Abstract
Pig-to-human organ transplantation is a feasible solution to resolve the shortage of organ donors for patients that wait for transplantation. To overcome immunological rejection, which is the main hurdle in pig-to-human xenotransplantation, various engineered transgenic pigs have been developed. Ablation of xeno-reactive antigens, especially the 1,3-Gal epitope (GalT), which causes hyperacute rejection, and insertion of complement regulatory protein genes, such as hCD46, hCD55, and hCD59, and genes to regulate the coagulation pathway or immune cell-mediated rejection may be required for an ideal xenotransplantation model. However, the technique for stable and efficient expression of multi-transgenes has not yet been settled to develop a suitable xenotransplantation model. To develop a stable and efficient transgenic system, we knocked-in internal ribosome entry sites (IRES)-mediated transgenes into the α 1,3-galactosyltransferase (GGTA1) locus so that expression of these transgenes would be controlled by the GGTA1 endogenous promoter. We constructed an IRES-based polycistronic hCD55/hCD39 knock-in vector to target exon4 of the GGTA1 gene. The hCD55/hCD39 knock-in vector and CRISPR/Cas9 to target exon4 of the GGTA1 gene were co-transfected into white yucatan miniature pig fibroblasts. After transfection, hCD39 expressed cells were sorted by FACS. Targeted colonies were verified using targeting PCR and FACS analysis, and used as donors for somatic cell nuclear transfer. Expression of GalT, hCD55, and hCD39 was analyzed by FACS and western blotting. Human complement-mediated cytotoxicity and human antibody binding assays were conducted on peripheral blood mononuclear cells (PBMCs) and red blood cells (RBCs), and deposition of C3 by incubation with human complement serum and platelet aggregation were analyzed in GGTA1 knock-out (GTKO)/CD55/CD39 pig cells. We obtained six targeted colonies with high efficiency of targeting (42.8% of efficiency). Selected colony and transgenic pigs showed abundant expression of targeted genes (hCD55 and hCD39). Knocked-in transgenes were expressed in various cell types under the control of the GGTA1 endogenous promoter in GTKO/CD55/CD39 pig and IRES was sufficient to express downstream expression of the transgene. Human IgG and IgM binding decreased in GTKO/CD55/CD39 pig and GTKO compared to wild-type pig PBMCs and RBCs. The human complement-mediated cytotoxicity of RBCs and PBMCs decreased in GTKO/CD55/CD39 pig compared to cells from GTKO pig. C3 was also deposited less in GTKO/CD55/CD39 pig cells than wild-type pig cells. The platelet aggregation was delayed by hCD39 expression in GTKO/CD55/CD39 pig. In the current study, knock-in into the GGTA1 locus and GGTA1 endogenous promoter-mediated expression of transgenes are an appropriable strategy for effective and stable expression of multi-transgenes. The IRES-based polycistronic transgene vector system also caused sufficient expression of both hCD55 and hCD39. Furthermore, co-transfection of CRISPR/Cas9 and the knock-in vector not only increased the knock-in efficiency but also induced null for GalT by CRISPR/Cas9-mediated double-stranded break of the target site. As shown in human complement-mediated lysis and human antibody binding to GTKO/CD55/CD39 transgenic pig cells, expression of hCD55 and hCD39 with ablation of GalT prevents an effective immunological reaction in vitro. As a consequence, our technique to produce multi-transgenic pigs could improve the development of a suitable xenotransplantation model, and the GTKO/CD55/CD39 pig developed could prolong the survival of pig-to-primate xenotransplant recipients.
Similar content being viewed by others
Introduction
Xenotransplantation is a powerful source to solve the shortage of organ transplant donors. In pig-to-human xenotransplantation, ablation of the alpha 1,3-Gal epitope (GalT) synthesized by α 1,3-galactosyltransferase (GGTA1) gene is essential to inhibit hyperacute rejection, which is the most severe barrier to xenotransplantation. Since the GGTA1 knock-out (GTKO) pig was developed1, the survival days of heart and kidney pig-to-primate xenotransplantation were prolonged from 2 to 6 months2,3,4. Although hyperacute rejection was inhibited in GTKO pigs, early graft failure was observed 3 days after pig-to-non-human primate organ transplantation5. To overcome early graft failure after GTKO pig-to-non-human primate organ transplantation, additional knock-out of cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) synthesizing N-glycolylneuraminic acid (Neu5Gc) and beta‐1,4‐N‐acetyl‐galactosaminyltransferase 2 (β4GalNT2) synthesizing Sda antigen have been suggested6,7,8,9,10. In spite of the double or triple knock-out of these genes in pigs, delayed graft rejection characterized by complement activation and thrombotic microangiopathy has still persisted after pig-to-non-human cardiac or renal xenotransplantation11,12,13. These findings suggest that modulation of complement activation and coagulation may be cooperatively needed with knock-out of xeno-antigens.
Transgenic pigs for complement regulatory protein such as hCD46, hCD55, and hCD59 were developed to overcome complement-mediated cytotoxicity or early graft failure compared to wild-type pigs or GTKO pigs5,14,15,16,17. GTKO/hCD46/hTBM pigs showed the longest survival (945 days for heterotopic, 195 days for orthotopic) in cardiac pig-to-primate xenografts18,19.
CD55 (decay-accelerating factor, DAF) can inhibit the complement cascade by dissociating C3 convertase into its constituent proteins20,21. Several convincing studies have shown that hCD55 is an applicable complement regulatory protein to protect against complement-mediated injury in xenografts22,23,24. The transgenic porcine heart with only hDAF was life-supported for 39 days, and GTKO/hCD55 transgenic pigs survived over 400 days in pig-to-non-human primate renal xenotransplantation17,25. Furthermore, recent research found that deficiency of hCD55 is related to the occurrence of angiopathic thrombosis26.
CD39 (ectonucleoside triphosphate diphosphohydrolase-1, ENTPD1) is one of the thromboregulatory molecules expressed on the luminal surface and caveolar microdomains of quiescent endothelial cells27,28, and is able to prevent coagulation by inactivating platelet aggregation. Platelet aggregation is stimulated when the endothelium is damaged from immunological rejection. CD39 has the role of NTPDase to regulate platelet aggregation. CD39 converts ADP, which is a potent agonist to trigger platelet aggregation, to AMP, and AMP is broken down by ecto-5’-nucleotidase (CD73) to adenosine27,29. Generated adenosine acts by inactivating platelet aggregation.
For these multiple immune-protective effect in pig-to-human xenotransplantation, many attempts using the internal ribosome entry sites (IRES) and the self-cleavable 2A sequence, which are widely studied for basic science, and therapeutic research, have been developed for the production of multi-transgenic pigs30,31,32,33. Recently, knock-out of four genes/transgenic for nine genes was developed using 2A and the piggy-bag mediated integration system34.
Over expression of transgenes is a fundamental option across the broad scientific research using genetically engineering animal models. Induction of transgene expression can be applied to study gene function, gene therapy, and biopharmaceuticals35,36,37. Despite these various uses for the expression of transgenes, transfection by a plasmid, lentiviral, and transposon systems have still shown to induce gene silencing, or insertional mutagenesis, or unknown chromosome position effects, or position-effect variegations38,39,40,41,42. Exogenous promoters for the expression of transgenes also results in gene silencing of the transgene43,44,45,46,47,48,49. Multi-transgenic pigs could be produced by breeding of each single gene expressed transgenic pigs, but it is not efficient in costs and time, and it is unpredictable. In addition, multi-transgenic pigs engineered using IRES-mediated tricistronic vector systems showed pretty low expression of the third gene of the inserted vector32.
On the contrary, targeting of the transgene into specific genome locus revealed abundant expression of transgene50,51,52, and expression of the transgene under the control of endogenous promoters of target gene loci for knock-in revealed stable expression over a long time53,54.
The objective of this report is to demonstrate that endogenous GGTA1 promoter-mediated multi-transgene expression by gene-targeting was stable and effective to create a transgenic pig model aimed at preventing immunological rejection towards humans in vitro.
Results
Construction of GTKO/CD55/CD39 knock-in vector and Establishment of targeted donor cell.
A total 785 reconstructed embryos derived from the GTKO/CD55/CD39 donor cells were transferred into four surrogates. Two surrogates became pregnant, and one of those surrogates gave birth to four live born piglets, and one piglet was survived (supplementary Fig. S4). The cloning efficiency was 2.1% in this single transfer. In total, the cloning efficiency was 0.5% (total piglets/total transferred embryos) (Table 1). The targeting strategy to knock-out the GGTA1 gene and knock-in the hCD55 and hCD39 genes and guide RNA sequences, to target initiation codon in exon 4 of the GGTA1 gene, were designed as described in supplementary Fig. S1A and S1B. Two homologous arms were designed to target exon 4, which contains the ATG initiation codon. Between two homologous arms, the cDNA for hCD55 and hCD39 were inserted under control of the GGTA1 endogenous promoter. We chose electroporation, which shows high efficiency of transfection for primary cultured cells, such as ear skin fibroblasts to transfect targeting vectors into pig ear skin fibroblasts. FACS sorting using hCD39 expression was performed to select targeted cells because we expected that only targeted cells would express hCD39. hCD39 positive cells were 20% of the total cells and sorted (supplementary Fig. 1C). After selection using FACS sorting, 24 single colonies were picked and 14 colonies were analyzed to verify targeting through targeting PCR (supplementary Fig. 1D). In six colonies the vector was successfully targeted on exon 4 of the GGTA1 gene with high efficiency (42.8% of efficiency) (Table 2). Colony #15 was used as donor for somatic nuclear transfer (SCNT).
Ablation of GalT and GGTA1 endogenous promoter-mediated expression of hCD55 and hCD39
We selected one of the targeted six colonies, which had greater cell morphology and proliferation. hCD55 and hCD39 were abundantly expressed on the cell surface of targeted donor cells and expression of the proteins was also confirmed (supplementary Fig. S1E and S1F). The targeting vector was knocked-in to the one allele of the GGTA1 locus, with consequent heterozygous knock-out of the GGTA1 gene. However, the other allele which was not knocked-in was also disrupted by CRISPR/Cas9, thereby inducing homozygous knock-out of the GGTA1 gene (Fig. 1A and supplementary Fig. S4C).
Ablation of GalT and targeted hCD55 and hCD39 gene at GGTA1 locus on produced GTKO/CD55/CD39 targeted cloned pig. (A) Schematic of targeting strategy to ablate GalT and knock-in of hCD55 and hCD39 gene. (B) Targeting PCR analysis in GTKO/CD55/CD39 transgenic pig (Donor; targeted colony, WT; wild-type, TG; transgenic pig). (C) Cell surface expression of GalT, hCD55, and hCD39 on variety cell source from wild type (blue line) and GTKO/CD55/CD39 transgenic pig (red line). (D) Comparison of hCD55 and hCD39 expression on GTKO/CD55/CD39 pig aorta endothelial cells (red line), and human aorta endothelial cells (blue line). Wild-type pig aorta endothelial cells (black line) was used as negative control. The original gels are presented in supplementary Fig. S3.
Production of GTKO/CD55/CD39 knock-in targeted transgenic cloned pig
Cloned piglets possessing the targeting vector inserted into the GGTA1 locus were produced and their genotype verified by targeting PCR (Fig. 1B). hCD55 and hCD39 was abundantly expressed in ear skin fibroblasts cells, aorta endothelial cells, cornea endothelial cells, peripheral blood mononuclear cells (PBMCs), red blood cells (RBCs), and kidney cells from GTKO/CD55/CD39 transgenic pig. The transgenic animals also revealed ablation of the aGal epitope on the surface of these cells (Fig. 1C). Moreover, the expression level of hCD55 was similar between human and GTKO/CD55/CD39 pig aorta endothelial cells. In the case of hCD39, the expression level in GTKO/CD55/CD39 pig aorta endothelial cells was higher than in human endothelial cells (Fig. 1D).
Human antibody binding
Human antibody binding to PBMCs and RBCs from GTKO/CD55/CD39 pigs was greatly reduced compared with binding to PBMCs and RBCs from wild-type pigs. However, human antibody binding to PBMCs (Fig. 2A) and RBCs (Fig. 2B) was not significantly different between GTKO and GTKO/CD55/CD39 pig.
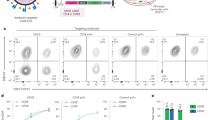
Prevention of humoral rejection on GTKO/CD55/CD39 pig in vitro. Human antibody binding to PBMCs (A) and RBCs (B). Both human IgG and IgM binding to cells from GTKO and GTKO/CD55/CD39 pig decreased compared with cells from wild-type pig. Data represented relative mean fluorescence intesnsity (MFI). Experiments were performed in triplicated (n = 1, ***P < 0.05, one-way analysis of variance). Inhibition of human complement mediated rejection on cells from GTKO/CD55/CD39 pig. PBMCs (C) and RBCs (D) from wild-type, GTKO/CD55/CD39 pig and human. These cells were incubated with various human complement serum concentration, and the viability was represented by calculation with absorbance of CCK-8 (PBMCs) or viable remaining cells (RBCs). Experiments were performed in triplicated (n = 1; * P < 0.05; **P < 0.01; ***P < 0.001; ns, non-significant; two-way analysis of variance).
Inhibition of immunological rejection by human complement
The viability against human complement was estimated using peripheral blood mononuclear cells (PBMCs) and red blood cells (RBCs) from wild-type, GTKO, and GTKO/CD55/CD39 pig. Both GTKO/CD55/CD39 cell types remarkably prevented human complement-mediated cytotoxicity against various human complement serum concentration (0%, 3.125%, 6.25%, 12.5%, and 25%) compared with wild-type and GTKO cells. Against most concentrations of human complement serum, the viability of PBMCs and RBCs from GTKO/CD55/CD39 pig was not significantly different from that of human PBMCs or RBCs. As shown in Fig. 2C, PBMCs from wild-type and GTKO pigs survived only 24.95–42.09% (wild-type), and 24.19–76.28% (GTKO), whereas the viability was 45.81–94.72% against various human complement serum concentration in PBMCs from GTKO/CD55/CD39 (Fig. 2C). As shown in Fig. 2D, the mean viability of wild-type pig RBCs against human complement serum was only 9.1–80.6%. The mean viability of GTKO pig RBCs was 67.25–85.7%, and that of GTKO/CD55/CD39 pig RBCs was significantly higher (89.3–91.5%).
C3 deposition
Deposition of C3 was estimated following activation of complement through incubation of RBCs with 0%, 25%, and 50% of human complement serum. MFI of C3 on RBCs from GTKO/CD55/CD39 pigs were 81.26–149 at 0, 25, and 50% concentration, whereas mean fluorescence intensity (MFI) of C3 on RBCs from GTKO were 85.7–523 at 0, 25, and 50% concentration, respectively. It was impossible to detect C3 deposition in RBCs from wild-type due to the dramatic hemolysis which occurred following human complement activation (Fig. 3A).
Functional hCD55 and hCD39 gene expressed on GTKO/CD55/CD39 pig. (A) Decrease of C3 deposition on RBCs from GTKO/CD55/CD39 compared with RBCs from GTKO pig at 0, 25, and 50% of human complement serum concentration. Experiments were performed in triplicated (n = 1, t-test; *P < 0.05; **P < 0.01; ns, non-significant). (B) Inhibition of platelet aggregation in GTKO/CD55/CD39 pig by platelet aggregation test response to ADP (10 µM) and collagen (2 mg/mL). Experiments were performed in independently triplicated (n = 1, *P value = 0.0169; **P value = 0.0014, one-way analysis of variance).
Platelet aggregation
Platelet aggregation was tested in wild-type and GTKO/CD55/CD39 transgenic pigs’ PRP.
The maximal aggregation of platelets in wild-type and GTKO pigs was between 38 and 64%, whereas maximal aggregation of platelets was only 14–18% in GTKO/CD55/CD39 pigs against 10 µM of ADP. Against collagen (4 µg/mL), the maximal aggregation of platelets in GTKO/CD55/CD39 pigs was only 35–39% (47 and 78% aggregation were estimated in wild-type and GTKO pigs) (Fig. 3B).
Discussion
Production of transgenic pigs is an imperative technology for the success of pig-to-human xenotransplantation. Many genes have to be engineered to overcome various immunological rejection issues in pig-to-human xenotransplantation. Many transgenic pigs with single gene or multi-transgenic pigs with more than three engineered genes were developed in the xenotransplantation field30,33,55,56. Recently, the successful first clinical heart and kidney xenotransplantation57 was carried out using transgenic pig with ten genetic modification. Nevertheless, an effective transgenic system has not been agreed upon. In our previous study52, we chose the GGTA1 locus and a gene trap strategy for transgene expression due to stable and various expression of GalT58,59,60,61,62. As a result, our transgenic pig expressed the transgene abundantly without an exogenous promoter, and the transgene was well maintained in expression for several generations. Therefore, we tried to produce multi-transgenic pigs by a gene trap strategy targeting the GGTA1 locus, for the stable and efficient expression of transgenes to solve the issues with exogenous promoter-mediated transgene expression and random integration of transgenes.
The homologous recombination strategy is an effective method for gene modification in mouse embryonic stem cells by knock-out and knock-in63,64. Since gene-editing technologies such as ZFN, TALEN, and CRISPR/Cas9 have been developed, some studies have attempted to use TALEN or CRISPR/Cas9 systems to increase the efficiency of homologous recombination-mediated knock-in65,66,67,68. In this sense, we developed a system that combined CRISPR/Cas9 and a hCD55/hCD39 knock-in vector to increase efficiency. We chose to target exon 4, which includes the initiation codon of the GGTA1 gene, so that expression of hCD55 and hCD39 would be under the control of the GGTA1 gene endogenous promoter, due to the wide expression of GalT in pig, and to induce the stable expression of the transgene. We constructed a targeting vector to ablate the GGTA1 gene and express hCD55 and hCD39 by the gene trap principle. The combination of CRISPR/Cas9 and knock-in systems increased the frequency of homologous recombination, so that the targeted gene was detectable by FACS sorting (20% expression of hCD39 after neomycin selection, supplementary Fig. 1C). After FACS sorting, we obtained six precise targeted colonies of a total of 20 colonies analyzed (42.8% efficiency), with a much higher efficiency than in our previous study (2.6% of efficiency)52 (Table 2). Furthermore, another allele apart from the targeted allele was also mutated by the CRISPR/Cas9 effect, whereas knock-in usually resulted in heterozygous targeting (Fig. 1A and supplementary Fig. S4C).
To overcome disadvantage such as gene silencing, insertional mutagenesis, unknown chromosome position effects and position-effect variegation of random integration or exogenous promoter-mediated expression of transgene38,39,40,41,42,43,44,45,46,47,48,49, we chose the endogenous promoter-mediated method. The accurate targeting of transgene is essential for endogenous promoter-mediated expression. The results of our study, precisely targeting of hCD55/hCD39 knock-in vector is confirmed. However, additional review on the number of copies is required.
Anti-GalT antibodies are naturally developed in human in the first few months of infancy69,70,71. It is probably due to the fact that the infant is exposed to viruses or microorganisms expressing GalT in the gastrointestinal tract72. Because of this phenomenon, GalT expressed on the cell surface in pigs is the main hurdle causing hyperacute rejection in pig-to-human xenotransplantation. Antigen-antibodies mediated rejection still remains the main cause for xenotransplantation failure following knock-out of GalT. Other non-gal antigens, such as Neu5Gc or Sda, have been knocked out to decrease anti-pig antibody binding in human9,73,74. However, the survival of Neu5Gc deficient pig kidney was shorter than GTKO pig kidney in pig-to-nonhuman primate xenotransplantation, and antibody binding to Neu5Gc deficient pig were also increased in old world monkey because unlike humans, old world monkey have activated CMAH gene, and possess naturally-existing antibodies directed to Neu5Gc deficient pig74,75,76. Therefore, the current model of pig-to-non-human primate xenotransplantation is less than ideal given the expectation for improved survival in the first pig-to-human trials with a Neu5Gc deficient pig compared to the pig-to-non-human primate model. These findings and our results imply that triple knock-out of GGTA1, CMAH and β4GalNT2 and insertion of hCD55 and hCD39 genes could be effective to prevent immune rejection in pig-to-human xenotransplantation.
hCD55 plays a role in the regulation of complement activation, which is caused by the antigen–antibody complex via the classical pathway. The mechanism between hCD55 and NK cells is unknown, however hCD55 was associated with NK cell-mediated lysis, whereas other complement regulatory proteins including hCD46 and hCD59 were not22. In this study, we successfully eliminated the GalT epitope through targeting of the hCD55/hCD39 knock-in vector into the GGTA1 gene locus. Ablation of the GalT epitope considerably decreased human complement-mediated cytotoxicity and human antibody binding compared to wild-type pig cells. Although human antibody binding to cells from GTKO/CD55/CD39 pig was not greater than to cells from GTKO pigs, our transgenic pig cells showed further prevention against human complement-mediated lysis compared to GTKO pig cells (Fig. 2).
Decrease of C3 deposition on GTKO/CD55/CD39 pig cells following activation of the complement cascade was comparable to that in cells from GTKO, that also proved the inhibitory effect of the complement cascade by targeting hCD55 (Fig. 3A).
These data imply that the hCD55 gene targeted in our GTKO/CD55/CD39 pig could be capable of dramatically controlling the complement reaction in pig-to-human humoral rejection. Although precluding hyperacute rejection or complement activation, coagulation disorders also remain a barrier for successful pig-to-primate xenotransplantation. At the sites of vascular injury, platelet adhesion is initiated and activated by agonists, including ADP and collagen. Therefore, platelets, which have a role in the interaction between endothelium and leukocytes in the form of platelet microthrombi, have been thought to be a major player in the process of improving survival of organ xenotransplantation77,78. To regulate platelet-mediated thrombosis, we also aimed to knock-in hCD39 with hCD55. As a result, the platelet aggregation was much more delayed in PRP from GTKO/CD55/CD39 pig than PRP from wild-type and GTKO pigs, for not only ADP-induced, but also collagen-induced platelet aggregation (Fig. 2B). These results showed that our GTKO/CD55/CD39 pig could prevent not only humoral rejection but also platelet-mediated thrombosis in pig-to-primate xenotransplantation.
Our endogenous-mediated expression of transgenes by knock-in, targeting of hCD55 and hCD39 into the GGTA1 locus, induced abundant and stable expression of functioning proteins as well. Ablation of the GalT epitope and concomitant expression of hCD55 improved the inhibition of complement-mediated cytotoxicity compared to wild-type and GTKO pigs. The function of hCD39 was also proven by platelet aggregation analysis. Although hCD55 and hCD39 are not novel genes for xenotransplantation, endogenous promoter-mediated expression of hCD55 and hCD39 in a transgenic pig had not been tried in pig-to-non-human primate xenotransplantation. Moreover, our transgenic pig showed similar expression levels of hCD55 and higher expression of hCD39 compared to human aorta endothelial cells (Fig. 1D). In this phenomenon, our transgenic pig could be a comparable model to various transgenic pigs for xenotransplantation.
Methods
Ethical statements and animal carep
All experimental methods were carried out according to ARRIVE guidelines. All methods were performed in accordance with relevant guidelines and regulations. All protocols of animal care and use procedure were approved by the Institutional Animal Care and Use Committee of Optipharm, Inc., Life Science Institute (IACUC approval No. OPT-140103-1, D-grade). All pigs used in the experiments were white Yucatan miniature pigs and approved by institutional animal care. The animal facility for all pigs at Optipharm, Inc., was a specific pathogen-free environment. Filtered water and air, and sterilized feed were supplied, and the rooms were maintained at 24 °C ± 2 °C and 12 h light/12 h dark cycles. For experiments of this study, one-year-old healthy pigs, wild-type, GTKO, and GTKO/CD55/CD39 were humanely euthanized by intravenous injection of 2 mM/kg potassium chloride solution under general anesthesia. After a veterinarian certified the death of the pigs, their organs were harvested.
Construction of hCD55/hCD39 knock-in vector and CRISPR/Cas9 vector for GalT
Two homologous arms (left and right arms) were designed to target exon4 of the GGTA1 gene as described in Fig. 1. These arms were amplified from pig genomic DNA by PCR. hCD55 and hCD39 fragments were synthesized from human cDNA by PCR. Left and right arm DNA fragments were inserted into modified PGKneolox2DTA.2 (Addgene plasmid 13449). hCD55 and hCD39 fragments were inserted between the left arm and the neomycin fragment. IRES sequences were inserted between the hCD55 and hCD39 fragments to express hCD39. Small guide RNA sequences (5’-AATGAATGTCAAAGGAAGAG-3’) for CRISPR/Cas9 vector were targeted for the ATG codon of the GGTA1 gene.
Culture, transfection, and selection of pig primary ear skin fibroblasts
Ear skin fibroblasts were isolated from 2 month-old white yucatan miniature pigs. Isolated ear skin fibroblasts were cultured in Dulbecco's modified eagles medium (DMEM, Welgene, Korea) containing 15% fetal bovine serum (FBS, Hyclone, USA), 1% non-essential amino acid, 0.1 mM β-mercaptoethanol, and 1% antibiotic–antimycotic (Gibco, USA). Transfection occurred in 1 × 106 suspended ear skin fibroblasts at 90% confluency, with 5 μg of GTKO/hCD55/hCD39 knock-in vector and 5 μg of CRISPR/Cas9 for GGTA1 using electroporation (Amaxa 4D, Lonza, USA), according to manufacturer’s instructions. After 2 days of transfection, the cells were incubated with neomycin (300 μg/mL) for further 14 days. After 14 days of transfection, the fibroblasts were harvested and stained with anti-hCD39 antibody (Santacruz, USA). CD39 positive cells were sorted by flowcytometry (Aria II, BD, USA).
Production of cloned pig from SCNT and embryo transfer
SCNT and embryo transfers were performed as described previously in our study37. The pregnancy status was monitored with an ultrasound scanner (Mysono 201, Medison Co., LTD, Korea).
Verification of targeted donor cells and transgenic pigs
From the single cell culture, 24 colonies were picked and 20 colonies were expanded to be analyzed. Each colony was harvested in 12-well plate culture vessels. Genomic DNA was extracted using DNeasy blood and tissue kit (QIAGEN, USA) from each colony and cloned pig tissue. Left arm, right arm, and long PCR amplification were performed to screen targeted colony and cloned pig using primers as described in supplementary Table S1.
Expression of GalT, hCD55, and hCD39
For flow cytometry analysis, harvested cells were suspended in 100 µL of Dulbecco’s phosphate buffered saline (DPBS, Gibco) and stained with 2 µL of anti-hCD55 (sc-57133, Santacruz) or anti-hCD39 (sc-65232, Santacruz) for 2 h at room temperature. Fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were then incubated for 1 h on ice to analyze the expression of hCD55 and hCD39. To analyze GalT expression, 2 µL of Alexa Flour 488®-conjugated isolectin-ib4 (I21411, Invitrogen, USA) was incubated with the suspended cells for 3 h on ice. Expression of GalT, hCD55, and hCD39 on the cell surface was estimated by flow cytometry (Calibur-S system, BD). To identify translational expression of hCD55 and hCD39, 2 × 106 cells were harvested and lysed with 30 µL of mammalian protein extract reagent (Thermo, USA) for 4 h on ice. Proteins extracted from the cells were quantified using the Bradford assay (BioRad, USA) and resolved on 8% sodium dodecyl sulfate polyacrylamide gels. The proteins were then transferred to nitrocellulose membrane, which was blocked with 1X Tris-buffered saline, 0.1% Tween-20 containing 5% skimmed milk for 1 h at room temperature. Incubation with anti-hCD39 (sc-33558, Santacruz), or anti-hCD55 (ab54595, Abcam), or anti-beta-actin (sc-47778, Santacruz) as primary antibodies happened overnight at 4 °C. The membrane was then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Human hCD55 and hCD39 were detected with the ECL chemiluminescence kit for western blot analysis (GE Healthcare, UK), according to the manufacturer’s protocol.
Isolation of PBMCs and RBCs
A total of 5 mL of whole blood from wild-type, GTKO (developed in our previous study79), and GTKO/CD55/CD39 pigs was collected into EDTA tubes (BD). PBMC and RBCs were separated from collected blood using density media ficoll-paque plus (GE), according to manufacturer’s protocol. Isolated PBMCs and RBCs were washed with DPBS (Gibco), and immediately used for complement-mediated cytotoxicity or human antibody binding assays, or for confirmation of gene expression.
Culture of Aorta endothelial cells, corneal endothelial cells, and kidney cells
Pig aortas were incubated with 0.005% collagenase type IV (sigma, USA), and the isolated cells were washed with DPBS(Gibco). The cells were then culture with endothelial cell growth medium-2 bullet kit (Lonza, Switzerland). Pig corneal endothelial cells were isolated and cultured, as described in our previous study80. Pig kidney tissues were minced into 1–2-mm sections, and digested with 3% collagenase type IV. The digested tissues were passed through a 100-m cell strainer (BD). The isolated cells were washed with DPBS (Gibco), and cultured in DMEM (Welgene) containing 15% FBS (Hyclone), 1% non-essential amino acid, 0.1 mM β-mercaptoethanol, and 1% antibiotic–antimycotic (Gibco).
Complement-mediated cytotoxicity
2 × 105 of PBMCs and RBCs isolated from pigs were incubated with 0%, 3.125%, 6.25%, 12.5%, and 25% of pooled human complement serum (Innovative research, USA) for 2 h (PBMCs) or overnight (RBCs) on v-bottom 96-well plates (Corning, USA). After incubation, human complement serum was removed following centrifugation. PBMCs were then re-suspended with 100 µL of RPMI 1640 media (Gibco) containing 5% fetal bovine serum (Gibco), 15 mM HEPES (Sigma), and 0.2 M EDTA (Bioneer, Korea). To estimate viability against human complement serum, 10 µL of CCK-8 solution was added to suspended PBMCs, and the absorbance of each well at 450 nm was measured. After incubation of RBCs with human complement serum, viability was estimated by measurement of the absorbance (405 nm) of the remaining viable RBCs.
Human antibody binding assay
Pig PBMCs and RBCs were incubated with 10% normal human serum (Millipore, USA) for 30 min at 4 °C. PBMCs and RBCs were then washed by centrifugation and incubated with 100 μL of PBS containing 3 µL of anti-human IgG (62-8411, Invitrogen) or anti-human IgM (F5384, Sigma) for 1 h at 4 °C. Stained PBMCs and RBCs were analyzed using flow cytometry (CantoII, BD). Human antibody binding was calculated using the MFI formula below:
C3 deposition
RBCs from wild-type, GTKO, and GTKO/CD55/CD39 pigs (5 × 105) were incubated with human complement serum (Innovative technology, USA) at a concentration range of 0%, 25%, and 50% for 24 h. The cells were then incubated with an anti-C3 antibody (Santacruz) for 2 h at room temperature. The FITC-conjugated secondary antibody (Abcam) was applied for 1 h on ice. After each incubation, the cells were washed three times with 1 mL of PBS. Stained cells were analyzed by flow cytometry (CantoII, BD, USA).
Platelet aggregation analysis
Blood was drawn from wild-type, GTKO and GTKO/CD55/CD39 pigs into trisodium citrate (0.109 M, 3.2%) tubes (BD). Pig platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were separated by centrifugation. Platelet aggregation response against ADP (10 µM) and collagen (2 mg/mL) was measured by light transmission aggregometry (chrono-log, USA).
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Lai, L. et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295, 1089–1092. https://doi.org/10.1126/science.1068228 (2002).
Kuwaki, K. et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat. Med. 11, 29–31. https://doi.org/10.1038/nm1171 (2005).
Yamada, K. et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 11, 32–34. https://doi.org/10.1038/nm1172 (2005).
Chen, G. et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat. Med. 11, 1295–1298. https://doi.org/10.1038/nm1330 (2005).
Azimzadeh, A. M. et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation 22, 310–316. https://doi.org/10.1111/xen.12176 (2015).
Park, J. Y. et al. alpha1,3-galactosyltransferase deficiency in germ-free miniature pigs increases N-glycolylneuraminic acids as the xenoantigenic determinant in pig-human xenotransplantation. Cell. Reprogram. 14, 353–363. https://doi.org/10.1089/cell.2011.0083 (2012).
Park, J. Y. et al. Alpha 1,3-galactosyltransferase deficiency in pigs increases sialyltransferase activities that potentially raise non-gal xenoantigenicity. J. Biomed. Biotechnol. 2011, 560850. https://doi.org/10.1155/2011/560850 (2011).
Byrne, G., Ahmad-Villiers, S., Du, Z. & McGregor, C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation 25, e12394. https://doi.org/10.1111/xen.12394 (2018).
Lutz, A. J. et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 20, 27–35. https://doi.org/10.1111/xen.12019 (2013).
Song, K. H. et al. Cloning and functional characterization of pig CMP-N-acetylneuraminic acid hydroxylase for the synthesis of N-glycolylneuraminic acid as the xenoantigenic determinant in pig-human xenotransplantation. Biochem. J. 427, 179–188. https://doi.org/10.1042/BJ20090835 (2010).
Zhang, J. et al. Potential antigens involved in delayed xenograft rejection in a Ggta1/Cmah Dko pig-to-monkey model. Sci. Rep. 7, 10024. https://doi.org/10.1038/s41598-017-10805-0 (2017).
Ariyoshi, Y. et al. Antibody reactivity with new antigens revealed in multi-transgenic triple knockout pigs may cause early loss of pig kidneys in baboons. Xenotransplantation 28, e12642. https://doi.org/10.1111/xen.12642 (2021).
Cooper, D. K. et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation 23, 83–105. https://doi.org/10.1111/xen.12219 (2016).
Cozzi, E. et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation 70, 15–21 (2000).
Ramirez, P. et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase). Transplant. Proc. 37, 4103–4106. https://doi.org/10.1016/j.transproceed.2005.09.186 (2005).
McGregor, C. G. et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J. Thorac. Cardiovasc. Surg. 130, 844–851. https://doi.org/10.1016/j.jtcvs.2005.04.017 (2005).
Vial, C. M. et al. Life supporting function for over one month of a transgenic porcine heart in a baboon. J. Heart Lung Transplant. 19, 224–229 (2000).
Mohiuddin, M. M. et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat. Commun. 7, 11138. https://doi.org/10.1038/ncomms11138 (2016).
Langin, M. et al. Author Correction: Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 568, E7. https://doi.org/10.1038/s41586-019-1108-4 (2019).
Lublin, D. M. & Atkinson, J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu. Rev. Immunol. 7, 35–58. https://doi.org/10.1146/annurev.iy.07.040189.000343 (1989).
Lukacik, P. et al. Complement regulation at the molecular level: the structure of decay-accelerating factor. Proc. Natl. Acad. Sci. USA 101, 1279–1284. https://doi.org/10.1073/pnas.0307200101 (2004).
Miyagawa, S. et al. Effect of tandem forms of DAF(CD55) on complement-mediated xenogeneic cell lysis. Xenotransplantation 13, 433–439. https://doi.org/10.1111/j.1399-3089.2006.00331.x (2006).
Diaz-Roman, T. M. et al. Human DAF on pig cells protects against human and non-human primate sera cytotoxicity mediated by exogenous or endogenous complement, as determined by flow cytometry. Transpl. Immunol. 16, 125–130. https://doi.org/10.1016/j.trim.2006.03.008 (2006).
Murakami, H. et al. Transgenic pigs expressing human decay-accelerating factor regulated by porcine MCP gene promoter. Mol. Reprod. Dev. 61, 302–311. https://doi.org/10.1002/mrd.10043 (2002).
Kim, S. C. et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surg. 19, 2174–2185. https://doi.org/10.1111/ajt.15329 (2019).
Ozen, A. et al. CD55 deficiency, early-onset protein-losing enteropathy, and thrombosis. N. Engl. J. Med. 377, 52–61. https://doi.org/10.1056/NEJMoa1615887 (2017).
Kaczmarek, E. et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J. Biol. Chem. 271, 33116–33122 (1996).
Goepfert, C. et al. CD39 modulates endothelial cell activation and apoptosis. Mol. Med. 6, 591–603 (2000).
Zimmermann, H. 5’-Nucleotidase: molecular structure and functional aspects. Biochem. J. 285(Pt 2), 345–365 (1992).
Kwon, D. J. et al. Generation of alpha-1,3-galactosyltransferase knocked-out transgenic cloned pigs with knocked-in five human genes. Transgenic Res. 26, 153–163. https://doi.org/10.1007/s11248-016-9979-8 (2017).
Bouabe, H., Fassler, R. & Heesemann, J. Improvement of reporter activity by IRES-mediated polycistronic reporter system. Nucleic Acids Res. 36, e28. https://doi.org/10.1093/nar/gkm1119 (2008).
Chinnasamy, D. et al. Multicistronic lentiviral vectors containing the FMDV 2A cleavage factor demonstrate robust expression of encoded genes at limiting MOI. Virol. J. 3, 14. https://doi.org/10.1186/1743-422X-3-14 (2006).
Jeong, Y. H. et al. Production of multiple transgenic Yucatan miniature pigs expressing human complement regulatory factors, human CD55, CD59, and H-transferase genes. PLoS ONE 8, e63241. https://doi.org/10.1371/journal.pone.0063241 (2013).
Yue, Y. et al. Extensive germline genome engineering in pigs. Nat. Biomed. Eng. 5, 134–143. https://doi.org/10.1038/s41551-020-00613-9 (2021).
Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 36, 1136–1145. https://doi.org/10.1038/nbt.4305 (2018).
Prelich, G. Gene overexpression: uses, mechanisms, and interpretation. Genetics 190, 841–854. https://doi.org/10.1534/genetics.111.136911 (2012).
Glover, D. J., Lipps, H. J. & Jans, D. A. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 6, 299–310. https://doi.org/10.1038/nrg1577 (2005).
Eszterhas, S. K., Bouhassira, E. E., Martin, D. I. & Fiering, S. Transcriptional interference by independently regulated genes occurs in any relative arrangement of the genes and is influenced by chromosomal integration position. Mol. Cell Biol. 22, 469–479. https://doi.org/10.1128/mcb.22.2.469-479.2002 (2002).
Chiaromonte, F., Miller, W. & Bouhassira, E. E. Gene length and proximity to neighbors affect genome-wide expression levels. Genome Res. 13, 2602–2608. https://doi.org/10.1101/gr.1169203 (2003).
Akhtar, W. et al. Chromatin position effects assayed by thousands of reporters integrated in parallel. Cell 154, 914–927. https://doi.org/10.1016/j.cell.2013.07.018 (2013).
Tchasovnikarova, I. A. et al. GENE SILENCING. Epigenetic silencing by the HUSH complex mediates position-effect variegation in human cells. Science 348, 1481–1485. https://doi.org/10.1126/science.aaa7227 (2015).
Karpen, G. H. Position-effect variegation and the new biology of heterochromatin. Curr. Opin. Genet. Dev. 4, 281–291. https://doi.org/10.1016/s0959-437x(05)80055-3 (1994).
Zuniga, R. A. et al. Development of a new promoter to avoid the silencing of genes in the production of recombinant antibodies in chinese hamster ovary cells. J. Biol. Eng. 13, 59. https://doi.org/10.1186/s13036-019-0187-y (2019).
Radhakrishnan, P., Basma, H., Klinkebiel, D., Christman, J. & Cheng, P. W. Cell type-specific activation of the cytomegalovirus promoter by dimethylsulfoxide and 5-aza-2’-deoxycytidine. Int. J. Biochem. Cell Biol. 40, 1944–1955. https://doi.org/10.1016/j.biocel.2008.02.014 (2008).
Choi, K. H., Basma, H., Singh, J. & Cheng, P. W. Activation of CMV promoter-controlled glycosyltransferase and beta-galactosidase glycogenes by butyrate, tricostatin A, and 5-aza-2’-deoxycytidine. Glycoconj. J. 22, 63–69. https://doi.org/10.1007/s10719-005-0326-1 (2005).
Knust, B., Bruggemann, U. & Doerfler, W. Reactivation of a methylation-silenced gene in adenovirus-transformed cells by 5-azacytidine or by E1A trans activation. J. Virol. 63, 3519–3524. https://doi.org/10.1128/JVI.63.8.3519-3524.1989 (1989).
Krishnan, M. et al. Effects of epigenetic modulation on reporter gene expression: implications for stem cell imaging. FASEB J. 20, 106–108. https://doi.org/10.1096/fj.05-4551fje (2006).
Palmer, T. D., Rosman, G. J., Osborne, W. R. & Miller, A. D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc. Natl. Acad. Sci. USA 88, 1330–1334. https://doi.org/10.1073/pnas.88.4.1330 (1991).
Verma, I. M. & Somia, N. Gene therapy—promises, problems and prospects. Nature 389, 239–242. https://doi.org/10.1038/38410 (1997).
Rieblinger, B. et al. Strong xenoprotective function by single-copy transgenes placed sequentially at a permissive locus. Xenotransplantation 25, e12382. https://doi.org/10.1111/xen.12382 (2018).
Kong, Q. et al. Rosa26 locus supports tissue-specific promoter driving transgene expression specifically in pig. PLoS ONE 9, e107945. https://doi.org/10.1371/journal.pone.0107945 (2014).
Choi, K. et al. Production of heterozygous alpha 1,3-galactosyltransferase (GGTA1) knock-out transgenic miniature pigs expressing human CD39. Transgenic Res. 26, 209–224. https://doi.org/10.1007/s11248-016-9996-7 (2017).
Larson, J. D. & Baker, S. J. Engineering inducible knock-in mice to model oncogenic brain tumor mutations from endogenous loci. Methods Mol. Biol. 207–230, 2019. https://doi.org/10.1007/978-1-4939-8805-1_18 (1869).
Ijaz, F. & Ikegami, K. Knock-in of labeled proteins into 5’UTR enables highly efficient generation of stable cell lines. Cell Struct. Funct. 46, 21–35. https://doi.org/10.1247/csf.21002 (2021).
Yang, L. et al. Porcine germline genome engineering. Proc. Natl. Acad. Sci. USA 118, 25. https://doi.org/10.1073/pnas.2004836117 (2021).
Park, S. J. et al. Production and characterization of soluble human TNFRI-Fc and human HO-1(HMOX1) transgenic pigs by using the F2A peptide. Transgenic Res. 23, 407–419. https://doi.org/10.1007/s11248-013-9780-x (2014).
Porrett, P. M. et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surg. https://doi.org/10.1111/ajt.16930 (2022).
Huai, G., Qi, P., Yang, H. & Wang, Y. Characteristics of alpha-Gal epitope, anti-Gal antibody, alpha1,3 galactosyltransferase and its clinical exploitation (Review). Int. J. Mol. Med. 37, 11–20. https://doi.org/10.3892/ijmm.2015.2397 (2016).
Thorlacius-Ussing, L., Ludvigsen, M., Kirkeby, S., Vorum, H. & Honore, B. Proteomic analysis of tissue from alpha1,3-galactosyltransferase knockout mice reveals that a wide variety of proteins and protein fragments change expression level. PLoS ONE 8, e80600. https://doi.org/10.1371/journal.pone.0080600 (2013).
Shao, Y. et al. The expression and distribution of alpha-Gal gene in various species ocular surface tissue. Int. J. Ophthalmol. 5, 543–548. https://doi.org/10.3980/j.issn.2222-3959.2012.05.01 (2012).
Lu, Y. et al. A standardized quantitative method for detecting remnant alpha-Gal antigen in animal tissues or animal tissue-derived biomaterials and its application. Sci. Rep. 8, 15424. https://doi.org/10.1038/s41598-018-32959-1 (2018).
McKenzie, I. F. et al. Distribution of the major xenoantigen (gal (alpha 1–3)gal) for pig to human xenografts. Transpl. Immunol. 2, 81–86. https://doi.org/10.1016/0966-3274(94)90032-9 (1994).
Capecchi, M. R. Generating mice with targeted mutations. Nat. Med. 7, 1086–1090. https://doi.org/10.1038/nm1001-1086 (2001).
Smithies, O. Forty years with homologous recombination. Nat. Med. 7, 1083–1086. https://doi.org/10.1038/nm1001-1083 (2001).
Kwon, D. N. et al. Production of biallelic CMP-Neu5Ac hydroxylase knock-out pigs. Sci. Rep. 3, 1981. https://doi.org/10.1038/srep01981 (2013).
Cui, C. et al. Gene targeting by TALEN-induced homologous recombination in goats directs production of beta-lactoglobulin-free, high-human lactoferrin milk. Sci. Rep. 5, 10482. https://doi.org/10.1038/srep10482 (2015).
Yao, X. et al. Tild-CRISPR allows for efficient and precise gene knockin in mouse and human cells. Dev. Cell 45, 526–536 e525. https://doi.org/10.1016/j.devcel.2018.04.021 (2018).
Zhang, J. P. et al. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol. 18, 35. https://doi.org/10.1186/s13059-017-1164-8 (2017).
Rood, P. P. et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl. Int. 20, 1050–1058. https://doi.org/10.1111/j.1432-2277.2007.00546.x (2007).
Dons, E. M. et al. T-cell-based immunosuppressive therapy inhibits the development of natural antibodies in infant baboons. Transplantation 93, 769–776. https://doi.org/10.1097/TP.0b013e3182481168 (2012).
Neethling, F., Cooper, D. K., Xu, H. & Michler, R. E. Newborn baboon serum anti-alpha galactosyl antibody levels and cytotoxicity to cultured pig kidney (PK15) cells. Transplantation 60, 520–521 (1995).
Galili, U., Mandrell, R. E., Hamadeh, R. M., Shohet, S. B. & Griffiss, J. M. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 56, 1730–1737 (1988).
Burlak, C. et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surg. 14, 1895–1900. https://doi.org/10.1111/ajt.12744 (2014).
Estrada, J. L. et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 22, 194–202. https://doi.org/10.1111/xen.12161 (2015).
Iwase, H. et al. Evidence suggesting that deletion of expression of N-glycolylneuraminic acid (Neu5Gc) in the organ-source pig is associated with increased antibody-mediated rejection of kidney transplants in baboons. Xenotransplantation, e12700. https://doi.org/10.1111/xen.12700 (2021).
Yamamoto, T. et al. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci. Rep. 10, 9771. https://doi.org/10.1038/s41598-020-66311-3 (2020).
Alwayn, I. P. et al. Inhibition of platelet aggregation in baboons: therapeutic implications for xenotransplantation. Xenotransplantation 7, 247–257 (2000).
Alwayn, I. P. et al. Mechanisms of thrombotic microangiopathy following xenogeneic hematopoietic progenitor cell transplantation. Transplantation 71, 1601–1609 (2001).
Choi, K., Shim, J., Ko, N. & Park, J. No excessive mutations in transcription activator-like effector nuclease-mediated alpha-1,3-galactosyltransferase knockout Yucatan miniature pigs. Asian Australas. J. Anim. Sci. 33, 360–372. https://doi.org/10.5713/ajas.19.0480 (2020).
Shim, J. et al. Human immune reactivity of GGTA1/CMAH/A3GALT2 triple knockout Yucatan miniature pigs. Transgenic Res. 30, 619–634. https://doi.org/10.1007/s11248-021-00271-w (2021).
Acknowledgements
We thank to Dr. Jeong-Woong Lee from KRIBB who provided the CRISPR-Cas9 plasmid vectors for this study. We would also like to thank Youn-Sang Kweon, SeongHoon Kim, Jae hoon Choi, and Jae Bin Lee for their supporting about expertly animal care.
Funding
This study was supported by a grant of the Korea health Technology R&D project through the Korea Health Industry Development Institute (KHDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI20C0056020021).
Author information
Authors and Affiliations
Contributions
N.K. wrote the main manuscript text and prepared all figures and tables. N.K., J.-S., H.-J.L., Y.L., J.-K.P., K.K., and K.C. contributed to produce the transgenic pig. J.-W. Lee provided the CRISPR-Cas9 plasmid vector for this research. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ko, N., Shim, J., Kim, HJ. et al. A desirable transgenic strategy using GGTA1 endogenous promoter-mediated knock-in for xenotransplantation model. Sci Rep 12, 9611 (2022). https://doi.org/10.1038/s41598-022-13536-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13536-z
This article is cited by
-
Recent advances in therapeutic CRISPR-Cas9 genome editing: mechanisms and applications
Molecular Biomedicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.