration cathode for bioelectricity generation and simultaneous industrial wastewater treatment. Iran. J. Health Environ. 14, 473–486 (2021).
Li, N., Liu, L. & Yang, F. Power generation enhanced by a polyaniline–phytic acid modified filter electrode integrating microbial fuel cell with membrane bioreactor. Sep. Purif. Technol. 132, 213–217 (2014).
Singh, S. et al. A nickel oxide-decorated in situ grown 3-D graphitic forest engrained carbon foam electrode for microbial fuel cells. Chem. Commun. 57, 879–882 (2021).
Navidjouy, N., Soltani, F. & Rahimnejad, M. Biological Fuel Cells 301–320 (Elsevier, 2023).
Zhao, Y., Ma, Y., Li, T., Dong, Z. & Wang, Y. Modification of carbon felt anodes using double-oxidant HNO3/H2O2 for application in microbial fuel cells. RSC Adv. 8, 2059–2064 (2018).
Wei, J., Liang, P. & Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 102, 9335–9344 (2011).
Wu, X., Shi, Z., Zou, L., Li, C. M. & Qiao, Y. Pectin assisted one-pot synthesis of three dimensional porous NiO/graphene composite for enhanced bioelectrocatalysis in microbial fuel cells. J. Power Sour. 378, 119–124 (2018).
Ouis, M., Kameche, M., Innocent, C., Charef, M. & Kebaili, H. Electro-polymerization of pyrrole on graphite electrode: Enhancement of electron transfer in bioanode of microbial fuel cell. Polym. Bull. 75, 669–684 (2018).
Karthikeyan, R. et al. Effect of composites based nickel foam anode in microbial fuel cell using Acetobacter aceti and Gluconobacter roseus as a biocatalysts. Bioresour. Technol. 217, 113–120 (2016).
Yang, Q. et al. Boosting the anode performance of microbial fuel cells with a bacteria-derived biological iron oxide/carbon nanocomposite catalyst. Chemosphere 268, 128800 (2021).
Zhang, H., Zhang, R., Zhang, G., Yang, F. & Gao, F. Modified graphite electrode by polyaniline/tourmaline improves the performance of bio-cathode microbial fuel cell. Int. J. Hydrogen Energy 39, 11250–11257 (2014).
Jeremiasse, A. W., Hamelers, H. V., Saakes, M. & Buisman, C. Ni foam cathode enables high volumetric H2 production in a microbial electrolysis cell. Int. J. Hydrogen Energy 35, 12716–12723 (2010).
Nambiar, S., Togo, C. & Limson, J. Application of multi-walled carbon nanotubes to enhance anodic performance of an Enterobacter cloacae-based fuel cell. Afr. J. Biotechnol. 8 6927–6932, (2009).
Jiang, Z. et al. Enhanced catalytic capability of electroactive biofilm modified with different kinds of carbon nanotubes. Anal. Chim. Acta 1035, 51–59 (2018).
Acknowledgements
This article is the result of a Master’s thesis in Environmental Health Engineering in 2023 and project code (IR.UMSU.REC.1401.270), which was financially supported by the Urmia University of Medical Sciences, and was conducted in the research laboratory of the School of Health. We would like to express our sincere gratitude to all those who assisted us in conducting this thesis.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: N.N., F.M., S.A., M.R.; data collection: F.M., N.N.; analysis and interpretation of results: F.M., N.N., S.A.; draft manuscript preparation: F.M., N.N., S.A., M.R. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoodzadeh, F., Navidjouy, N., Alizadeh, S. et al. Investigation of microbial fuel cell performance based on the nickel thin film modified electrodes. Sci Rep 13, 20755 (2023). https://doi.org/10.1038/s41598-023-48290-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48290-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.