Abstract
In countries with a high incidence of tuberculosis, the typical clinical features of Crohn's disease (CD) may be covered up after tuberculosis infection, and the identification of atypical Crohn's disease and intestinal tuberculosis (ITB) is still a dilemma for clinicians. Least absolute shrinkage and selection operator (LASSO) regression has been applied to select variables in disease diagnosis. However, its value in discriminating ITB and atypical Crohn's disease remains unknown. A total of 400 patients were enrolled from January 2014 to January 2019 in second Xiangya hospital Central South University.Among them, 57 indicators including clinical manifestations, laboratory results, endoscopic findings, computed tomography enterography features were collected for further analysis. R software version 3.6.1 (glmnet package) was used to perform the LASSO logistic regression analysis. SPSS 20.0 was used to perform Pearson chi-square test and binary logistic regression analysis. In the variable selection step, LASSO regression and Pearson chi-square test were applied to select the most valuable variables as candidates for further logistic regression analysis. Secondly, variables identified from step 1 were applied to construct binary logistic regression analysis. Receiver operating characteristic (ROC) curve analysis was performed on these models to assess the ability and the optimal cutoff value for diagnosis. The area under the ROC curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy rate, together with their 95% confidence and intervals (CIs) were calculated. MedCalc software (Version 16.8) was applied to analyze the ROC curves of models. 332 patients were eventually enrolled to build a binary logistic regression model to discriminate CD (including comprehensive CD and tuberculosis infected CD) and ITB. However, we did not get a satisfactory diagnostic value via applying the binary logistic regression model of comprehensive CD and ITB to predict tuberculosis infected CD and ITB (accuracy rate:79.2%VS 65.1%). Therefore, we further established a binary logistic regression model to discriminate atypical CD from ITB, based on Pearsonchi-square test (model1) and LASSO regression (model 2). Model 1 showed 89.9% specificity, 65.9% sensitivity, 88.5% PPV, 68.9% NPV, 76.9% diagnostic accuracy, and an AUC value of 0.811, and model 2 showed 80.6% specificity, 84.4% sensitivity, 82.3% PPV, 82.9% NPV, 82.6% diagnostic accuracy, and an AUC value of 0.887. The comparison of AUCs between model1 and model2 was statistically different (P < 0.05). Tuberculosis infection increases the difficulty of discriminating CD from ITB. LASSO regression showed a more efficient ability than Pearson chi-square test based logistic regression on differential diagnosing atypical CD and ITB.
Similar content being viewed by others
Introduction
Crohn's disease (CD) is a transmural inflammatory disease, which can affect the entire digestive tract from the mouth to the anus. In the early years, Crohn's disease was prevalent in western countries. However, with ethnic migration and the improvement of people's living standards, the incidence of Crohn's disease in developing countries, especially China, with an incidence increased from 0.28/100,000 in 1950–2002 to 0.848/100,000 in 1950–20071. China has the second highest incidence of tuberculosis (TB) in the world. As a TB highly endemic country and considering the increasing incidence of CD, TB infection may cover up the typical appearance of CD. So it is called atypical CD, which is more difficult to distinguish from tuberculosis and also the typical CD2,3.
Though CD and ITB have different etiologies, there are many clinical, radiological, endoscopic manifestations overlapped (Fig. 1), especially in epidemic ITB areas, where the incidence of CD is increasing4,5. Acid fast bacilli (AFB) and granulomas with caseous necrosis are identified as golden criteria for diagnosing ITB, however, its value is limited with a positive rate at about 50%6. Generally, most of the studies report diarrhea, hematochezia, perianal disease, presence of longitudinal ulcers, aphthous ulcers, cobblestoning, and skip lesions are more common in CD, whereas presence of transverse ulcers and patulous ileocaecal valve are more common in ITB7,8. However, there is no gold standard for the diagnosis of CD. Clinicians make a decision largely relying on a comprehensive analysis of clinical, radiological, endoscopic manifestations. Nowadays, with the help of guide consensus, most Chinese patients with CD or ITB with typical disease characteristics obtain correctly and timely treatment. Gastroenterologists have also proposed multiple methods to improve the diagnostic value of the two diseases. He et al. applied random forest to screen variables and built two regression models based on 7 differential variables9. Wu et al. used t test and chi-square test to select variables and proposed a predictive model to discriminate CD and ITB10. Mao et al. established a model through univariate and multiple logistic regression analyses based on clinical and computed tomographic enterography (CTE) characteristics11. Models built via those methods were convenient and reliable for the differentiation of part of CD and ITB patients. Unfortunately, a study reported a misdiagnosis rate of the two disease was still at 50–70%12. Based on this, The methodology for the identification of these two diseases still needs to be further explored.
In clinical practice, anti-tuberculosis treatment was often used to discriminate the two disease. However, if patients with CD use anti-tuberculosis drugs, it will delay the course of the disease and bear the side effects of anti-tuberculosis drugs. Conversely, if ITB patients receive a treatment of CD, it will cause the spread of tuberculosis. In our previous study, a novel grouping strategy was already presented to separate CD into typical CD (TCD) and atypical CD (UCD). We proposed the phenotype of UCD is deeply influenced by TB infection history, which is also a major reason of misdiagnosis. we enrolled 141 atypical CD and ITB patients and built some predictive models based on clinical, radiological, endoscopic manifestations. However, we have not systematically and comprehensively explored the identification of CD and ITB, especially for the identification between atypical CD and ITB. Also, when identifying with ITB, we did not verify that different phenotypes of CD need to be discussed separately13. Therefore, more comprehensive information need to be defined when come across analysis of clinical phenotypes.
Conventional methods, such as stepwise selection, are usually applied for variable selection in diseases14,15,16,17,18. However, they have the disadvantage of overfitting19. LASSO (least absolute shrinkage and selection operator) is a variable selection method proposed by statistician Robert Tibshirani in 199620.Compared to traditional regression approaches, LASSO regression can handle a larger set of potential predictors, picking out the variables most associated with the disease. Based on this, LASSO has been utilized in the screening of disease risk factors and establishment of prediction models21,22,23,24,25.Few studies have used LASSO regression to explore risk factors for CD26,27,28. However, to our knowledge, in the identification of CD and ITB, especially in the identification of atypical CD and ITB, whether LASSO regression can help differentiate the two diseases remains unknown.
In this study, we enrolled 332 CD and ITB patients, including 118 typical CD, 109 atypical CD and 105 ITB patients. We wish to deepen the understanding of the identification between CD and ITB and reduce the rate of misdiagnosis of the disease.
Materials and methods
Participants
This retrospective study was approved by the Ethics Committee of second Xiangya hospital Central South University, Changsha, China. All subjects provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
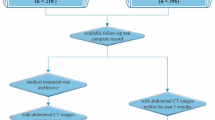
A total of 400 patients were enrolled from January 2014 to January 2019 in second Xiangya hospital Central South University. After a year-follow-up, 68 patients were excluded, including 14 patients with intestinal lymphomas, 12 patients with Behcet's disease, 14 patients with ulcerative colitis, 8 patients with unexplained intestinal ulcer, and 20 patients lost of follow up. Finally, we selected 332 patients to conduct our study, with 105 patients diagnosed with ITB and 227 patients diagnosed with CD. Among CD patients, 109 patients were defined as untypical CD (UCD), and 118 CD patients were defined as typical CD (TCD). UCD means the CD patient does not exclude tuberculosis; TCD means the CD patient excludes tuberculosis. Whether a patient with CD has tuberculosis infection is evaluated by a specialist. Detailed information was showed in Fig. 2.
Inclusion criteria and exclusion criteria
The inclusion criteria were as follows: (1) Patients older than 18 years of age; (2) Patients were initially suspected of CD or ITB based on clinical data. The exclusion criteria were as follows: (1) ITB patients with caseous granuloma detected in pathological section when enrolled in the study; (2) Patients with HIV infection; (3) Patients diagnosed with other disease in the follow-up such as intestinal lymphomas, ulcerative colitis, Behcet's disease; (4) Patients lost of follow up.
Data collection and definition
57 indexes were collected in this study, including demographic and clinical features, laboratory features, computed tomography enterography features, endoscopic features and site of involvement. All the continuous variables like albumin, platelets were changed into categorical variables. The detailed information was shown in supporting information (Table S1). Comprehensive assessment of T-SPOT, PPD and Lung CT results to assess the type of CD. If the assessment result does not exclude tuberculosis, then UCD is considered; if the result excludes tuberculosis, then TCD is considered.
Diagnostic criteria and follow-up
Patients diagnosed with ITB should meet the following criteria: Patients who received regular anti-tuberculosis treatment at least 3 months and diagnosed with ITB after a-year follow-up according to clinical, laboratory, radiological, endoscopic features. Patients diagnosed with CD should meet at least 1 of the following criteria: (1) Noncaseating granuloma detected in pathological section during follow-up; (2) Patients who received regular immunosuppressive or biological agents at least 3 months and diagnosed with CD after a- year follow-up according to clinical, laboratory, radiological, endoscopic features; (3) Patients who received anti-tuberculosis drug treatment for 3 months, but did not get better after re-examination, and got better after receiving immunosuppressive agents or biological agents after a- year follow-up according to clinical, laboratory, radiological, endoscopic features.
Statistical analysis
SPSS 20.0 and R software version 3.6.1 and the “glmnet” package (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses. The prediction model was developed using a 2-step approach. Firstly, Pearson chi-square test was used to compare enumeration data. Statistical significance was determined as a P value of less than 0.05. All variables with statistical significance (P < 0.05) in Pearson chi-square test was taken as candidates for further binary logistic regression analyses. We also used the LASSO regression to select the most valuable variables. The feature selection step was performed on complete data. Secondly, variables identified from step 1 were applied to constructed a binary logistic regression model. The regression coeffcients of the predictive model were regarded as the weights for the respective variables, and the score for each patient was calculated. Receiver operating characteristic (ROC) curve analysis was performed on these models to assess the ability and the optimal cutoff value for diagnosis. The area under the ROC curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy rate, together with their 95% confidence and intervals (CIs) were calculated. MedCalc software (Version 16.8) was applied to analyze the ROC curves of models.
Results
Univariate analysis for differentiation of CD and ITB
Univariate analysis for differentiation of comprehensive CD and ITB
We performed univariate analysis of 57 indicators including demographic characteristics, clinical characteristics, laboratory findings, imaging characteristics, and endoscopic characteristics between comprehensive CD and ITB, which was evaluated by Pearson chi-square analysis on 332 CD and ITB patients. 29 indicators were statistically different (P < 0.05, Table S2).
Univariate analysis for differentiation of UCD and ITB
Of the 57 indicators, 18 indicators were statistically different which were evaluated by Pearson chi-square test on 214 UCD and ITB patients. Among them, abdominal pain, diarrhea, perianal abscess, perianal fistula, ileus, bowl resection history, elevated platelets, decreased albumin, elevated CRP, FOBT positive, comb sign, segmental distribution of lesion, cobblestone appearance, longitudinal ulcers, jejunal involvement, rectal involvement were seen more frequently in CD, whereas positive PPD skin test and positive T-SPOT were more frequently identified in ITB (P < 0.05, Table 1).
Since we have many variables and relatively few cases, to pick out the variables most associated with UCD and ITB, LASSO regression was applied to filter the variables on 214 UCD and ITB patients. We utilized ten-fold cross-validation to select the penalty term, lambda(λ). log(λ) = − 3.662 (λ = 0.000217771) when the error of the model is minimized, and 26 variables were selected for further logistic regression analysis (Table S1, Fig. 3A,B).
Development of a predictive model for differentiation between comprehensive CD and ITB
To establish a predictive model, we divided these 332 patients randomly into a training set (70%) and a validation set (30%). The training set comprised 236 cases (69 ITB patients and 167 CD patients), and the validation set comprised 96 cases (36 ITB patients and 60 CD patients). All variables with statistical significance (P < 0.05) selected by univariate analysis were taken as candidates for further logistic regression analyses. Based on binary logistic regression analysis, the regression was set as
(X1, night sweat; X2,perianal fistula; X3, illeus; X4, bowl resection history; X5, elevated PLT; X6, elevated CRP; X7, positive T-SPOT; X8, transverse ulcer; X9, involvement of jejunum). The cutoff value was 0.738, when P > 0.738, it was diagnosed with CD; when P < 0.738, it was diagnosed with ITB. The AUC, sensitivity, specificity, PPV, NPV, and accuracy rate of the prediction model were 0.907, 78.4%, 89.9%, 94.9%, 63.2% and 81.8%, respectively (Table 2). This predictive model was validated using the validation data set. ROC analysis showed that the AUC of the predictive model was 0.832 (95%CI, 0.750–0.915). With a cutoff value of 0.738, The sensitivity, specificity, PPV, NPV, and accuracy rate were 80%, 77.6%, 85.7%, 70% and 79.2%.
Validation data set from UCD and ITB patients
To validate the diagnostic value of the predictive regression model, we applied a validation data set of UCD and ITB patients (27UCD and 36 ITB patients), which was selected from the 96 cases of mentioned validation set. With a cutoff value of 0.738, the sensitivity, specificity, PPV, NPV, and accuracy rate were 77.8%, 55.6%, 56.8%, 76.9% and 65.1% in differentiating ITB from UCD (Table 2).
Development of a predictive model for differentiation between UCD and ITB
Since we did not get a satisfactory diagnostic value via applying the regression model of comprehensive CD and ITB to predict UCD and ITB. We tried to explore a predictive model for the differentiation between UCD and ITB. In the first step, we applied all the 214 UCD and ITB patients to select features, using Pearson chi-square test (for model1) and LASSO regression (for medel2) respectively. In the second step, 214 UCD and ITB patients were randomly divided into a training set (70%) and a validation set (30%). Two binary logistic regressions were established with the variables selected by Pearson chi-square test (for model 1) and LASSO regression (for model 2), using the same training set (Table 3).We applied a validation data set of UCD and ITB patients to validate the efficacy of the two models (Table 4). Model 1 for diagnosing CD at the cutoff value of 0.665 showed 69.4% specificity, 66.67% sensitivity, 62% PPV, 73.5% NPV, and 68.3% diagnostic accuracy; and with the cutoff value of 0.421, model 2 showed 75.8% specificity, 56.2% sensitivity, 69.2% PPV, 64.1% NPV, and 66.2% diagnostic accuracy. By statistically analyzing the ROC curves of the two models using MedCalc software (Version 16.8), we got a P value of < 0.05. This result indicated that the diagnostic value of model 2 was better than model 1.
Development of a predictive model for differentiation between UCD and TCD
Univariate analysis was also performed between TCD and UCD. Interestingly, 16 indicators were statistically different (P < 0.05, Table S3). This prompted us to further explore the predictive equations that could identify these two phenotypes. 227 CD patients were randomly divided into a training set (70%) and a validation set (30%). The training set comprised 164 cases (82 TCD patients and 82 UCD patients), and the validation set comprised 63 cases (36 TCD patients and 27 UCD patients). All variables with statistical significance (P < 0.05) selected by univariate analysis were taken as candidates for further binary logistic regression analysis. Based on binary logistic regression analysis, the regression was set as
(X1, bowl resection history; X2, intestinal wall edema; X3, intestinal stenosis; X4, increased fat density; X5 shallow ulcer; X6 swollen ileocecal valve; X7 involvement of ileum; X8 involvement of sigmoid colon). The cutoff value was 0.66, when P > 0.54, it was diagnosed with TCD; when P < 0.54, it was diagnosed with UCD. The AUC, sensitivity, specificity, PPV, NPV, and accuracy of the prediction model were 0.825, 80.5%, 70.7%, 78.4%, 73.3% and 75.6%, respectively. This predictive model was validated using the validation data set. The sensitivity, specificity, PPV, NPV, and accuracy rate were 47.2%, 52.9%, 60.7%, 45.7%,and 52.3%.
Discussion
Due to many overlapping symptoms, CD and ITB are difficult to distinguish. In recent years, with the increasing incidence of CD in China, physicians have gradually deepened their understanding of CD and formed a consensus on the diagnosis of CD. Under the guidance of consensus, most typical CD patients were correctly diagnosed. However, for those CD patients accompanied by latent tuberculosis infection, though the guidelines recommend diagnostic anti-tuberculosis treatment for identification, it may not an optimal choice. Based on this, our study was the first time to systematically demonstrate the differentiation between CD and ITB, we clarified that tuberculosis infection increased the difficulty of discriminating CD from ITB,and CD patients under different circumstances need to be considered separately when distinguishing from ITB.
There are multiple studies on the identification of CD and ITB11,29,30,31. Jung et al.30 built a seven-marker model to discriminate CD and ITB, with a sensitivity, specificity of 98.0, 92.4, respectively. Their study enrolled 261 patients and indicated that age, sex, ring shape ulcers, suspicious pulmonary tuberculosis, diarrhea, longitudinal ulcers, and involvement of the sigmoid colon were the important indexes for discrimination. We also created a predictive model for the same purpose. However, compared to Jung’s study, our model showed 9 indicators including night sweat, perianal fistula, intestinal obstruction, intestinal surgery, elevated PLT, elevated CRP, T-SPOT positive, transverse ulcer, involvement of jejunum were the vital markers to differentiate the two diseases,with a sensitivity, specificity of 78.4%, 89.9%, respectively. Although we have a larger sample size, our results have a lower diagnostic efficacy. We suspected that TB infection may affect the CD phentype as times change, making it more difficult to identify the two disease.
With the help of guidelines as well as the multivariate mathematical models, most typical CD patients could receive a correctly and timely treatment. However, there is still a high rate of misdiagnosis for CD when distinguishing from ITB. We speculated the reason is that previous studies did not consider typical CD and atypical CD separately. These methods were not applicable to the discrimination between ITB and atypical CD patients. To verify this, in this study, we substituted the UCD and ITB data into the previously established model, and found that the diagnostic deficiency was getting worse as we suspected, with accuracy rate decreased from 81.8 to 65.1%. This finding prompted us to look for ways to identify atypical CD and ITB. Until now, few studies were carried out to differentiate UCD from ITB. Zhao et al.32 pointed that the level of tuberculosis interferon gamma release assay (TB-IGRA) could help discriminate the UCD and ITB, and if TB-IGRA ≥ 100 pg/ml, the possibility of ITB should be considered first, and diagnostic anti-TB treatment should be recommended. However, previous TB infection history may lead to false positives in TB-IGRA results. Due to the small sample size of this study, the conclusions need to be verified. Our previous research also explored the distinction between UCD and ITB. We enrolled 43 UCD and 56 UITB patients, and built 4 regression equations from clinical, laboratory, endoscopic, and radiological features, respectively13. Though AUC of clinical prediction model was 0.834, the model is not suitable for the application in clinical practice due to the subjectivity of its variable collection. Hence, in this study, we comprehensively analyzed the clinical, laboratory, endoscopic, and radiological features of UCD and ITB. We used Chi-square test and LASSO regression to filter variables. Results of the two methods revealed abdominal pain, perianal fistula, ileus, elevated CRP, cobblestone appearance, involvement of jejunum were favor of UCD, while T-SPOT positive were favor of ITB, which indicated these three indexes were reliable for the identification of the the two disease. We constructed two equations to predict the two disease, with the AUC over 80%. though these two equations had a partial missed diagnosis rate, they still provided a valuable method for the identification of UCD and ITB. It is worth mentioning that LASSO regression was considered to be a better method to choose variables when the size of predicted variables were relatively large33, our results showed the diagnostic value of training set in model 2 was prior to model 1, however, it is not much different when comparing the validation set, which may be related to the sample size.
Our research further analyzed the characteristics of typical CD and atypical CD. Results showed that swollen ileocecal valve, involvement of ileum ulcer were favor of UCD, while intestinal surgery, intestinal wall edema, intestinal obstruction, blurred fat gap, shallow ulcer involvement of sigmoid colon were favor of TCD. Most of these indicators come from radiological examination, which suggested that when we diagnose CD, we should pay attention to the results of radiological examination, which may help reduce misdiagnosis. We also noticed that the indicators that support the diagnosis of UCD were similar to those of intestinal tuberculosis mentioned in previous studies34,35,36. We are not surprised because China is a country with a high incidence of tuberculosis, and the phenotype of UCD may be affected by tuberculosis infection to some extent. We further established the regression equation based on the indexes obtained by the multivariate regression analysis. Interestingly, we found that the two different subgroups of CD can be identified by the equation, with the AUC, sensitivity, specificity, PPV, NPV, and accuracy of the prediction model were 0.825, 80.5%, 70.7%, 78.4%, 73.3% and 75.6%, respectively. The diagnostic value was similar to that of the discrimination between UCD and ITB.This indicated that in the identification of ITB and CD, it is necessary to consider tuberculosis-infected CD separately,especially in areas where tuberculosis is epidemic.
In conclusion, there were several advantages in our study. Firstly, we proposed a new method to distinguish between CD and ITB, and we confirmed that identification of UCD and ITB required additional attention,especially in areas with a high incidence of tuberculosis,the existence of UCD affected the misdiagnosis rate of CD and ITB that should not be ignored. Secondly, we constructed two equations for the discrimination between UCD and ITB, and we were the first to apply LASSO regression to select variables and build a model for the identification of the two diseases. Thirdly, we constructed a equation to discriminate TCD and UCD, this validated that when discriminating between ITB and CD, we should consider UCD and TCD separately. Our research has enriched the identification methods of CD and ITB, and provided valuable guidance for clinical practice. However, there are also some limitations. This study is a single-center research and lack of an independent external dataset for testing the models. Although LASSO regression helps us to select and confirm some identification of parameters, these parameters may not be widely used due to geographical differences, multi-center data needs to be collected. In the future, to reduce the misdiagnosis rate of CD, new biomarker exploration is needed. Other methods such as neural networks and random forests can also be used in larger data sets to build predicted models. In epidemic ITB areas, efforts are still needed to increase the detection rate of Mycobacterium tuberculosis.
Data availability
The datasets generated and/or analysed during the current study are not available since we are still collecting more data for further study, but are available from the corresponding author on reasonable request.
References
Zheng, J. J., Zhu, X. S., Huangfu, Z., Shi, X. H. & Guo, Z. R. Prevalence and incidence rates of Crohn’s disease in mainland China: A meta-analysis of 55 years of research. J. Dig. Dis. 11, 161–166 (2010).
Riumallo-Herl, C., Canning, D. & Salomon, J. A. Measuring health and economic wellbeing in the Sustainable Development Goals era: Development of a poverty-free life expectancy metric and estimates for 90 countries. Lancet Glob. Health 6, e843–e858 (2018).
Zumla, A. et al. The WHO 2014 global tuberculosis report–further to go. Lancet Glob. Health 3, e10–e12 (2015).
Singh, P., Ananthakrishnan, A. & Ahuja, V. Pivot to Asia: Inflammatory bowel disease burden. Intest. Res. 15, 138–141 (2017).
Ahuja, V. & Tandon, R. K. Inflammatory bowel disease in the Asia-Pacific area: A comparison with developed countries and regional differences. J. Dig. Dis. 11, 134–147 (2010).
Pulimood, A. B. et al. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn’s disease. J. Gastroenterol. Hepatol. 20, 688–696 (2005).
Kedia, S. et al. Computerized tomography-based predictive model for differentiation of Crohn’s disease from intestinal tuberculosis. Indian J. Gastroenterol. 34, 135–143 (2015).
Kedia, S. et al. Differentiating Crohn’s disease from intestinal tuberculosis. World J. Gastroenterol. 25, 418–432 (2019).
He, Y. et al. Development and validation of a novel diagnostic nomogram to differentiate between intestinal tuberculosis and crohn’s disease: A 6-year prospective multicenter study. Am. J. Gastroenterol. 114, 490–499 (2019).
Wu, X. et al. Diagnostic performance of a 5-Marker predictive model for differential diagnosis between intestinal tuberculosis and Crohn’s disease. Inflamm. Bowel Dis. 24, 2452–2460 (2018).
Mao, R. et al. Computed tomographic enterography adds value to colonoscopy in differentiating Crohn’s disease from intestinal tuberculosis: A potential diagnostic algorithm. Endoscopy 47, 322–329 (2015).
Ng, S. C. et al. Systematic review with meta-analysis: Accuracy of interferon-gamma releasing assay and anti-Saccharomyces cerevisiae antibody in differentiating intestinal tuberculosis from Crohn’s disease in Asians. J. Gastroenterol. Hepatol. 29, 1664–1670 (2014).
Meng, Y., Li, Y., Hao, R., Li, X. & Lu, F. Analysis of phenotypic variables and differentiation between untypical Crohn’s disease and untypical intestinal tuberculosis. Dig. Dis. Sci. 64, 1967–1975 (2019).
Xu, Y. et al. Predicting ICU mortality in rheumatic heart disease: Comparison of XGBoost and logistic regression. Front. Cardiovasc. Med. 9, 847206 (2022).
Ali, A. H. et al. The utility and diagnostic accuracy of transient elastography in adults with morbid obesity: A prospective study. J. Clin. Med. 11, 1201 (2022).
Alshanbari, H. M. et al. Prediction of COVID-19 severity from clinical and biochemical markers: A single-center study from Saudi Arabia. Eur. Rev. Med. Pharmacol. Sci. 26, 2592–2601 (2022).
Violi, F. et al. The ADA (age-d-dimer-albumin) score to predict thrombosis in SARS-CoV-2. Thromb. Haemost. https://doi.org/10.1055/a-1788-7592(2022).
Varshney, K., Glodjo, T. & Adalbert, J. Overcrowded housing increases risk for COVID-19 mortality: An ecological study. BMC Res. Notes 15, 126 (2022).
McNeish, D. M. Using lasso for predictor selection and to assuage overfitting: A method long overlooked in behavioral sciences. Multivariate Behav. Res. 50, 471–484 (2015).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Methodol. 58, 267–288 (1996).
Li, Z. et al. Identification of potential early diagnostic biomarkers of sepsis. J. Inflamm. Res. 14, 621–631 (2021).
Ouyang, N., Li, G., Wang, C. & Sun, Y. Construction of a risk assessment model of cardiovascular disease in a rural Chinese hypertensive population based on lasso-Cox analysis. J. Clin. Hypertens. 24, 38–46 (2022).
Jin, L., Deng, L. & Wang, W. Candidate genes of allergic dermatitis are associated with immune response. J. Healthc. Eng. 2022, 8745722 (2022).
Au, E. H. et al. Factors associated with advanced colorectal neoplasia in patients with CKD. Am. J. Kidney Dis. 79, 549–560 (2022).
Chong, G. O. et al. Prediction model for tumor budding status using the radiomic features of f-18 fluorodeoxyglucose positron emission Tomography/Computed tomography in cervical cancer. Diagnostics 11, 1517 (2021).
Kuenzig, M. E. et al. Serum newborn screening blood metabolites are not associated with childhood-onset inflammatory bowel disease: A population-based matched case-control study. Inflamm. Bowel Dis. 26, 1743–1747 (2020).
Liu, Y., Duan, Y. & Li, Y. Integrated gene expression profiling analysis reveals probable molecular mechanism and candidate biomarker in anti-TNFalpha non-response IBD patients. J. Inflamm. Res. 13, 81–95 (2020).
Garza-Hernandez, D., Estrada, K. & Trevino, V. Multivariate genome-wide association study models to improve prediction of Crohn’s disease risk and identification of potential novel variants. Comput. Biol. Med. 145, 105398 (2022).
Pratap, M. V. et al. Endoscopic and clinical responses to anti-tubercular therapy can differentiate intestinal tuberculosis from Crohn’s disease. Aliment. Pharmacol. Ther. 45, 27–36 (2017).
Jung, Y. et al. Predictive factors for differentiating between Crohn’s disease and intestinal tuberculosis in koreans. Am. J. Gastroenterol. 111, 1156–1164 (2016).
Bae, J. H. et al. Development and validation of a novel prediction model for differential diagnosis between Crohn’s disease and intestinal tuberculosis. Inflamm. Bowel Dis. 23, 1614–1623 (2017).
Zhao, Y., Xu, M., Chen, L., Liu, Z. & Sun, X. Levels of TB-IGRA may help to differentiate between intestinal tuberculosis and Crohn’s disease in patients with positive results. Ther. Adv. Gastroenterol. 13, 320856429 (2020).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. Roy. Stat. Soc.: Ser. B (Methodol.) 58, 267–288 (1996).
Patel, B. & Yagnik, V. D. Clinical and laboratory features of intestinal tuberculosis. Clin. Exp. Gastroenterol. 11, 97–103 (2018).
Kentley, J. et al. Intestinal tuberculosis: A diagnostic challenge. Trop. Med. Int. Health 22, 994–999 (2017).
Sharma, R., Madhusudhan, K. S. & Ahuja, V. Intestinal tuberculosis versus Crohn’s disease: Clinical and radiological recommendations. Indian J. Radiol. Imaging 26, 161–172 (2016).
Acknowledgements
We sincerely thank the support from the Natural Science Foundation of Hunan Province (NO.2022JJ40841), the Fundamental Research Funds for the Central Universities of Central South University (NO.2021zzts0343), and sincerely thank Prof. Renhe Yu for his statistical analysis support. We also sincerely thank the editor and reviewers for their contributions and suggestions.
Author information
Authors and Affiliations
Contributions
F.G.L. and Y.N.Y. designed research, Y.L. and Y.N.Y. analyzed the results and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Lu, F. & Yin, Y. Applying logistic LASSO regression for the diagnosis of atypical Crohn's disease. Sci Rep 12, 11340 (2022). https://doi.org/10.1038/s41598-022-15609-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15609-5
This article is cited by
-
Integrated analysis of single-cell and bulk RNA sequencing data reveals prognostic characteristics of lysosome-dependent cell death-related genes in osteosarcoma
BMC Genomics (2024)
-
Age and mean platelet volume-based nomogram for predicting the therapeutic efficacy of metoprolol in Chinese pediatric patients with vasovagal syncope
World Journal of Pediatrics (2024)
-
Exploring natural killer cell-related biomarkers in multiple myeloma: a novel nature killer cell-related model predicting prognosis and immunotherapy response using single-cell study
Clinical and Experimental Medicine (2024)
-
Machine learning-based prediction models for parathyroid carcinoma using pre-surgery cognitive function and clinical features
Scientific Reports (2023)
-
Leveraging machine learning to distinguish between bacterial and viral induced pharyngitis using hematological markers: a retrospective cohort study
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.