Abstract
In ischemic stroke patients undergoing endovascular treatment (EVT), we aimed to test the hypothesis that cerebral microbleeds (CMBs) are associated with clinical outcomes, while estimating the mediating effects of hemorrhagic transformation (HT), small-vessel disease burden (white matter hyperintensities, WMH), and procedural success. From a multicenter EVT registry, patients who underwent pretreatment MR imaging were analyzed. They were trichotomized according to presence of CMBs (none vs. 1–4 vs. ≥ 5). The association between CMB burden and 3-month mRS was evaluated using multivariable ordinal logistic regression, and mediation analyses were conducted to estimate percent mediation. Of 577 patients, CMBs were present in 91 (15.8%); 67 (11.6%) had 1–4 CMBs, and 24 (4.2%) had ≥ 5. Increases in CMBs were associated with hemorrhagic complications (β = 0.27 [0.06–0.047], p = 0.010) in multivariable analysis. The CMB effect on outcome was partially mediated by post-procedural HT degree (percent mediation, 14% [0–42]), WMH (23% [7–57]) and lower rates of successful reperfusion (6% [0–25]). In conclusion, the influence of CMBs on clinical outcomes is mediated by small-vessel disease burden, post-procedural HT, and lower reperfusion rates, listed in order of percent mediation size.
Similar content being viewed by others
Introduction
Cerebral microbleeds (CMBs) are characterized by magnetic resonance imaging (MRI) findings of small foci of chronic blood products in the brain tissue1. CMBs are associated with an increased future risk of hemorrhagic and ischemic stroke2. They also influence acute stroke treatment, as their presence is associated with an increased risk of intracranial hemorrhage with anticoagulation/antiplatelet therapy3 or thrombolytic therapy4. Interestingly, increases in CMBs seems to be associated with a greater risk of hemorrhage, with reported increased risks with ≥ 5 CMBs5 or ≥ 10 CMBs4.
CMBs are also known to contribute to neurological dysfunction. The presence of CMBs has been associated with cognitive impairment and clinical disability6,7 and a significant small-vessel disease burden in the involved patients. There is a possibility that CMBs may influence endovascular treatment (EVT) outcomes by hindering a functional recovery in multiple domains following ischemic stroke8. Such a baseline injury to the brain may be quantitatively measured by white matter hyperintensity (WMH)9.
Mechanical thrombectomy for acute large-vessel occlusive stroke has become the standard of therapy10. It has broad efficacy over almost all subgroups, irrespective of age, sex, and stroke severity11. However, procedural hemorrhagic complications still substantially influence mechanical thrombectomy outcomes, and identification of predictive factors is needed to minimize and effectively manage such complications12. In this regard, it is of value to evaluate whether the presence of CMBs is associated with the risk of hemorrhagic complications after mechanical thrombectomy. Unfortunately, there is currently only limited literature addressing the safety of mechanical thrombectomy in patients with CMBs at presentation; moreover, meta-analyses have included only a small number of patients13.
For this study, we hypothesized that an increase in CMBs will negatively influence outcomes after mechanical thrombectomy, and that this effect will be mainly mediated through increases in hemorrhagic complications. We further sought to evaluate whether this negative influence was also mediated by small-vessel disease burden or other potential variables.
Methods
Patient enrollment and evaluations
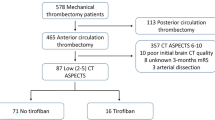
Patients were retrospectively identified from the Acute Stroke due to Intracranial Atherosclerotic occlusion and Neurointervention-Korean Retrospective (ASIAN KR) registry which included 720 consecutive patients who had undergone EVT for an emergent large-vessel occlusion between January 2011 and May 201614,15. The ASIAN KR registry included patient data from three comprehensive stroke centers. All three centers used noncontrast computed tomography (CT) and CT angiography for baseline imaging. In two centers, pretreatment MRI was routinely co-performed in nearly all patients. In a third center, pretreatment MRI was usually taken in the early phase of the study period, while multiphase CT alone was used in the later phases of the study. From this registry, patients who met the following criteria were included; (1) available 3 month functional outcomes and data regarding hemorrhagic complications; (2) symptoms onset to arterial puncture time less than 24 h; and (3) pretreatment MR imaging with both T2-weighted fluid-attenuated inversion recovery and gradient-recall echo (GRE) protocols to ensure reliable CMB and WMH grading (Fig. 1). Detailed MR protocols are shown in the supplementary methods.
The 3-month modified Rankin Scale (mRS) score, which was achieved by each center through retrospective review of prospectively collected stroke registry, was utilized as clinical outcomes. After de-identification and blinding of clinical data, core laboratory imaging analysis was performed to ensure consistent grading and eliminate bias.
Regarding preprocedural diffusion-weighted images, the infarct volume was evaluated using the NordicICE semi-automated software (NordicNeuroLab, Bergen, Norway) (J.W.C., interventional neuroradiologist, 10 years of clinical experience). Successful reperfusion was defined as a modified Treatment In Cerebral Ischemia grade 2b–316 (J.S.L., interventional neurologist, over 10 years of clinical experience, and Y.H.H., vascular neurologist, over 10 years of clinical experience). Hemorrhagic complications were evaluated by brain CT images in most cases or on GRE sequence MR images for CT-ineligible cases at around 5–7 days after onset. Hemorrhagic transformation (HT) degrees were graded according to the European Cooperative Acute Stroke Study II criteria17 (S.I.S., vascular neurologist, over 10 years of clinical experience). For statistical analysis, the grades were analyzed as ordinal; grade 0, no hemorrhage; grade 1, hemorrhagic infarct type 1 (small petechiae); grade 2, hemorrhagic infarct type 2 (more confluent petechiae); grade 3, parenchymal hematoma (PH) type 1 (≤ 30% of the infarcted area with some slight space-occupying effect); and grade 4, PH type 2 (> 30% of the infarcted area with substantial space-occupying effect).
The data collection protocol was approved by the institutional review board of each participating hospital (Ajou University Hospital IRB, Kyungpook National University Hospital IRB, and Keimyung University Dongsan Hospital IRB) and implemented under the ethical standards of the 1964 Declaration of Helsinki and its later amendments. The corresponding IRB waived the need for written informed consent due to the retrospective nature of the study.
Identification and analysis of CMBs and WMHs
CMBs were identified in pretreatment GRE images (S.J.L., interventional neurologist, over 5 years of clinical experience) as a blooming effect. Punctate, homogenous, round/ovoid, hypointense lesions < 10 mm were identified. Previous intracranial hemorrhage was not regarded as CMBs. The exclusion of mimics was performed through the analysis of other sequences and CT imaging if needed1. The number of CMBs was trichotomized to: none, 1–4, and ≥ 5 according to previous literatures18,19,20.
WMHs were evaluated in the initial MR fluid-attenuated inversion recovery images or T2-weighted images according to the CREDOS WMH visual rating scale: normal, minimal, moderate, and severe ischemia (S.J.L.)9. Chronic territorial infarcts were not regarded as WMHs. To exclude confounding effects of acute ischemic changes to the current infarct, WMH was usually measured in the unaffected hemisphere, as there is high interhemispheric severity correlation21. The analysis of CMBs and WMH was performed on separate readings to ensure blinding between each other.
Statistics
Clinical characteristics and reperfusion outcomes were compared between trichotomized CMB groups (none, 1–4 CMBs, and ≥ 5 CMBs). Univariate analysis was performed to evaluate the influence of CMBs on HT and poorer functional outcomes. For comparison of three groups, univariate analysis was performed with the χ2 test for categorical variables or analysis of variance with Bonferroni post-hoc tests for continuous variables. For comparison of two groups, univariate analysis was performed with the χ2 test for categorical variables and student’s t-test for continuous variables. The variables trichotomized CMB number, HT degree, and WMH were used as continuous variables based on trend analysis, whereas successful reperfusion and 3-month mRS was analyzed as ordinal. To identify factors associated with ordinal increases in 3-month mRS, ordinal logistic regression analysis was performed including trichotomized CMB numbers, WMH, and other clinically significant variables. To identify factors associated with increases in HT degree, multiple linear regression was performed including trichotomized CMB numbers, CMB distributions, WMH, and other clinically significant variables.
To assess HT and WMH as potential mediators of the relationship between CMBs and poorer functional outcomes, mediation analysis was performed with the degree of HT and WMH as mediating variable, respectively. Successful reperfusion was also incorporated in the mediation analysis after univariate analyses. Mediation analysis was performed using the template described by Baron and Kenny22. To assess mediation, the independent variable (X) is first shown to be significantly associated with the dependent variable (Y) (Fig. 2, pathway c). Second, X must be significantly associated with the mediator (M) (Fig. 2, pathway a). Third, M is significantly associated with Y, while controlling for X (Fig. 2, pathway c’). If all the above associations are confirmed, mediation (indirect effect) can be established by estimation of the direct causal relationship.
Model of the hypothetical causal pathway for the influence of CMBs on functional outcomes. (A) Post-procedural hemorrhagic complications measured by hemorrhagic transformation degree as a mediator. (B) Small-vessel disease burden (measured by white matter hyperintensities using the CREDOS criteria) as a mediator. (C) Successful reperfusion as a mediator.
The pathway between CMBs and mediator was tested using univariable and multivariable linear regression analyses. All other pathways were tested using univariable and multivariable ordinal regression analyses and reported as unadjusted and adjusted common odds ratio. Multivariable modeling included clinically relevant variables. The causal relationship's proportion between CMB burden and poor functional outcome attributable to the mediator was measured by dividing the log odds ratio of the indirect effect of CMB in pathway A-B by the log odds ratio of the direct effect of CMB in pathway C23,24. The confidence intervals for the proportion of the mediated effect were estimated using bootstrap resampling with 1000 resamples. In this approach, the 95% confidence interval can exceed 0% and 100%; however, we manually truncated the lower bound to 0% and the upper bound to 100%25.
Data are presented as the mean ± standard deviation, number (%), or median [interquartile range] as appropriate for data type and distribution. All statistical analyses were performed with IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY) and R software, version 3.6.3. A p < 0.05, was considered to be statistically significant. The VGAM and boot package was used to perform the mediation analysis.
Results
Baseline characteristics and reperfusion outcomes
A total of 577 patients (mean age: 67 years ± 13; 322 men [55.8%]) were included in the analysis. CMB was present in 91 (15.8%) patients. Sixty-seven (11.6%) had 1–4 CMBs, whereas 24 (4.2%) had ≥ 5 CMBs. When baseline characteristics were compared between the three groups (Table 1), the mean age was higher in the CMB ≥ 5 group (66.7 ± 12.8 vs. 70.4 ± 10.6 vs. 72.0 ± 9.9, p = 0.01; No CMB vs. CMB ≥ 5, p = 0.07, post-hoc Bonferroni test), while the rates of comorbid hypertension increased with the CMB burden (288/486 [59.3%] vs. 49/67 [73.1%] vs.22/24 [91.7%], p = 0.001). Rates of prior stroke were higher in the presence of CMBs (78/486 [16.0%] vs. 19/67 [28.4%] vs. 5/24 [20.8%], p = 0.04), and WMH grades were also significantly higher with increases in the CMB burden (p < 0.001). However, no differences were found in the premorbid mRS (0.0 [0.0–0.0] vs. 0.0 [0.0–0.0] vs. 0.0 [0.0–0.0], p = 0.41).
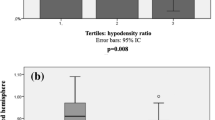
Regarding procedural characteristics and reperfusion outcomes (Table 2), significant differences were observed in the rates of reperfusion success, with lower rates of successful reperfusion in the CMB ≥ 5 group (382/486 [78.6%] vs. 54/67 [80.6%] vs. 13/24 [54.2%], p = 0.02). While the overall rate of difference in HT was not significant between groups, there was a significant intergroup difference in PH rates (59/486 [12.1%] vs. 9/67 [13.4%] vs. 8/24 [33.3%], p = 0.01), especially for the CMB ≥ 5 group. Median 3-month mRS was significantly higher in the CMB ≥ 5 group (2.0 [1.0–4.0] vs. 3.0 [2.0–4.0] vs. 5.0 [4.0–6.0], p < 0.001; No CMB and CMB 0–4 vs. CMB ≥ 5, p < 0.005, post-hoc Bonferroni test). An ordinal distribution of the 3 month mRS is shown for each CMB group in Fig. 3.
Factors associated increases in 3 month mRS and HT degree
In the multivariable analysis identifying factors associated with increases in 3 month mRS, increases in trichotomized CMB numbers (OR: 1.42 [95% CI 1.04–1.94], p = 0.028), increases in HT grades (OR: 1.48 [95% CI 1.31–1.68], p < 0.001), and increases in WMH severity (OR: 1.27 [95% CI 1.06–1.53], p = 0.01) were associated with increases in 3 month mRS with successful reperfusion, age, sex, comorbid hypertension, infarct volume, and premorbid mRS as co-variables (Table 3).
In the multivariable analysis identifying factors associated with increases in HT, increases in trichotomized CMB numbers (β = 1.00 [95% CI 0.39–1.61], p = 0.001) were associated with increases in HT, while WMH (β = − 0.1 [95% CI − 0.22 to 0.02], p = 0.108), and IV thrombolysis (β = − 0.14 [95% CI − 0.34 to 0.06], p = 0.163) was not, along with age, sex, comorbid hypertension, infarct volume, and successful reperfusion as co-variables (Table 4).
Mediation by HT concerning the association between CMBs and poorer functional outcomes
In the first step of the mediation analysis, CMBs were significantly predictive of a higher 3-month mRS with an adjusted common odds ratio (acOR) of 1.77 [95% CI 1.31–2.38]. In the second step, CMBs were significantly associated with HT degree with a beta of 0.27 [95% CI 0.06–0.47]. In the third step, the direct effect of CMBs on poorer functional outcome remained statistically significant after adjustment for HT degree with an acOR 1.63 [95% CI 1.21–2.20]. The mediator HT was an independent variable and significantly associated with poorer functional outcomes with an acOR of 1.43 [95% CI 1.26–1.62]. HT was attributed to 14% [95% CI 0–42] of the total influence of CMBs on functional outcomes (Table 5). Age, sex, comorbid hypertension, infarct volume, successful reperfusion, and IV thrombolysis were included as covariates in all multivariable analyses. In the analysis of CMB influence on HT degree, there was no collinearity effect between CMB and successful reperfusion (VIF for CMB: 1.04, VIF for successful reperfusion: 1.03).
In the eight patients with ≥ 5 CMBs and a post-procedural PH, hemorrhage occurred at the anatomical CMBs location in three cases (37.5%), whereas in five cases (63.5%), PH occurred at an anatomically irrelevant area.
Mediation by WMH concerning the association between CMBs and poorer functional outcomes
In the first step of mediation analysis, CMBs were significantly predictive of higher 3-month mRS with an acOR of 1.77 [95% CI 1.31–2.38]. In the second step, CMBs were significantly associated with WMH with a beta of 0.50 [95% CI 0.36–0.64]. In the third step, the direct effect of CMBs on poorer functional outcome remained statistically significant after adjustment for WMH with an acOR 1.55 [95% CI 1.13–2.11]. The mediator WMH was an independent variable and significantly associated with poorer functional outcomes with an acOR of 1.31 [95% CI 1.10–1.57]. WMH was attributed to 23% [95% CI 7–57] of the total influence of CMBs on functional outcomes (Table 5). Age, sex, comorbid hypertension, infarct volume, successful reperfusion, and IV thrombolysis were included as covariates in all multivariable analyses.
Mediation by successful reperfusion concerning the association between CMBs and poorer functional outcomes
As the lower rate of successful reperfusion was an unexpected finding for groups with higher CMB burden, further mediation analysis was performed with successful reperfusion as mediator. In the first step of mediation analysis, CMBs were significantly predictive of higher 3-month mRS with an acOR of 1.84 [95% CI 1.37–2.47]. In the second step, CMBs showed a significant inverse correlation with successful reperfusion with an acOR of 0.67 [95% CI 0.46–0.97]. In the third step, the direct effect of CMBs on poorer functional outcome remained statistically significant after adjustment for successful reperfusion with an acOR 1.77 [95% CI 1.31–2.38]. The mediator successful reperfusion was an independent variable and significantly associated with poorer functional outcomes with an acOR of 0.34 [95% CI 0.24–0.49]. Lower rates of successful reperfusion were attributed to 6% [95% CI 0–25] of the total influence of CMBs on poorer functional outcomes (Table 5). Age, sex, comorbid hypertension, infarct volume, and IV thrombolysis were included as covariates in all multivariable analyses.
Discussion
This study shows that increases in CMB burden are associated with worsened functional outcomes after endovascular treatment, and reveals the factors that act as mediators of outcomes. As per previous concerns, CMB burden was associated with an increased incidence of post-procedural hemorrhagic complications, resulting in worse functional outcomes. However, hemorrhagic complications were only partly accountable for the poorer functional outcomes. The influence of a high CMB burden on outcomes was partially mediated by white matter hyperintensity at a greater degree, while lower rates of successful reperfusion played a smaller role.
The current study revealed that the rate of hemorrhagic complications significantly increased with the CMB burden, independent of other covariates. Previous reports regarding this issue are somewhat controversial. However, advances in endovascular treatment methods and MR imaging methods may be the cause of the different results. Results of a recent meta-analysis involving 598 patients regarding the association between post-mechanical thrombectomy hemorrhagic complications and CMBs13 did not show a positive association. When we take a closer look into the studies analyzed, a previous study including Asian patients from 2002 to 2012 using 1.5T MRI GRE failed to show an association between CMB burden and hemorrhagic complications. However, in the study, only a small number of patients with ≥ 5 CMBs were included, and Merci retrievers were used in most cases26. Another study including European patients from 2010 to 2013 used a 1.5-T MRI and susceptibility weighted imaging. They reported the percentage of patients with ≥ 5 CMBs to be 2.3% (9/392) and a marginally increased risk for CMB to be associated with the risk for ICH27. Another study, which was not included in the meta-analysis, enrolled 1532 patients treated with intravenous thrombolysis or mechanical thrombectomy using modern devices between 2007 and 201719. Contrary to the total population, in the 595 patients with recanalization, CMBs—especially with a high burden and lobar location—were independently associated with poor 3-month clinical outcomes and risk of symptomatic intracranial hemorrhage. Considering that our study used 3T MRI and included patients between 2011 and 2016, it is likely that more sensitive quantification of CMB burden and more consistent reperfusion with modern thrombectomy devices may result in the positive associations between CMB burden and hemorrhagic complications.
Functional outcomes were also negatively influenced by CMBs presence significantly, while this association was only partially mediated by hemorrhagic complications. CMBs are known to be markers of small-vessel disease burden, which may influence outcomes28. Thus, CMB influence may be potentially mediated by pathways other than hemorrhagic complications. If this is the case, CMB degree may be associated with stroke outcomes overall, and not just thrombolysis or EVT populations. However, data regarding functional outcomes are scarce, and only recently, was association between CMB burden and functional outcomes shown in a group of minor ischemic stroke patients29. To clarify this interrelation, we used mediation analysis to explain the influence of CMBs on outcomes mediated by both HT and WMH (small-vessel disease burden); showing that both factors mediated the negative influence of CMBs on outcomes. While both CMBs and WMHs are considered as imaging biomarkers of small-vessel disease, WMHs were used as a proxy for overall small-vessel disease burden in this study to represent the chronic brain injury associated with the small-vessel disease, and also because WMHs are relatively frequent, with well-validated grading systems. There is increasing evidence that WMH represents macroscopic injury to the white matter, and its extent influences functional recovery in multiple domains following ischemic stroke8. Further, WMH predicts post-stroke cognitive performance after stroke well among small-vessel disease markers30. Clinically, CMBs and WMHs share common risk factors, such as hypertension and diabetes. Our results remained statistically significant even after controlling for such factors.
An unexpected result of our study is the lower rate of reperfusion with increasing CMB burden. Due to retrospective imaging analysis, cause of reperfusion failure could not be clearly identified in this study. Recent studies have reported associations between small vessel disease markers and intracranial arterial dolichoectasia31. Such increases in the arterial tortuosity may theoretically interfere with lesion access, device delivery, and clot retrieval32. However, the lower reperfusion rates accounted for 6% of the total effect of CMB burden on functional outcomes, which was lower than that of other variables. Furthermore, the CMB effect on outcomes mediated by post-procedural HT degree was independent of successful reperfusion as per our hypothesis.
The study results must be interpreted with some caution. First, although poor outcomes were observed in the high CMB burden group, this effect was not purely due to increases in hemorrhagic complications. The results of this study do not suggest absence of treatment effect of EVT in the high CMB population, and should not be used as evidence for excluding patients from a powerful treatment modality. A recent study using data from a randomized IV thrombolysis has shown no reduced treatment effect of alteplase in acute ischemic stroke patients with one or more CMBs33, while reaching inconclusive conclusions regarding larger numbers of CMBs. Similarly designed studies will be needed regarding EVT treatment effect. In the time being, our results support the cumulative evidence of CMBs as a risk factor of HT, establishing CMB as an imaging biomarker that deserves attention. Second, there were differences in baseline characteristics according to increasing CMB count, such as increased age or reperfusion rates; however, after correcting for these factors, our results showed that both the influence on outcomes and the mediation effect remained statistically significant. Third, a simple 4-point visual scale was used to grade WMH, which might attenuate its effect. There is a chance that when WMH is quantitatively analyzed34, the percent mediation may increase. Third, current treatment recommendations advocate a reduction of door-to-puncture time35, and the use of advanced imaging for routine patient selection may be debatable. Although the most appropriate imaging method requires further evidence, screening for high CMB burden may be an advantage of MR-based imaging.
Summary
In conclusion, a high CMB burden was associated with poorer outcomes in stroke patients undergoing mechanical thrombectomy. Our results show that this can be explained by higher small vessel disease burden that may hinder neurological recovery, increases in post-procedural HT complications, and lower reperfusion rates, listed in order of percent mediation size.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- acOR:
-
Adjusted common odds ratio
- CMB:
-
Cerebral microbleed
- CI:
-
Confidence interval
- EVT:
-
Endovascular treatment
- GRE:
-
Gradient echo protocol
- HT:
-
Hemorrhagic transformation
- mRS:
-
Modified Rankin Scale
- PH:
-
Parenchymal hematoma
- WMH:
-
White matter hyperintensity
References
Greenberg, S. M. et al. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 8, 165–174. https://doi.org/10.1016/S1474-4422(09)70013-4 (2009).
Wilson, D. et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: A meta-analysis. Neurology 87, 1501–1510. https://doi.org/10.1212/WNL.0000000000003183 (2016).
Wang, D. N. et al. Quantity of cerebral microbleeds, antiplatelet therapy, and intracerebral hemorrhage outcomes: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 24, 2728–2737. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.08.003 (2015).
Tsivgoulis, G. et al. Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: A meta-analysis. JAMA Neurol. 73, 675–683. https://doi.org/10.1001/jamaneurol.2016.0292 (2016).
Soo, Y. O. et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J. Neurol. 255, 1679–1686. https://doi.org/10.1007/s00415-008-0967-7 (2008).
Haller, S. et al. Cerebral microbleeds: Imaging and clinical significance. Radiology 287, 11–28. https://doi.org/10.1148/radiol.2018170803 (2018).
Li, X. et al. The significant effects of cerebral microbleeds on cognitive dysfunction: An updated meta-analysis. PLoS ONE 12, e0185145. https://doi.org/10.1371/journal.pone.0185145 (2017).
Etherton, M. R., Wu, O. & Rost, N. S. Recent advances in leukoaraiosis: White matter structural integrity and functional outcomes after acute ischemic stroke. Curr. Cardiol. Rep. 18, 123. https://doi.org/10.1007/s11886-016-0803-0 (2016).
Noh, Y. et al. A new classification system for ischemia using a combination of deep and periventricular white matter hyperintensities. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 23, 636–642. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.06.002 (2014).
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 50, e344–e418. https://doi.org/10.1161/STR.0000000000000211 (2019).
Goyal, M. et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 387, 1723–1731. https://doi.org/10.1016/S0140-6736(16)00163-X (2016).
Balami, J. S., White, P. M., McMeekin, P. J., Ford, G. A. & Buchan, A. M. Complications of endovascular treatment for acute ischemic stroke: Prevention and management. Int. J. Stroke Off. J. Int. Stroke Soc. 13, 348–361. https://doi.org/10.1177/1747493017743051 (2018).
Wu, X. et al. Pre-treatment cerebral microbleeds and intracranial hemorrhage in patients with ischemic stroke receiving endovascular therapy: A systematic review and meta-analysis. J. Neurol. 267, 1227–1232. https://doi.org/10.1007/s00415-019-09210-6 (2020).
Lee, J. S. et al. Temporal changes in care processes and outcomes for endovascular treatment of acute ischemic stroke: Retrospective registry data from three Korean centers. Neurointervention 13, 2–12. https://doi.org/10.5469/neuroint.2018.13.1.2 (2018).
Lee, J. S. et al. Prognosis of acute intracranial atherosclerosis-related occlusion after endovascular treatment. J. Stroke 20, 394–403. https://doi.org/10.5853/jos.2018.01627 (2018).
Tomsick, T. et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am. J. Neuroradiol. 29, 582–587. https://doi.org/10.3174/ajnr.A0843 (2008).
Hacke, W. et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352, 1245–1251. https://doi.org/10.1016/s0140-6736(98)08020-9 (1998).
Sun, J. et al. Different distribution patterns of cerebral microbleeds in acute ischemic stroke patients with and without hypertension. Eur. Neurol. 62, 298–303. https://doi.org/10.1159/000235850 (2009).
Choi, K. H. et al. Impact of microbleeds on outcome following recanalization in patients with acute ischemic stroke. Stroke https://doi.org/10.1161/STROKEAHA.118.023084 (2018).
Dannenberg, S. et al. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke 45, 2900–2905. https://doi.org/10.1161/STROKEAHA.114.006448 (2014).
Gurol, M. E. et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 66, 23–29. https://doi.org/10.1212/01.wnl.0000191403.95453.6a (2006).
Baron, R. M. & Kenny, D. A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182. https://doi.org/10.1037//0022-3514.51.6.1173 (1986).
Vanderweele, T. J. & Vansteelandt, S. Odds ratios for mediation analysis for a dichotomous outcome. Am. J. Epidemiol. 172, 1339–1348. https://doi.org/10.1093/aje/kwq332 (2010).
Freedman, L. S., Graubard, B. I. & Schatzkin, A. Statistical validation of intermediate endpoints for chronic diseases. Stat. Med. 11, 167–178. https://doi.org/10.1002/sim.4780110204 (1992).
Compagne, K. C. J. et al. Follow-up infarct volume as a mediator of endovascular treatment effect on functional outcome in ischaemic stroke. Eur. Radiol. 29, 736–744. https://doi.org/10.1007/s00330-018-5578-9 (2019).
Shi, Z. S. et al. Mechanical thrombectomy for acute ischemic stroke with cerebral microbleeds. J. Neurointerventional Surg. 8, 563–567. https://doi.org/10.1136/neurintsurg-2015-011765 (2016).
Gratz, P. P. et al. Preexisting cerebral microbleeds on susceptibility-weighted magnetic resonance imaging and post-thrombolysis bleeding risk in 392 patients. Stroke 45, 1684–1688. https://doi.org/10.1161/STROKEAHA.114.004796 (2014).
Henninger, N. et al. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc. Dis. 33, 525–531. https://doi.org/10.1159/000337335 (2012).
Sakuta, K. et al. Cerebral microbleeds load and long-term outcomes in minor ischemic stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 30, 105973. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105973 (2021).
Molad, J. et al. Only white matter hyperintensities predicts post-stroke cognitive performances among cerebral small vessel disease markers: Results from the TABASCO study. J. Alzheimer’s Dis. JAD 56, 1293–1299. https://doi.org/10.3233/JAD-160939 (2017).
Zhang, D. P. et al. Association between intracranial arterial dolichoectasia and cerebral small vessel disease and its underlying mechanisms. J. Stroke 22, 173–184. https://doi.org/10.5853/jos.2019.02985 (2020).
Alverne, F. et al. Unfavorable vascular anatomy during endovascular treatment of stroke: Challenges and bailout strategies. J. Stroke 22, 185–202. https://doi.org/10.5853/jos.2020.00227 (2020).
Schlemm, L. et al. Cerebral microbleeds and treatment effect of intravenous thrombolysis in acute stroke: An analysis of the WAKE-UP randomized clinical trial. Neurology https://doi.org/10.1212/WNL.0000000000013055 (2021).
Boulouis, G. et al. White matter hyperintensity burden in patients with ischemic stroke treated with thrombectomy. Neurology 93, e1498–e1506. https://doi.org/10.1212/WNL.0000000000008317 (2019).
Saver, J. L. et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 316, 1279–1288. https://doi.org/10.1001/jama.2016.13647 (2016).
Funding
This work was partly supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (NRF-2018R1A2B6007094; J.S.L.), and (NRF No. 2014R1A5A2010008: S.I.S.).
Author information
Authors and Affiliations
Contributions
Guarantors of integrity of entire study, S.I.S.,Y.H.H., J.S.L.; literature research, S.J.L., J.S.L.; clinical studies, S.J.L., J.M.H., J.W.C., D.H.K., Y.W.K., Y.S.K., J.H.H., J.Y., C.H.K., S.I.S., Y.H.H., J.S.L.; statistical analysis, S.J.L., J.H.P., B.P., J.S.L.; original manuscript preparation, S.J.L.; manuscript editing, S.J.L., S.I.S., Y.H.H., J.S.L.; data acquisition and data analysis/interpretation, all authors; manuscript revision for important intellectual content, all authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, SJ., Hwang, YH., Hong, J.M. et al. Influence of cerebral microbleeds on mechanical thrombectomy outcomes. Sci Rep 12, 3637 (2022). https://doi.org/10.1038/s41598-022-07432-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07432-9
This article is cited by
-
Intracranial hemorrhage risk in patients with cerebral microbleeds after mechanical thrombectomy for acute ischemic stroke: a systematic review and meta-analysis
Neurological Sciences (2024)
-
Factors influencing hemorrhagic transformation in ischemic stroke patients with atrial fibrillation: a hospital based-study
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.