Abstract
Because of its extremely rare incidence, the safety and efficacy of bronchial artery embolization (BAE) for the treatment of hemoptysis caused by pulmonary metastasis from HCC are not well known. We therefore evaluated the safety and efficacy of BAE in these patients. Data from 18 patients with hepatocellular carcinoma (HCC) and pulmonary metastasis who received BAE for the treatment of hemoptysis between 2003 and 2021 were retrospectively reviewed. Technical and clinical success were achieved in 100% and 94% of patients, respectively. Of the 18 embolization procedures, six were performed using polyvinyl alcohol (PVA) particles only, five were performed using gelfoam only, three were performed using gelfoam plus microcoils, one was performed using PVA plus microcoils, one was performed using embospheres, one was performed using lipiodol plus PVA and gelfoam, and one was performed using hystoacryl with microballoon protection. In eight patients for whom CT just before BAE and at follow-up were available, the mean size of the largest metastatic tumor decreased from 5.1 to 3.7 cm (P = 0.035). Hemoptysis recurred in three patients (17%) during follow-up. The median overall and hemoptysis-free survival periods were 149 days and 132 days, respectively. BAE is an effective and safe option for the treatment of hemoptysis in patients with pulmonary metastasis from HCC, with a favorable clinical success rate and a low rate of hemoptysis recurrence. In addition, we also observed BAE to have a positive antitumor effect on pulmonary metastases from HCC, but this requires confirmation in a future study.
Similar content being viewed by others
Introduction
With the introduction of regular follow-up examinations for high risk groups, the detection rate of early-stage hepatocellular carcinoma (HCC) has increased, resulting in an increase in the number of patients eligible for curative treatment and increased long-term survival1. However, 10–20% of patients receiving curative treatment suffer from extra-hepatic metastases 5–10 years after curative surgery2. Furthermore, in those patients whose HCC cannot be cured with radical treatment and who undergo palliative treatment instead, the rate of extra-hepatic metastasis is even higher3. Moreover, some patients are diagnosed as having super-induced extra-hepatic metastases, even during their first visit for palliative care2,4.
Currently, the lungs are considered the most favored organs for metastatic colonization by HCC, accounting for 51% of all extrahepatic metastasis5,6. The results of a large-scale population study in patients diagnosed with HCC lung metastasis showed 1-year overall survival and cancer-specific survival rates of 10% and 12.6%, respectively7,8.
Hemoptysis is symptomatic of a variety of diseases. Malignant neoplasms such as primary bronchogenic carcinoma, endobronchial metastatic carcinoma (most commonly from melanoma or breast, colon, or renal cell carcinoma), and bronchial carcinoid can all cause hemoptysis. Because of its rarity, the incidence of hemoptysis in pulmonary metastasis of HCC is not well known, with only a few cases being reported in the literature9,10. However, peribronchial pulmonary metastasis from HCC can cause life-threatening hemoptysis that is related to poor prognosis when treated conservatively, with a mortality rate exceeding 50%11.
Bronchial artery embolization (BAE) is considered a safe and effective option for hemoptysis, regardless of its severity12,13,14,15,16,17. BAE was shown to effectively stop or decrease hemoptysis caused by benign etiology18,19,20,21. In addition, BAE is also effective for the management of hemoptysis caused by malignancy22,23,24,25,26,27. Because of its extremely rare incidence, the safety and efficacy of BAE for treating hemoptysis caused by metastatic HCC are not well known. To the best of our knowledge, there has been only one case report describing the use of BAE to successfully manage hemoptysis caused by metastatic HCC10.
Thus, the aim of this study was to report our experience of BAE in the treatment of hemoptysis caused by metastatic HCC. This study covers the use of the procedure in 18 patients treated in a single large-volume liver cancer center.
Materials and methods
Patients
Our institutional review board (institutional review board of Asan Medical Center) approved the study protocol (IRB No. 2021–1558) and waived written informed consent. All methods were performed in accordance with relevant guidelines and regulations.
Between July 2003 and May 2021, 49,050 consecutive patients were diagnosed with HCC at our institution. Among these 49,050 patients, 1346 were identified as having pulmonary metastasis. Among these 1346 patients, 19 were identified as having hemoptysis. Among these 19 patients, one patient developed hemoptysis from nontuberculous mycobacterium infection. Finally, 18 patients (1.3%, 18/1346; all male; mean age, 60.7 ± 9.6 years) who underwent BAE for hemoptysis caused by pulmonary metastasis were included in this retrospective study. In all 18 study patients, the locations of hemoptysis were correlated with HCC lung metastases.
Table 1 shows the patient characteristics. All patients were male and had advanced-stage HCC (Barcelona Clinic Liver Cancer [BCLC] stage C) before undergoing BAE. All patients had previously undergone either curative intent (surgical resection or radiofrequency ablation) or palliative (chemoembolization, sorafenib, or radiotherapy) antitumor treatment. The amount of hemoptysis before initial BAE was considered to be trivial (blood-tinged sputum) in two (11%) patients, moderate (< 300 mL per 24 h) in 10 (56%) patients, and massive (> 300 mL per 24 h) in six (33%) patients.
To locate the source of hemoptysis and identify the underlying etiology, chest radiography and chest CT were carried out on all patients except for one who only underwent serial chest radiographs. Four patients received bronchoscopy prior to BAE: three patients received diagnostic bronchoscopy only, whereas the other patient underwent saline lavage and epinephrine treatment in addition to localization of the bleeding site.
Prior to BAE, patients received supportive measures, such as monitoring of cardiorespiratory parameters, correction of hypoxia, stabilization of blood pressure, and transfusion with blood products when necessary. Some patients with massive hemoptysis were managed in an intensive care unit before and after the BAE procedure. But there was no patient who underwent endotracheal intubation.
Indications for embolization included moderate to massive hemoptysis, or small volume hemoptysis refractory to conservative management and/or flexible bronchoscopy. Decision to pursue BAE was made by the primary clinical service (pulmonology or thoracic surgery) in a coordinated interdisciplinary fashion of interventional radiology teams.
BAE procedure
Femoral artery access was used to perform BAE in all cases. For visualization of the bronchial artery and identification of other nonbronchial systemic arteries, a diagnostic angiography of the aortic arch and descending thoracic aorta was obtained with use of a 5-F multi-side-hole catheter (Pigtail; Cook). Shaped 5-F angiographic catheters (Cobra, Simmons, Headhunter; Cook) was used to obtain selective bronchial angiographies.
A 1.9–2.4-F microcatheter (Progreat, Progreat Lambda, Terumo; ASAHI Tellus, Asahi Intecc; Renegade, Boston Scientific) was coaxially introduced through the 5-F angiographic catheter into the bronchial artery as distally as possible. The choice of embolic material was made at the discretion of the physician’s personal preferences who performed BAE. The embolic materials used for BAE were 355–500 µm polyvinyl alcohol particles (PVA) (Contour, Boston Scientific; Bearing, Merit Medical), 100–300 µm microspheres (Embosphere, Merit Medical), 1 mm-diameter gelfoam slurry (Spongostan, Ferrosan Medical Devices), iodized oil (Lipiodol, Guerbet), NBCA (n-Butyl cyanoacrylate; Histoacryl; BRAUN), and microcoils (Tornado, extended length: 14 cm, diameter: 3 mm, Cook).
Definitions and data analysis
Outcomes were defined according to the Society of Interventional Radiology Standards of Practice Committee28. Technical success was defined as the ability to selectively catheterize and embolize the abnormal bronchial or nonbronchial systemic arteries. Clinical success was defined as complete (cessation of hemoptysis) or partial success (a marked decrease in hemoptysis with a positive effect on the clinical course of the patient) within 48 h. Clinical failure was defined as persistent or recurrent hemoptysis during the admission period. In-hospital mortality was defined as death occurring during the same hospital period as the first BAE. The early recurrence was defined as a recurrence occurred within the first month, and late recurrence was defined as a recurrence occurred 1 month after BAE in the management of hemoptysis29. Six month mortality was defined as death occurring during the 6 months following the first BAE. The hemoptysis-free survival period was defined as the time elapsed from the initial BAE to the recurrence of hemoptysis or death from any cause. The overall survival period was defined as the interval between the initial BAE and death from any cause. Major complications were defined as sequelae that were permanent or a cause of death, requiring treatment or prolonged hospitalization. Minor complications were complications that did not require treatment and were self-limited.
When the antitumor effect of BAE was evaluated in those patients who had both chest CT just before BAE and follow-up chest CT (at least 1 month after BAE), the Response Evaluation Criteria in Solid Tumors (version 1.1) was used30. In addition, the size of the largest metastatic tumor was compared before and after BAE in these patients.
Statistical analysis
Survival curves for survival and freedom from recurrence of hemoptysis were constructed according to the Kaplan–Meier method. The Wilcoxon signed rank test was used to evaluate the change in size of the largest metastatic lung tumor. All statistical analyses were performed using SPSS for Windows version 21.0 (SPSS, Inc.). Two-sided p values < 0.05 were considered to be statistically significant.
Results
Technical and clinical outcomes
Details of the BAE procedures and clinical outcomes are shown in Table 2. BAE was technically successful in all 18 patients (100%). Bronchial arteries only were embolized in 16 patients (89%), whereas both bronchial and nonbronchial systemic arteries were embolized in two patients (11%). Of the 18 embolization procedures, six were performed using PVA only, five were performed using gelfoam only, three were performed using gelfoam plus microcoils, one was performed using PVA plus microcoils, one was performed using embospheres, one was performed using lipiodol plus PVA and gelfoam, and one was performed using histoacryl with microballoon protection. Table 3 shows the summary of angiographic findings in all patients and examples are presented in Figs. 1 and 2.
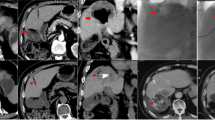
A 56-year-old man with pulmonary metastasis from HCC who presented with a moderate amount of hemoptysis. (A) Selective common bronchial trunk arteriography using a 5-F catheter shows multiple mediastinal tumor blushes and an extensive bronchopulmonary shunt (black arrowhead) in the right middle lobe. (B) Selective left upper bronchial arteriography using a 2.2-F microcatheter shows a bronchopulmonary shunt (black arrow head) and a metastatic lesion responsible for the tumor blush (black arrow). (C) Axial CT image before embolization shows the bronchial artery (black arrow) and pulmonary artery (white arrow) encased by an interlobar metastatic lymph node (black asterisk) between the right middle lobe and right lower lobe bronchus. This finding corresponds with the angiographic finding of a bronchopulmonary shunt.
An 80-year-old man with pulmonary metastasis from HCC who presented with a trivial amount of hemoptysis and a large amount of right hemothorax. (A) Coronal reconstructed CT image before embolization shows a large enhancing mass (black asterisk) with suspicious contrast media extravasation (black arrow) in the right lower lobe and a large amount of right hemothorax. (B)Selective right bronchointercostal trunk arteriography using a 5-F catheter shows a large hypervascular tumor blush fed by the right bronchial artery in the right lower lobe. (C–D) Embolization of the right bronchial artery was successfully performed using lipiodol, gelfoam, and PVA particles, and this patient showed complete resolution of hemoptysis after bronchial artery embolization. Note the extravasation (black arrow) of lipiodol into the pleural cavity and a vascular lake phenomenon (arrowheads) resulting from intratumoral bleeding.
Clinical success was achieved in 17 patients (94%): complete clinical success in 14 patients (78%) patients and partial clinical success in three (16%) patients. One patient showed recurrent hemoptysis 2 days after BAE and within the same admission period. A second BAE was performed, with the recanalized right bronchial artery being embolized with PVA. Partial clinical success was achieved after the second BAE, although the patient died of disease progression 2 months later.
Of the 18 study patients, evaluation of the antitumor effect of BAE was possible in eight. These eight patients did not receive any antitumor treatment during the period between the pre-BAE CT and the initial follow-up CT. Response of treated metastases one to four months (median, 2 months) after BAE according to the Response Evaluation Criteria in Solid Tumors was partial response in three patients (38%, 3/8), stable disease in four (50%, 4/8), and progressive disease in one patient (12%, 1/8). The mean size of the largest metastatic tumor had decreased from 5.1 cm to 3.7 cm (P = 0.035, Wilcoxon signed rank test; Figs. 3, 4, 5, 6 and 7).
An 81-year-old man with pulmonary metastasis from HCC who presented with a moderate amount of hemoptysis. (A) Axial CT image before embolization shows obstructive pneumonitis (asterisk) in the left upper lobe lingular division caused by a metastatic mass (white arrows). (B) Selective left bronchial arteriography shows a tortuous hypertrophied left bronchial artery with a hypervascular tumor blush (black arrows) in the proximal portion of the lingular division. Embolization of the left bronchial artery was successfully performed using gelfoam, and this patient showed complete resolution of hemoptysis following bronchial artery embolization. (C) Axial CT image 4 months after embolization of the bronchial artery shows the metastatic mass in the lingular division to be markedly decreased in size, as well as an improvement in obstructive pneumonitis.
A 72-year-old man with pulmonary metastasis from HCC who presented with a moderate amount of hemoptysis. (A) Axial chest CT image before embolization shows an intraluminal soft-tissue mass in the proximal right lower lobe lobar bronchus (white arrow). (B) Selective right bronchointercostal trunk arteriography using a 5-F catheter shows multiple hypervascular tumor blushes along the bronchial artery. Note that a hypervascular tumor blush (black arrows) in the proximal right lower lobe lobar bronchus corresponds with endobronchial soft tissue mass in the axial CT image. Embolization of the right bronchial artery was successfully performed using gelfoam and a microcoil, and this patient showed complete resolution of hemoptysis following bronchial artery embolization. (C) Axial CT image 4 months after embolization of the bronchial artery shows a marked decrease in the size of the metastatic mass in the right lower lobe lobar bronchus.
A 63-year-old man with a pulmonary metastasis from HCC who presented with a massive amount of hemoptysis. (A) Selective left bronchial arteriography using a 5-F catheter shows a tortuous hypertrophic left bronchial artery and a large hypervascular tumor blush (white arrow). Embolization of the left bronchial artery was successfully performed using gelfoam, and this patient showed complete resolution of hemoptysis after bronchial artery embolization. (B) Axial CT image before embolization shows a huge metastatic mass (white arrow) in the left upper lobe. (C) Axial CT image 3 months after embolization of the bronchial artery shows a marked decrease in the size of the metastatic mass (white arrow) in the left upper lobe.
A 57-year-old man with pulmonary metastasis from HCC who presented with a moderate amount of hemoptysis. (A–C) Selective right bronchial arteriography using a 5-F catheter shows a tortuous hypertrophic right bronchial artery and multiple nodular hypervascular tumor blushes. Embolization of the right bronchial artery was successfully performed using hystoacryl (NBCA: lipiodol = 1:10) and the patient showed complete resolution of hemoptysis after bronchial artery embolization. (D) Axial CT image before embolization shows multiple nodular pulmonary metastases in both lungs, particularly prominent in the right lower lobe, with patchy ground glass opacities suggesting aspirated blood. (E) Axial CT image 1 month after embolization of the right bronchial artery shows the metastatic nodules in the right lung to be decreased in size and number, but an aggravated pulmonary metastasis that was not in the embolized territory is visible in the left lung.
A 61-year-old man with pulmonary metastasis from HCC who presented with a moderate amount of hemoptysis. (A) Contrast-enhanced axial CT image before embolization shows a well-enhancing mass (largest metastatic tumor, 5 cm in diameter, white arrow) in the left lower lobe of the lung. (B) Selective left bronchial arteriography shows a tortuous hypertrophic left bronchial artery and large hypervascular tumor blush (white arrowheads) corresponding to the largest metastatic tumor on CT. Embolization of the left bronchial artery was successfully performed using PVA, and this patient showed complete resolution of hemoptysis after bronchial artery embolization. (C) Contrast-enhanced axial CT image 1 month after embolization of the bronchial artery shows that the largest metastatic mass has decreased in size (white arrow, decreased from 5 to 3 cm in diameter) and seems to be devascularized after bronchial artery embolization.
Of the 17 patients with initial clinical success, two showed recurrent hemoptysis, 132 and 134 days after initial BAE. In one of these patients who showed a moderate amount of recurrent hemoptysis 134 days after first BAE, follow-up chest CT images and angiography showed progression of pulmonary metastasis and that both bronchial arteries were tortuous and enlarged. The right and left bronchial arteries were successfully embolized using PVA, gelfoam, and hystoacryl, and partial clinical success was achieved. The other patient experienced a massive amount of hemoptysis 132 days after the first BAE. Follow-up chest CT images suggested aggravated endobronchial metastatic tumor, while angiography showed recanalization of the previously embolized left bronchial artery. Complete clinical success was achieved after embolization of the left bronchial artery with PVA.
The overall recurrence rate (one patient with initial clinical failure in the in-hospital period plus two patients with recurrence after initial clinical success) during follow-up was therefore 17%. There was no procedure-related complications.
Overall survival and hemoptysis-free survival
The in-hospital mortality rate was 11% (2 of 18 patients). In-hospital death was attributed to respiratory failure from progression of lung metastasis in one patient and hepatic failure from progression of primary HCC in the other patient. The 6 month mortality rate was 50% (9 of 18 patients).
Of the 18 patients, one was still alive at the time of writing and 17 had died from causes other than hemoptysis. There were 16 deaths attributed to progression of HCC and one death attributed to sepsis. After BAE, the median overall survival was 149 days (95% confidence interval, 0.7–297.3 days) and the median hemoptysis-free survival was 132 days (95% confidence interval, 97.3–166.7 days; Fig. 8).
Discussion
In the present study, the incidence of hemoptysis requiring BAE was rare, with it occurring in 1.3% of those patients who had metastatic HCC in the lung. We found that BAE was safe and effective at stopping hemoptysis in patients with pulmonary metastasis from HCC. After BAE, technical and clinical success were achieved in 100% and 94% of study patients, respectively. There was no procedure-related complication. In the majority of patients, embolization of the bronchial artery was sufficient to treat HCC-related hemoptysis, and only two patients (11%) required additional embolization of a non-bronchial systemic artery.
During follow-up, three patients (17%; early in one, late in two) in the current study showed recurrent hemoptysis. In the present study, the median overall and hemoptysis-free survival periods after BAE were 149 days and 132 days, respectively. However, considering that all three patients with recurrent hemoptysis were successfully treated with a second BAE, the period with hemoptysis formed a very small portion of the patients’ lives following BAE. There was no hemoptysis-related death in the present study.
An interesting finding in the present study was the positive antitumor effect of BAE in patients with pulmonary metastasis from HCC. The majority of patients showed hypervascular pulmonary tumor staining on angiography, and during the short-term follow-up period after BAE, the mean size of the largest metastatic tumor significantly decreased. In particular, in some patients with endobronchial HCC metastasis, post-obstructive pneumonia improved because of a decrease in the size of the endobronchial tumor. As in the liver, the lung has a dual blood supply, being fed by pulmonary and bronchial arteries. The precise percentages of the blood supplied to metastatic HCC from the bronchial and pulmonary arteries remains unclear, although the majority of the blood supply to pulmonary metastases seems to be from the bronchial artery31,32,33. Chemoembolization or bland embolization is typically indicated for intermediate-stage HCC in the liver34,35,36,37; however, a recent study showed promising outcomes for chemoembolization in the palliative treatment of pulmonary metastatic tumors from HCC, colorectal cancer, or melanoma31,33. As well as chemoembolization, our study may indicate the potential of bland embolization of the bronchial artery in the palliative treatment of metastatic HCC in the lung. However, the role of embolotherapy for pulmonary metastatic HCC still remains poorly defined, and future studies are required to determine the specific patient groups that may benefit from chemoembolization or bland embolization of the bronchial artery in the treatment of pulmonary metastatic HCC.
This study has several limitations. First, it is a retrospective analysis from a single center. Second, the sample size is relatively small and the study period is rather long (> 18 years) because of the low incidence of hemoptysis in patients with pulmonary HCC metastases. Indeed, to the best of our knowledge, no sizable study (except for one case report) has reported the clinical outcomes of BAE in this specific patient group.
In summary, we found that BAE was an effective and safe option for the management of hemoptysis associated with pulmonary metastasis from HCC. In addition, we also observed a positive antitumor effect of BAE on pulmonary HCC metastasis, but this requires confirmation in a future study.
References
Maluccio, M. & Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 62, 394–399 (2012).
Kanda, M. et al. Extrahepatic metastasis of hepatocellular carcinoma: Incidence and risk factors. Liver Int. 28, 1256–1263 (2008).
Lin, S. C., Shih, S. C., Kao, C. R. & Chou, S. Y. Transcatheter arterial embolization treatment in patients with hepatocellular carcinoma and risk of pulmonary metastasis. World J. Gastroenterol. 9, 1208–1211 (2003).
Katyal, S. et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology 216, 698–703 (2000).
Abbas, A. et al. Epidemiology of metastatic hepatocellular carcinoma, a nationwide perspective. Dig. Dis. Sci. 59, 2813–2820 (2014).
Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet 391, 1301–1314 (2018).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018).
El-Serag, H. B. Hepatocellular carcinoma. N. Engl. J. Med. 365, 1118–1127 (2011).
Onofrei, C., Patel, D. & Noor, A. An unexpected source of hemoptysis. Chest 150, 682a–682a (2016).
Kawaguchi, T. et al. Successful treatment for bronchial bleeding from invasive pulmonary metastasis of hepatocellular carcinoma: A case report. Hepatogastroenterology 48, 851–853 (2001).
Fartoukh, M. et al. Early prediction of in-hospital mortality of patients with hemoptysis: An approach to defining severe hemoptysis. Respiration 83, 106–114 (2012).
Hayakawa, K. et al. Bronchial artery embolization for hemoptysis: Immediate and long-term results. Cardiovasc. Intervent. Radiol. 15, 154–158 (1992) (discussion 158–159).
Knott-Craig, C. J. et al. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J. Thorac. Cardiovasc. Surg. 105, 394–397 (1993).
Fernando, H. C., Stein, M., Benfield, J. R. & Link, D. P. Role of bronchial artery embolization in the management of hemoptysis. Arch. Surg. 133, 862–866 (1998).
Mal, H. et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 115, 996–1001 (1999).
Panda, A., Bhalla, A. S. & Goyal, A. Bronchial artery embolization in hemoptysis: A systematic review. Diagn. Interv. Radiol. 23, 307–317 (2017).
Xu, S., Guan, L. J., Shi, B. Q., Tan, Y. S. & Zhang, X. J. Recurrent hemoptysis after bronchial artery embolization: Prediction using a nomogram and artificial neural network model. AJR Am. J. Roentgenol. 215, 1490–1498 (2020).
Shin, B. S., Jeon, G. S., Lee, S. A. & Park, M. H. Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 15, 1093–1098 (2011).
Martin, L. N., Higgins, L., Mohabir, P., Sze, D. Y. & Hofmann, L. V. Bronchial artery embolization for hemoptysis in cystic fibrosis patients: A 17-year review. J. Vasc. Interv. Radiol. 31, 331–335 (2020).
Shin, B. et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis in patients with chronic pulmonary Aspergillosis. PLoS ONE 11, e0168373 (2016).
Takeda, K. et al. Long-term outcomes of bronchial artery embolization for patients with non-mycobacterial non-fungal infection bronchiectasis. Respiration 99, 961–969 (2020).
Mehta, A. S. et al. Bronchial artery embolization for malignant hemoptysis: A single institutional experience. J. Thorac. Dis. 7, 1406–1413 (2015).
Han, K., Yoon, K. W., Kim, J. H. & Kim, G. M. Bronchial artery embolization for hemoptysis in primary lung cancer: A retrospective review of 84 patients. J. Vasc. Interv. Radiol. 30, 428–434 (2019).
Wang, G. R., Ensor, J. E., Gupta, S., Hicks, M. E. & Tam, A. L. Bronchial artery embolization for the management of hemoptysis in oncology patients: Utility and prognostic factors. J. Vasc. Interv. Radiol. 20, 722–729 (2009).
Garcia-Olive, I. et al. Results of bronchial artery embolization for the treatment of hemoptysis caused by neoplasm. J. Vasc. Interv. Radiol. 25, 221–228 (2014).
Witt, C. et al. Value of bronchial artery embolisation with platinum coils in tumorous pulmonary bleeding. Eur. J. Cancer 36, 1949–1954 (2000).
Park, H. S. et al. Bronchial artery and systemic artery embolization in the management of primary lung cancer patients with hemoptysis. Cardiovasc. Interv. Radiol. 30, 638–643 (2007).
Angle, J. F. et al. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 21, 1479–1486 (2010).
Chun, J. Y. et al. Radiological management of hemoptysis: A comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc. Interv. Radiol. 33, 240–250 (2010).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Boas, F. E. et al. Bronchial or pulmonary artery chemoembolization for unresectable and unablatable lung metastases: A phase I clinical trial. Radiology 301, 474–484 (2021).
Georgiades, C. Chemoembolization for lung neoplasms: Exuberant expectations versus meticulous investigation. Radiology 301, 485–486 (2021).
Hori, A. et al. Transarterial chemoembolization for pulmonary or mediastinal metastases from hepatocellular carcinoma. Br. J. Radiol. 93, 20190407 (2020).
Park, C. et al. Clinical significance of the initial and best responses after chemoembolization in the treatment of intermediate-stage hepatocellular carcinoma with preserved liver function. J. Vasc. Interv. Radiol. 31, 1998e1991-2006e1991 (2020).
Brown, K. T. et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J. Clin. Oncol. 34, 2046–2053 (2016).
Roth, G. S. et al. Comparison of trans-arterial chemoembolization and bland embolization for the treatment of hepatocellular carcinoma: A propensity score analysis. Cancers (Basel) 13, 812 (2021).
Kishore, S. A., Bajwa, R. & Madoff, D. C. Embolotherapeutic strategies for hepatocellular carcinoma: 2020 update. Cancers (Basel) 12, 791 (2020).
Acknowledgements
English language editing assistance was provided by Patrick Hughes, MD.
Author information
Authors and Affiliations
Contributions
Study concepts and design were formulated by J.H.K. Acquisition, analysis, or interpretation of data was performed by all authors. Drafting of the manuscript was performed by S.K. Statistical analysis was performed by J.H.K. Critical revison of the manuscript for important intellectual content was performed by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S., Kim, J.H., Ko, GY. et al. Bronchial artery embolization for hemoptysis caused by metastatic hepatocellular carcinoma. Sci Rep 12, 6906 (2022). https://doi.org/10.1038/s41598-022-10972-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10972-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.