Abstract
The purpose of the present research was to assess the prognostic impact of marital status in hepatocellular carcinoma (HCC) patients with tumors ≤ 2 cm (stage Ia) based on the data from the Surveillance, Epidemiology, and End Results (SEER) database. Patients who received a histopathologic HCC diagnosis between 2004 and 2016 were recruited. Overall survival (OS) was the major outcome measure. The Cox regression model and the Fine-Gray regression model were used for the purpose of comparing and examining the prognostic value of marital status for OS. The data for a total of 2446 stage Ia HCC patients were extracted from the database. The median overall survival time was 96.0 months, with 5-year and 10-year overall survival rates of 58.2% and 45.8%, respectively. In both the Fine-Gray regression model and Cox regression model, marital status [married vs. unmarried and others, both P < 0.001, hazard ratio (HR) = 1.389 for Cox and HR = 1.378 for Fine-Gray], age at diagnosis, tumor grade, and surgery at the primary site independently served as prognostic indicators associated with OS. In conclusion, positive marital status was independently associated with better OS for stage Ia HCC patients, and its prognostic influence should be validated in the near future.
Similar content being viewed by others
Introduction
Primary liver cancer has been ranked as the seventh most prevalent malignant neoplasm and the second major contributor to cancer-related deaths on a global scale1,2,3. Among all types of liver cancers, hepatocellular carcinoma (HCC) is the dominant type and accounts for more than 75% of primary liver cancers4,5. Benefiting from early detection and timely treatment of some major risk factors, including alcoholic and/or nonalcoholic fatty liver disease and chronic HCV and/or HBV infection, the incidence rate of HCC has recently slowed in some areas6,7,8. Additionally, encouraging clinical results over the past three decades indicate that the overall survival (OS) rate of HCC has increased slightly9,10. Further, curative surgical resection, including liver transplantation or local ablation, for treating early-stage HCC has made great contributions11.
In addition, amid all of the indicators to defeat this disease, marital status has long been explored as a significant prognostic variable in a wide range of tumors. In 2013, Aizer et al. examined the influences of marital status on clinical outcomes among 10 major contributors to cancer-associated fatality in the United States using the Surveillance, Epidemiology and End Results (SEER) program12. For liver or intrahepatic bile duct cancers specifically, their results demonstrated that married patients exhibited a greater possibility of receiving definitive therapy (P < 0.01) and have significant survival benefits (P < 0.01) than unmarried persons (including divorced/separated, widowed, and single). However, in another large sample retrospective analysis conducted in Italy, a decreased risk of liver cancer was observed in unmarried patients13. Similarly, for HCC patients with poor or anaplastic differentiation who underwent surgical resection, a 2019 SEER report suggested that marital status had a non-significant benefit on survival outcomes14.
Considering that HCC patients with tumors ≤ 2 cm (stage Ia) could enjoy long-term survival and the unknown correlation between stage Ia HCC and marital status, we extracted and analyzed data from the SEER database to further evaluate the influences of marital status on survival status in this setting.
Methods
Patient selection
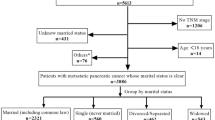
This retrospective study obtained data from the SEER 18 population-based registries (SEER* Stat 8.3.6), which includes approximately 30% of the United States population. Data of 88,559 patients with confirmed liver cancer diagnoses from 2004 to 2016 was obtained from the database (ICD-O-3 Histology recodes 8140-8389). The following were the inclusion criteria used in recruiting participants for the present study: (1) patients who have a histopathological diagnosis of HCC; (2) tumors less than 2 cm without lymph node metastasis; and (3) upon HCC diagnosis, the patient must be no younger than 18 years of age. Below were some of the major exclusion criteria: (1) patients’ records of other cancers or with metastatic diseases and for whom HCC was not the first diagnosed cancer, and (2) HCC patients whose survival time was less than 1 month or who had untraced data (Fig. 1).
Determination of study variables and outcomes
We queried the database for patient information including demographics, treatment history and outcomes (age, sex, race, marital status, local surgery, use of radiotherapy (RT), use of chemotherapy (CT), survival data including status (alive or dead), cause of death (COD) and COD to site recode, survival time in months, and cause-specific death classification). For HCC patients registered in 2016, eligible tumors were restaged according to the definitions in the AJCC 8th edition. We defined marital status as a binary factor by dividing married (including common law) by unmarried and others, which included divorced, separated, single (never married), widowed, unmarried or domestic partners, and unknown coded in the SEER data, as has been reported in other studies15,16,17. The items COD to site recode and cause-specific death classification indicated if a patient died from HCC (HCC-DSD: disease-specific death) or of causes other than HCC (HCC-NDSD: nondisease-specific death).
Data analysis
As the main endpoint in the present research, we measured OS, which was defined as the duration between the day of HCC diagnosis and the day of death or the final follow-up recorded in the database. CSS (cause-specific survival) was described as the duration between the day of initial diagnosis and the point of death attributable to HCC or the date of the final follow-up recorded in the program, whichever came first. Patients’ baseline characteristics were summarized by descriptive statistics and frequency tables. One-way analysis of variance (ANOVA) was used to compare the proportions of different groups. The non-linear correlation between age at diagnosis and all CODs was investigated by means of a restricted cubic spline (RCS)18,19. Methods for multivariate and univariate analysis by Cox regression model were described in our previous studies20,21. In addition, considering that stage Ia HCC patients could enjoy long-term survival, we further regarded HCC-NDSD as a competing event in this cohort. In this model, the cumulative incidence function (CIF) was employed to determine the possibility of each factor in the univariate analysis and was checked with Gray’s test. According to the results of the univariate analysis, variables with a p-value < 0.05 were selected and incorporated into a multivariate competing-risks survival analysis with the aid of a proportional subdistribution hazard model, as determined by the Fine-Gray test. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were also calculated in the analysis. A two-sided P value < 0.05 was established as a criterion of statically significant difference. Furthermore, to better illustrate the effects of marital status on liver cancer, the following librarians/databases were searched to trace eligible studies: Ovid MEDLINE®, PubMed, and Google Scholar updated until 31 December 2021 (present in the discussion section). The search strategy was developed and consisted of 2 main concepts: (1) liver cancer and (2) marital status. Methods for article selection were also described in our previous studies22,23 and we confirmed that the selection was performed in accordance with the PRISMA guidelines for systematic reviews.
The R software (version: 3.6.2; Institute for Statistics and Mathematics, Vienna, Austria; https://www.r-project.org) and SPSS software (version: 25.0 IBM Corporation, Armonk, NY, USA) was utilized to perform all analyses of statistical data.
Ethics statement
It was not necessary to get written informed consent for participating in the present research as the information contained in the SEER database has been de-identified and is publically available following authorization. The present research was exempted from ethical assessment by the Institutional Review Board of Zhejiang Provincial People’s Hospital. We hereby certify that the present research was conducted in conformity with the Declaration of Helsinki.
Results
Demographic and baseline characteristics
We extracted the data from 2446 eligible stage Ia HCC patients from the SEER database between 2004 and 2016. Table 1 presents the demographics and baseline features of the patients who were included in the present research. The ages of patients at diagnosis ranged between 20 and 90 years old with 59 years old as the median age. We further applied RCS with 3 knots (5th, 50th, and 95th centiles) to evaluate the association between age at diagnosis and all CODs (Fig. 2A). Based on the result, the appropriate inflection point to age at diagnosis was also 59 years old. Among all enrolled patients, 1397 (57.1%) patients were married, and 1049 (42.9%) HCC patients were classified as unmarried or other. A total of 1860 (76.0%) of the patients underwent cancer surgery. Only 64 (2.6%) HCC patients were treated with RT. The correlation coefficient of different variables is presented in Fig. S1. No factors were highly correlated with other baseline characteristics. Additionally, male patients were observed to have significant higher proportion with positive marital status than unmarried and others (45.0% vs. 29.4%, P < 0.001), while age had no significance with marital status.
Survival outcomes
The OS rates for 5- and 10-year were 58.2% (95% CI, 0.560–0.604) and 45.8% (95% CI, 0.431–0.485), respectively, with a median OS time of 96.0 months (95% CI, 82.920–109.080, Fig. 2B). Of the 2,446 HCC patients, 326 patients died of HCC-NDSD, such as other infectious and parasitic diseases including HIV (n = 122), other causes of death (n = 39), and diseases of the heart (n = 38), accounting for 61.0% of the total (Fig. S2). Table 1 presents the baseline features of HCC patients who died due to HCC-DSD and HCC-NDSD. The CSS rates over 5 and 10 years were 69.1% (95% CI, 0.669–0.713) and 60.6% (95% CI, 0.577–0.635), correspondingly, with a median CSS time not achieved at the time of analysis (Fig. 2B). Among married patients, the median OS duration was 130.0 ± 7.7 months (95% CI, 114.961–145.039), and in patients classified as unmarried and other, the median OS duration was 65.0 ± 5.6 months (95% CI, 53.951–76.049).
Fine-gray regression analysis
A univariate analysis using Fine-Gray test suggested that age at diagnosis (P < 0.001), sex (P = 0.004), marital status (P < 0.001), surgery at the primary site (P < 0.001), tumor differentiation (P < 0.001), and CT (P < 0.001) significantly impacted the prognosis of stage Ia HCC patients. The cumulative risk curves for marital status are shown in Fig. 2C. The CIF was found to be elevated over 36, 60, and 120 months and was elevated for advanced age, female sex, unmarried and others, poorly or undifferentiated or unknown tumor grade, no or unknown status of surgery at the primary site, and receiving CT among eight variables. The CIF values among married HCC patients were 18.8%, 25.0%, and 30.6% at 36, 60, and 120 months, respectively. The corresponding figures for patients recoded as unmarried and others were 25.4%, 33.5%, and 42.4%, correspondingly. Table 2 depicts the findings recorded from the CIF values and univariate analysis.
We then employed the six variables that had statistical significance in the univariate analysis entered into the Fine-Gray model. According to the findings from the Fine-Gray regression model, age at the time of diagnosis (< 59 vs. ≥ 59, P < 0.001, HR = 1.419, 95% CI: 1.246–1.618), marital status (married vs. unmarried and others, P < 0.001, HR = 1.378, 95% CI: 1.212–1.568), tumor differentiation (well or moderately differentiated vs. poorly or undifferentiated, P = 0.007, HR = 1.402, 95% CI: 1.095–1.795; well or moderately differentiated vs. unknown, P = 0.032, HR = 1.169, 95% CI: 1.014–1.349), surgical resection of the primary site (no/unknown vs. yes, P < 0.001, HR = 0.340, 95% CI: 0.293–0.393) and treatment of CT (no/unknown vs. yes, P = 0.009, HR = 0.819, 95% CI: 0.706–0.952) all served as prognostic indicators that were significantly associated with OS in an independent manner (Table 3). Furthermore, multivariate analysis of NDSD also indicated that marital status (married vs. unmarried and others, P < 0.001, HR = 1.481, 95% CI: 1.308–1.680) was an independent factor associated with NDSD (Table S1).
Cox regression analysis
According to the results of the Cox regression analysis, four clinicopathological characteristics, namely age at the time of diagnosis, sex, tumor differentiation, marital status, and one treatment-related parameter (surgery at the primary site) were significantly associated with OS (Table S2). The findings recorded from the multivariate analysis illustrated that the significant covariates were age at the time of diagnosis (< 59 vs. ≥ 59, P < 0.001, HR = 1.415, 95% CI: 1.244–1.611), marital status (married vs. unmarried and others, P < 0.001, HR = 1.389, 95% CI: 1.223–1.578), tumor differentiation (well or moderately differentiated vs. poorly or undifferentiated, P = 0.005, HR = 1.398, 95% CI: 1.106–1.767; well or moderately differentiated vs. unknown, P = 0.055 HR = 1.146, 95% CI: 0.997–1.318) and surgical resection of the primary site (no/unknown vs. yes, P < 0.001, HR = 0.357, 95% CI: 0.311–0.409; Table 3). Both the Fine-Gray regression model and the Cox regression model demonstrated that marital status independently served as a prognostic indicator for OS.
Discussion
The objective of the present research was to evaluate the effect of marital status on OS in stage Ia HCC patients since the influence of marital status remains controversial in this setting. While using available data in the SEER database, we demonstrated that positive marital status acted as a prognostic variable in an independent manner favoring improved OS in both the Fine-Gray regression model and Cox regression model.
The results exploring the effects of marital status on liver cancer patients are summarized in Table 4 following chronological order14,24,25,26,27,28,29,30,31,32,33,34,35,36. Fourteen studies that met the eligibility requirements were enrolled. It should be noted that marital status was not covered in the National Cancer Data Base, which is also a widely used database for the analysis of various malignancies37. Most studies (13/14, 92.9%) were retrospective analyses with data extracted from the SEER database, with only one report having external validation with patients from their own cancer center. Among the 13 reports, 3 studies showed no significant association between marital status and survival outcomes, one study investigated the benefit of RT in HCC patients with major vascular invasion (P = 0.834 in univariate analysis), one study assessed the impact of RT in unresectable HCC patients (P = 0.475), and the other study evaluated the effects of marital status on patients developing less differentiated HCC who underwent surgical resection (P = 0.370). The remaining 10 studies all supported the benefit of positive marital status for liver cancer patients despite different situations of patient enrollment. A similar study was reported by Peters and colleagues29. In this study, 13,694 HCC patients diagnosed with stage I-II disease were enrolled in the present research. Positive marital status was first demonstrated to have a significantly higher likelihood of patients receiving liver resection, and liver transplantation (both P < 0.001). The findings recorded from the Cox regression analysis correlated with DSS, being married was shown to have a significantly longer DSS (P = 0.010; HR = 0.71; 95% CI, 0.55–0.92). In the only prospective study36, Chiu et al. compared four models predicting quality of life (QoL) following hepatic resection in 332 stage I-III HCC patients during 2012–2015 at three institutions. The findings indicated that marital status was one of nine independent variables involved in the model for predicting the QoL scores obtained six months after hepatic resection.
Unfortunately, all of these enrolled studies concluded that marital status on survival outcomes was based on the findings revealed by the Cox proportional hazard models. In fact, for HCC patients diagnosed with stage Ia, 33.3% (326/979) of patients died due to various reasons other than HCC, as revealed in the current study (Fig. S2). This nonhomogeneity could certainly cause bias in calculating the effects of marital status on OS. Starting with this consideration and the knowledge that stage Ia HCC patients could enjoy prolonged survival duration, with a 10-year overall survival rate of 45.8%, we then employed the Fine-Gray regression model according to the findings recorded from the CIF analysis. Marital status still acted as prognostic parameter affecting OS in an independent manner (P < 0.001, HR = 1.378). Previously, Yang et al. compared the Fine-Gray regression model with the Cox regression model in penile cancers38. Survival analysis indicated that the findings derived with the aid of the Cox regression model were different from those obtained by the Fine-Gray regression model, while the Kaplan–Meier curve analysis led to an overestimation compared to the CIF analysis of penile cancer patients. Similar findings were also observed in esophageal cancer39 and cecum cancer40 in the literature. Therefore, when comparing survival outcomes among different variables, especially for competing events in survival outcomes, it is worth considering the possibility of CIF analysis to avoid overestimation results41.
This study is first limited by its inherent retrospective nature, with some heterogeneity in the analysis. Some important information, such as patients’ baseline characteristics, including lesbian, gay, bisexual, transgender, and queer status, liver function, alcoholic and/or nonalcoholic fatty liver disease, HBV ± HCV infection, cancer location, Child–Pugh score, and treatment complications, was not available in the SEER database. Secondly, the SEER database only recorded marital status at patients’ initial diagnosis, and changes in marital status might occur during the long-term follow-up periods, which could alter the influence of marital status. Third, a high incidence of receiving CT was observed within the cohort, which was mainly explained by these patients were re-staged based on the newly AJCC 8th staging manual. In previous guidelines for treating early stage HCC with/without microvascular invasion, adjuvant CT followed by surgical resection was recommended. Finally, potential interactions among sex, generation and socioeconomic status might influence marital status, and whether the findings generated in the current study apply to other populations or demographics around the world, need to be confirmed by large, well-designed, prospective studies in the future.
In conclusion, we evaluated the impact of marital status on the OS outcomes of HCC patients with tumors ≤ 2 cm registered in the SEER database between 2004 and 2016. Through comparison between two different regression models, positive marital status can be used to act as a prognostic indicator for better OS outcomes in an independent manner. Our results are consistent with earlier major studies supporting the benefit of being married for stage Ia HCC patients. When treating localized diseases with potential medical cures, additional interventions, including but not limited to family and social support, should be given to subpopulations with negative marital status.
Data availability
The datasets produced for this work (SEER database) are accessible via the following link: https://seer.cancer.gov/data/access.html. Further inquiries can be directed to the corresponding authors.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7, 6. https://doi.org/10.1038/s41572-020-00240-3 (2021).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Kumar, M., Zhao, X. & Wang, X. W. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: One step closer to personalized medicine?. Cell Biosci. 1, 5. https://doi.org/10.1186/2045-3701-1-5 (2011).
Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet 391, 1301–1314. https://doi.org/10.1016/S0140-6736(18)30010-2 (2018).
Gao, S. et al. Declining rates of hepatocellular carcinoma in urban Shanghai: Incidence trends in 1976–2005. Eur. J. Epidemiol. 27, 39–46. https://doi.org/10.1007/s10654-011-9636-8 (2012).
Singal, A. G., Pillai, A. & Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLOS Med. 11, e1001624. https://doi.org/10.1371/journal.pmed.1001624 (2014).
Kim, H. S. & El-Serag, H. B. The epidemiology of hepatocellular carcinoma in the USA. Curr. Gastroenterol. Rep. 21, 17. https://doi.org/10.1007/s11894-019-0681-x (2019).
Shaw, J. J. & Shah, S. A. Rising incidence and demographics of hepatocellular carcinoma in the USA: What does it mean?. Expert Rev. Gastroenterol. Hepatol. 5, 365–370. https://doi.org/10.1586/egh.11.20 (2011).
Mak, L. Y. et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am. Soc. Clin. Oncol. Educ. Book 38, 262–279. https://doi.org/10.1200/EDBK_200939 (2018).
Erstad, D. J. & Tanabe, K. K. Hepatocellular carcinoma: Early-stage management challenges. J. Hepatocell. Carcinoma 4, 81–92. https://doi.org/10.2147/JHC.S107370 (2017).
Aizer, A. A. et al. Marital status and survival in patients with cancer. J. Clin. Oncol. 31, 3869–3876. https://doi.org/10.1200/JCO.2013.49.6489 (2013).
Randi, G. et al. Marital status and cancer risk in Italy. Prev. Med. 38, 523–528. https://doi.org/10.1016/j.ypmed.2003.12.004 (2004).
Yan, B. et al. Does marital status impact postoperative survival in patients with less differentiated hepatocellular carcinoma? A population-based study. Cancer Med. 8, 6272–6279. https://doi.org/10.1002/cam4.2536 (2019).
Chang, S. M. & Barker, F. G. Marital status, treatment, and survival in patients with glioblastoma multiforme: A population based study. Cancer 104, 1975–1984. https://doi.org/10.1002/cncr.21399 (2005).
Yang, D. et al. Impact of sex on the survival of patients with hepatocellular carcinoma: A surveillance, epidemiology, and end results analysis. Cancer 120, 3707–3716. https://doi.org/10.1002/cncr.28912 (2014).
Ellis, L. et al. Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol. 36, 25–33. https://doi.org/10.1200/JCO.2017.74.2049 (2018).
Desquilbet, L. & Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 29, 1037–1057. https://doi.org/10.1002/sim.3841 (2010).
Li, X., Liu, Z., Ye, Z., Gou, S. & Wang, C. Impact of age on survival of patients with pancreatic cancer after surgery: Analysis of SEER data. Pancreatology 18, 133–138. https://doi.org/10.1016/j.pan.2017.11.008 (2018).
Song, T. et al. Multimodal treatment based on thyroidectomy improves survival in patients with metastatic anaplastic thyroid carcinoma: A SEER analysis from 1998 to 2015. Gland Surg. 9, 1205–1213. https://doi.org/10.21037/gs-20-503 (2020).
Song, T. et al. Trends and predictors of survival for small cell carcinoma of the cervix uteri: A SEER population study. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 35–41. https://doi.org/10.1016/j.ejogrb.2020.07.054 (2020).
Song, T., Liang, X., Fang, M. & Wu, S. High-dose versus conventional-dose irradiation in cisplatin-based definitive concurrent chemoradiotherapy for esophageal cancer: A systematic review and pooled analysis. Expert Rev. Anticancer Ther. 15, 1157–1169. https://doi.org/10.1586/14737140.2015.1074041 (2015).
Song, T., Fang, M. & Wu, S. Concurrent chemoradiation therapy tailored to the older adults with esophageal cancer: State of the art and the future. Clin. Interv. Aging 13, 2275–2287. https://doi.org/10.2147/CIA.S179014 (2018).
Chen, Z. et al. Influence of marital status on the survival of adults with extrahepatic/intrahepatic cholangiocarcinoma. Oncotarget 8, 28959–28970. https://doi.org/10.18632/oncotarget.16330 (2017).
Zhang, W. et al. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci. Rep. 7, 41695. https://doi.org/10.1038/srep41695 (2017).
Wu, C. et al. Effect of marital status on the survival of patients with hepatocellular carcinoma treated with surgical resection: An analysis of 13,408 patients in the surveillance, epidemiology, and end results (SEER) database. Oncotarget 7, 79442–79452. https://doi.org/10.18632/oncotarget.12722 (2016).
He, X. K. et al. Marital status and survival in patients with primary liver cancer. Oncotarget 8, 64954–64963. https://doi.org/10.18632/oncotarget.11066 (2017).
Wu, W., Fang, D., Shi, D., Bian, X. & Li, L. Effects of marital status on survival of hepatocellular carcinoma by race/ethnicity and gender. Cancer Manag. Res. 10, 23–32. https://doi.org/10.2147/CMAR.S142019 (2018).
Peters, N. A., Javed, A. A., He, J., Wolfgang, C. L. & Weiss, M. J. Association of socioeconomics, surgical therapy, and survival of early stage hepatocellular carcinoma. J. Surg. Res. 210, 253–260. https://doi.org/10.1016/j.jss.2016.11.042 (2017).
Lin, Q. et al. Improved survival with radiotherapy in hepatocellular carcinoma with major vascular invasion: A propensity-matched analysis of surveillance, epidemiology, and end results database. Cancer Med. 8, 515–526. https://doi.org/10.1002/cam4.1937 (2019).
Zhang, L. et al. A nomogram to predict prognosis of patients with unresected hepatocellular carcinoma undergoing radiotherapy: A population-based study. J. Cancer 10, 4564–4573. https://doi.org/10.7150/jca.30365 (2019).
Xiao, Z. et al. Development and external validation of prognostic nomograms in hepatocellular carcinoma patients: A population based study. Cancer Manag. Res. 11, 2691–2708. https://doi.org/10.2147/CMAR.S191287 (2019).
Wu, W. et al. Pattern of distant extrahepatic metastases in primary liver cancer: A SEER based study. J. Cancer 8, 2312–2318. https://doi.org/10.7150/jca.19056 (2017).
Guo, X. et al. Advanced hepatocellular carcinoma with bone metastases: prevalence, associated factors, and survival estimation. Med. Sci. Monit. 25, 1105–1112. https://doi.org/10.12659/MSM.913470 (2019).
Liang, Y., Wu, X., Lu, C. & Xiao, F. Impact of marital status on the prognosis of liver cancer patients without surgery and the critical window. Ann. Palliat. Med. 10, 2990–2999. https://doi.org/10.21037/apm-20-1885 (2021).
Chiu, C. C. et al. Comparison of models for predicting quality of life after surgical resection of hepatocellular carcinoma: A prospective study. J. Gastrointest. Surg. 22, 1724–1731. https://doi.org/10.1007/s11605-018-3833-7 (2018).
Quinn, T. J. & Kabolizadeh, P. Rectal cancer in young patients: Incidence and outcome disparities. J. Gastrointest. Oncol. 11, 880–893. https://doi.org/10.21037/jgo-20-197 (2020).
Yang, J. et al. Competing-risks model for predicting the prognosis of penile cancer based on the SEER database. Cancer Med. 8, 7881–7889. https://doi.org/10.1002/cam4.2649 (2019).
Yu, Z. et al. A competing risk analysis study of prognosis in patients with esophageal carcinoma 2006–2015 using data from the surveillance, epidemiology, and end results (SEER) database. Med. Sci. Monit. 26, e918686. https://doi.org/10.12659/MSM.918686 (2020).
Wu, W. et al. Competitive risk analysis of prognosis in patients with cecum cancer: A population-based study. Cancer Control 28, 1073274821989316. https://doi.org/10.1177/1073274821989316 (2021).
van Walraven, C. & McAlister, F. A. Competing risk bias was common in Kaplan–Meier risk estimates published in prominent medical journals. J. Clin. Epidemiol. 69, 170–173. https://doi.org/10.1016/j.jclinepi.2015.07.006 (2016).
Acknowledgements
We received no financial support for the present research. We thank the National Cancer Institute (NCI) for providing the SEER data after permission.
Author information
Authors and Affiliations
Contributions
S.Y. and F.C. conceived the study. Y.W. and H.X. searched the database and literature. F.C., T.S. and S.Y. discussed and analyzed the data. F.C. and T.S. wrote the manuscript. Y.W., H.X. and S.Y. revised the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, F., Wu, Y., Xu, H. et al. Impact of marital status on overall survival in patients with early-stage hepatocellular carcinoma. Sci Rep 12, 19923 (2022). https://doi.org/10.1038/s41598-022-14120-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14120-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.