Abstract
Stocking programs have been widely implemented to re-establish extirpated fish species to their historical ranges; when employed in species with complex life histories, such management activities should include careful consideration of resulting hybridization dynamics with resident stocks and corresponding outcomes on recovery initiatives. Genetic monitoring can be instrumental for quantifying the extent of introgression over time, however conventional markers typically have limited power for the identification of advanced hybrid classes, especially at the intra-specific level. Here, we demonstrate a workflow for developing, evaluating and deploying a Genotyping-in-Thousands by Sequencing (GT-seq) SNP panel with the power to detect advanced hybrid classes to assess the extent and trajectory of intra-specific hybridization, using the sockeye salmon (Oncorhynchus nerka) stocking program in Skaha Lake, British Columbia as a case study. Previous analyses detected significant levels of hybridization between the anadromous (sockeye) and freshwater resident (kokanee) forms of O. nerka, but were restricted to assigning individuals to pure-stock or “hybrid”. Simulation analyses indicated our GT-seq panel had high accuracy, efficiency and power (> 94.5%) of assignment to pure-stock sockeye salmon/kokanee, F1, F2, and B2 backcross-sockeye/kokanee. Re-analysis of 2016/2017 spawners previously analyzed using TaqMan® assays and otolith microchemistry revealed shifts in assignment of some hybrids to adjacent pure-stock or B2 backcross classes, while new assignment of 2019 spawners revealed hybrids comprised 31% of the population, ~ 74% of which were B2 backcross or F2. Overall, the GT-seq panel development workflow presented here could be applied to virtually any system where genetic stock identification and intra-specific hybridization are important management parameters.
Similar content being viewed by others
Introduction
Global fish populations are in decline due to agricultural development, rapidly rising riverine water temperatures, and reduced connectivity1,2,3,4,5,6. In particular, 80% of salmonid populations in the Columbia River have experienced losses linked to hydroelectric development and the destruction of spawning and rearing habitat7,8. In response, management strategies have included incorporating fish passage at dams to mitigate impacts to migratory fish life histories and supplementing populations in decline with hatchery fry for stock enhancement. For example, Coho salmon (Oncorhynchus kisutch) have been restored in the lower Columbia River using hatchery stocking, resulting in the establishment of local naturalized populations5,9. Additionally, the enhancement of spawning habitat has been an effective strategy to restore fish populations, where the replacement of river substrate improved water velocities, dissolved oxygen, and usage of the site by spawning Chinook salmon (O. tshawytscha)10. Ultimately, the success of fish stocking programs that target species with diverse migratory and resident forms should include careful consideration of resulting hybridization dynamics and corresponding outcomes on recovery initiatives.
Hybridization in fish species is well documented, including inter-specific hybrids such as rainbow (Oncorhynchus mykiss) and cutthroat trout (O. clarkii)11, as well as within-species hybrids between different life history forms, such as the case between anadromous sockeye salmon and freshwater resident kokanee12. The long-term effects of hybridization are often complex, with the potential for both positive and negative outcomes13. Hybridization can be a powerful conservation tool harnessed to rescue populations with low genetic diversity and increase fitness through the integration of favorable traits such as larger body size, more offspring, and longer lifespans13,14,15,16. On the other hand, detrimental effects have been observed such as introgression with maladapted gene complexes17, decrease in reproductive success18, and negative impacts on growth19, with hybrid fitness theorized to decrease as the divergence between parental phenotypes increases20. Overall, the impacts of intra-specific hybridization in the wild are still not well understood, warranting further study, especially when observed as part of an active management program.
Oncorhynchus nerka provides an excellent system for investigating the genetic and physiological outcomes of hybridization as it exhibits tremendous life history variation, shows natal homing behavior, and represents a valuable species targeted for population restoration through restocking21. This species exhibits two main migratory forms, including anadromous sockeye salmon (hereafter referred to as “sockeye salmon”) and freshwater resident kokanee (hereafter referred to as “kokanee”). Kokanee are much smaller than sockeye salmon (26 cm versus > 45 cm average adult fork length) and occur sympatrically in many lakes, but tend to exhibit different spawning habitat preferences and spawning periods22,23. Despite differences in spawning behavior, kokanee males are known to sneak on spawning sockeye salmon females24, and size-selective mating has been observed between male sockeye salmon and female kokanee25, allowing for gene flow between migratory forms. Sockeye-kokanee hybridization can lead to an increase in body size of resident hybrids that can increase angler satisfaction19 and bolster the overall genetic diversity of O. nerka in the system16. Conversely, hybrids can also experience negative impacts such as lower swimming capabilities than pure sockeye salmon26, medial seawater adaptabilities27, intermediate maturation time23, decreased egg survival rate28, and the loss of the iconic red coloration that is key in sexual selection25. With a broad range of potential outcomes, accurate assessment of the extent of introgression is critical for elucidating the long-term effects of hybridization associated with supplementation and reintroduction programs.
To address large-scale declines in sockeye salmon populations in the Columbia River, an experimental reintroduction program was initiated in Skaha Lake within the Okanagan Basin of the Southern Interior of British Columbia, Canada. This program provides an excellent opportunity to investigate the extent and outcomes of intra-specific hybridization between O. nerka migratory and resident forms outside of a laboratory setting. Previous research examining hybridization between sockeye salmon and kokanee found O. nerka hybrids occupy intermediate morphologies and exhibit a largely resident life history, although this latter finding requires further investigation12,29. These studies employed genetic marker sets that were effective at differentiating pure-stock from hybrid, however, they had limited power for the identification of advanced hybrid classes that has become increasingly important for genetic monitoring as the reintroduction program matures. The detection of advanced hybrid classes within a species can be challenging due to the proximity of backcrosses to pure genotype frequencies. For example, allozyme loci were only able to reveal intermediate allelic frequencies suggesting hybridization between transplanted and native sockeye30, while microsatellite assays have the ability to detect introgression to the F1 level31, but both marker types experience difficulty in the detection of advanced hybrid classes based on the conventional number of loci employed32,33. In that regard, traditional markers may not have the statistical power to identify advanced hybrid classes34, with an estimated minimum of 70 markers required to discriminate between pure parental species and advanced backcrosses35. A previous simulation-based sensitivity analysis found that a panel comprised of 300 highly differentiated SNPs has the resolution needed to detect advanced hybrid classes between sockeye salmon and kokanee in Skaha Lake36. With this in mind, advancements in massively parallel sequencing can be leveraged to improve the identification of hybridization in systems by pooling barcoded amplicons to increase the power of genetic panels for classification37; Genotyping-in-Thousands by sequencing (GT-seq) is a particularly useful approach in cases where large sample sizes need to be cost-effectively genotyped38.
Here, we demonstrate a workflow for developing, evaluating and deploying a GT-seq SNP panel with the power to detect advanced hybrid classes to assess the extent and trajectory of intra-specific hybridization, using the sockeye salmon reintroduction program in the Okanagan Basin as a case study. Using previously published restriction site associated DNA sequencing (RAD-seq) collected for this system, we first simulated two pure (kokanee, sockeye) and four hybrid classes (F1, F2, B2 backcross-kokanee, B2 backcross-sockeye) with three different SNP datasets (300 highest Fst SNPs; 600 highest Fst SNPs; 350 random SNPs selected from the 600 highest Fst SNPs) to assess information content to inform panel construction. We then evaluated the accuracy, efficiency, and power of the optimized GT-seq panel using simulation analyses. We further assessed panel performance by re-genotyping individuals sampled in Skaha Lake in 2016 and 2017 that were previously genetically assigned as pure-stock or F1 hybrid at 32 SNPs and subjected to otolith microchemistry analysis to reconstruct migratory history29. Finally, we genetically assigned O. nerka spawners sampled in 2019 to pure-stock or hybrid class (F1, F2, B2 backcross-kokanee, B2 backcross-sockeye) to estimate stock proportions and examine trends in this system over time.
Methods
Study system and samples
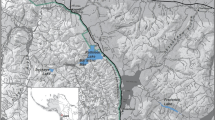
Skaha Lake is located in the Canadian portion of the Okanagan Basin, and flows south to join the Columbia River (Fig. 1). O. nerka spawn in the Okanagan River, upstream of Skaha Lake. Historically, sockeye salmon populations existed in the Okanagan Basin, however, the construction of a dam at McIntyre Bluff in 1921 blocked access to spawning grounds and channelization of the Okanagan River further degraded spawning habitat. These activities left Skaha Lake with a sole population of stream-spawning kokanee39. In attempts to restore sockeye salmon to the Okanagan Basin, a re-introduction program was implemented in 2004. Through this initiative, upstream fish passage was created by making structural improvements to migration barriers, restoring stream habitat, and stocking sockeye salmon fry annually into Skaha Lake. This system now supports a self-sustaining population of sockeye salmon39,40.
Map of the Columbia River displaying Skaha Lake, Okanagan River (extending southward originating at McIntyre Dam), and downstream lakes and dams. Size comparison of an average sockeye male and kokanee male included. The maps were created using a custom R script (https://github.com/changsarahl/BCmap) with R version 4.1.1 (https://www.R-project.org/). Geographic data were accessed under the open government license—British Columbia: https://catalogue.data.gov.bc.ca/dataset/freshwater-atlas-watersheds and the Washington geospatial open data license: https://geo.wa.gov/datasets/waecy::wa-hydrography-nhdwaterbody/about.
We genotyped tissue samples from O. nerka spawners in the Okanagan River upstream of Skaha Lake that were previously sampled in 2016 (n = 45) and 2017 (n = 59)29, and newly sampled in 2019 (n = 218; this study) as deadpitch (post-mortem carcass collection after spawning) by personnel from the BC Ministry of Forests, Lands, Natural Resource Operations, and Rural Development. The section of river that was sampled represents the only spawning habitat that exists for O. nerka populations from Skaha Lake and all forms spawn within this section of river. Samples were collected on multiple dates that spanned the duration of the spawning run. Biological data (length and sex) and tissue samples (operculum punches preserved in ethanol) were collected from all sampled fish. Moreover, the 2016 and 2017 samples were previously genetically assigned to pure-stock or F1 hybrid using a panel of 32 TaqMan® SNP assays and subjected to otolith microchemistry analysis36 to determine fish migratory history and investigate maternal migratory signatures that are passed transgenerationally to offspring41.
GT-seq SNP panel design
We used previously published genotypic data collected via RAD-seq42 for: (1) Skaha Lake kokanee (n = 20) collected in 2003 prior to the sockeye salmon restocking program; and (2) Okanagan River sockeye salmon (n = 35) collected in 2012 downstream of historical migration barriers. Using the populations module in STACKS version 2.0 beta 843, we required all loci to be present in at least 60% of individuals in both populations, with a minor allele frequency greater than 0.05. Due to the salmonid whole genome duplication event, we filtered out suspected homeologs by removing any locus with a negative Fis or Hobs > 0.5 that occurred in both reference populations44. The resulting SNP dataset was then filtered for quality using VCFtools45 to remove SNPs not in Hardy–Weinberg equilibrium and to calculate Weir and Cockerham (1984)’s θ, an unbiased estimate of Fst46, between Skaha Lake kokanee and Okanagan River sockeye salmon following36. We removed loci with insufficient flanking sequence required for primer design by retaining loci with the SNP positioned between the 40th and 70th base pairs of the RAD tag sequences and selected the top 650 loci exhibiting the highest Fst. We then assessed all loci pairs for deviation from linkage equilibrium using GENEPOP 4.547, removed loci that were linked, and finalized selection of 600 candidate loci.
To examine panel informativeness of candidate loci and forecasting of panel ability after primer dropout, we simulated parental and offspring individuals (n = 1000) for six hybrid classes (kokanee, sockeye, F1, F2, B2 backcross-kokanee, B2 backcross-sockeye) with recom-sim.py (https://github.com/salanova-elliott/recom-sim). Separate simulations were conducted at: 1) 300 highest Fst SNPs; 2) 600 highest Fst SNPs; and 3) 350 SNPs randomly selected from the 600 highest Fst SNPs. We assigned simulated individuals to parental or hybrid class by calculating the posterior probabilities of membership as implemented in NEWHYBRIDS48 with the reference populations flagged as known genotypes with the “z” option, and constructed confusion matrices for each panel assessment. After in-silico assessment, we sent the full RAD tag sequences that were associated with the pool of candidate SNPs to GTseek LLC (https://gtseek.com/) for custom locus-specific primer design.
GT-seq test library preparation
We constructed a GT-seq test library with the previously extracted DNA samples collected in 2016 (n = 45) and 2017 (n = 59) for which otolith microchemistry analysis was previously conducted29. Extracted DNA was quantified with a Qubit 3.0 Fluorometer and the dsDNA High Sensitivity Assay Kit (Invitrogen). Library preparation followed the original protocol38, with the exception that we diluted the PCR1 product to 1:10 (https://doi.org/10.17504/protocols.io.byvppw5n). The PCR2 product was quantified with Picogreen™ (Molecular Probes, Inc.) and each sample was normalized to a concentration of 10 ng/µL. The pooled library was purified with a MinElute PCR Purification Kit (Qiagen) and eluted into a final volume of 25µL. Test libraries were sequenced using a Mid Output Reagent Kit (300 cycles) on an Illumina MiniSeq within the Ecological and Conservation Genomics Laboratory at the University of British Columbia Okanagan.
GT-seq genotyping and primer optimization
Demultiplexed raw sequencing files were processed with the GT-seq pipeline available on GitHub (https://github.com/GTseq/GTseq-Pipeline). We removed primers with non-specific in silico probes, candidates that were overrepresented (exhibiting > 2% of the raw read count), observed primer dimers, potential PCR artefacts, off-target amplification or in silico probe variation following previously published work49. A second and third test library with the same sample composition as the first test library were prepared and tested iteratively with optimized primer pools from previous libraries using the protocols detailed above for sample preparation, sequencing, and primer dropout (Fig. 2).
To compile the final dataset based on the optimized SNP panel for downstream analyses, raw sequencing files from individuals were concatenated across sequencing runs and processed with the GT-seq pipeline available on GitHub (https://github.com/GTseq/GTseq-Pipeline). We filtered out individuals with > 25% missing data using PLINK50. We decided on a cutoff of 25% to minimize the proportion of missing data that can negatively impact the detection of population structure, particularly with the high resolution needed to elucidate hybrid classes within a species51.
Panel performance
We assessed the accuracy, efficiency and power of NEWHYBRIDS assignments with simulated individuals (n = 100) using two pools (top 300 Fst SNPs, optimized SNP panel) with hybridpowercomp as implemented in the R package hybriddetective34,52. Specifically, we evaluated assignment probabilities based on: (1) accuracy (correct assignments/total assignments per category); (2) efficiency (correct assignments/total number of individuals in a category); and (3) power (product of accuracy and efficiency). We also used hybriddetective34,52 to quantify error rates associated with individual assignment (top 300 Fst SNPs, optimized SNP panel) as follows: (1) Type I: false positive error rate (number of known pure individuals wrongly assigned to a hybrid genotype frequency class/total number of known pure individuals); and (2) Type II: false negative error rate (number of known hybrid individuals wrongly assigned to a pure genotype frequency class/total number of known hybrid individuals).
Re-analysis of 2016–2017 samples
We genetically assigned the 2016 and 2017 sampled individuals to pure-stock or hybrid class using NEWHYBRIDS48, the genotypic data from the optimized SNP panel, and reference baseline genetic data from 2003 Skaha Lake kokanee and 2012 Okanagan River sockeye salmon42. Five genotype frequency classes (kokanee, sockeye, F1, F2, B2 backcross-kokanee, and B2 backcross-sockeye) were specified, and the analysis initiated with a burn-in period of 10,000 followed by 50,000 iterations. We applied the s and z flags to the reference sockeye salmon and kokanee populations to designate individuals of known genotype that were sampled separately from the test individuals. We then selected the maximum probability of assignment to assign individuals to the most likely class. Lastly, we compared the NEWHYBRIDS48 genetic assignment to pure-stock or hybrid class to the migratory history of the individuals, as previously inferred29 by way of otolith microchemistry analysis.
Assignment of 2019 samples
For the new samples collected in 2019 (n = 218), DNA was extracted with a standard Chelex-based protocol in 96-well plates containing approximately 0.5 mm2 of tissue, 5 µl proteinase K (10 mg/ml), and 195 ul 10% Chelex solution53. Plates were incubated for 4 h at 55 °C to digest tissue, and then 95 °C for 15 min using an Applied Biosystems Veriti thermal cycler (Applied Biosystems, Foster City, CA, USA). Library preparation was conducted as above with the optimized SNP panel. The multiplexed pooled library was sequenced using a partial High Output Reagent Kit (300 cycles) on the Illumina MiniSeq within the Ecological and Conservation Genomics Laboratory at the University of British Columbia Okanagan. Samples with individual missing data > 25% were filtered out for downstream analyses. Individual assignment to hybrid class was conducted using NEWHYBRIDS48 and the same parameters as above. Fisher’s Exact Test was conducted in R to determine if proportions of hybrid class were significantly different within a sample year and between sample years.
Morphometric analyses
One-way ANOVA and post-hoc Tukey Tests were conducted in R to determine if mean fork length for the 2019 samples was significantly different between individuals assigned to pure-stock (kokanee, sockeye salmon) and the various hybrid classes (F1, F2, B2 backcross-kokanee, and B2 backcross-sockeye).
Results
Initial panel ability
The simulated SNP panels provided comparable accuracy to assign individuals to hybrid class based on initial NEWHYBRIDS assignment of simulated individuals (Table 1). The top 300 Fst SNP panel performed best with high accuracy (> 0.98), followed by the top 600 Fst SNP panel (> 0.96) and the random 350 SNPs selected from the top 600 Fst SNPs (> 0.92).
From the initial pool of top 600 Fst SNPs, primers were successfully designed for 515 SNPs after in silico testing. Following three rounds of multiplex amplicon sequencing and primer pool optimization, the optimized GT-seq panel consisted of 342 SNPs (Fig. 2, Supplementary Table S1). Our finalized 342 SNP panel had an accuracy of > 94% across all hybrid classes (Table 1). The lowest assignment accuracies were to F1 and F2 across all simulated panels (but all ≥ 0.92; Table 1), which is consistent with previous results36.
SNP panel assignment efficacy
The optimized 342 SNP GT-seq panel performed slightly worse than the top 300 Fst SNP pool, but still displayed high accuracy and efficiency in assigning simulated individuals to pure-stock and the different hybrid classes (Fig. 3). Pure-stocks and B2 backcrosses were detected at > 98% accuracy and > 99% efficiency at a critical posterior probability threshold of 50% (Fig. 3). F1 and F2 classes were detected at lower, but still meaningful levels, with efficiency at > 92% and accuracy at > 90% at the 50% critical probability threshold (Fig. 3). The power of assignment was comparable between the 342 SNP GT-seq panel and top 300 Fst SNPs where pure-stocks and B2 backcrosses remained stable over a wide range of probability thresholds: > 99% at a critical probability threshold of 50%, and > 98% at a threshold of 90% (Supplementary Fig. S1). However, the power of assignment for F1 and F2 classes declined in performance at higher critical posterior probability thresholds: > 90% at a critical probability threshold of 50% and > 79% at a threshold of 90% (Supplementary Fig. S1). The Type I false positive error rate and Type II false negative error rate were < 0.001% for both the 342 SNP GT-seq panel and top 300 Fst SNPs.
Re-analysis of 2016–2017 Okanagan river samples
We successfully genotyped 2016 (n = 36) and 2017 (n = 55) Okanagan River spawners with the 342 SNP GT-seq panel after filtering for 25% missing data across individuals (average read depth = 220.8; genotyping rate = 90.1%). Overall, assignments to pure-stock and hybrid class were generally similar between the different marker sets, although there was a slightly higher proportion of individuals assigned to pure-stock using the 342 SNP GT-seq panel (Table 2; Supplementary Table S2). Our 342 SNP GT-seq panel further refined assignments into multiple hybrid classes for those individuals previously classified as hybrids, both in 2016 (proportion F1: 0.22, B2 backcross-kokanee: 0.03) and 2017 (proportion F1: 0.20, B2 backcross-kokanee: 0.05) (Table 2).
A small number of assignment differences were found between the TaqMan® SNP assays29 and 342 SNP GT-seq panel. In 2016, four hybrids were reclassified as sockeye salmon with three instances of microchemistry conflict, where the latter results suggested that these individuals did not migrate to the ocean (Supplementary Table S2). Additionally, one sockeye salmon was genetically reclassified to F1 hybrid. One individual that was genetically assigned as sockeye salmon with both the 32 SNP TaqMan® assays29 and 342 SNP GT-seq panel had conflicting microchemistry that suggested no history of anadromy or maternal anadromy (Supplementary Table S2). Within the 2017 cohort, five individuals that were previously genetically assigned as hybrids were refined to: sockeye salmon (n = 1) and kokanee (n = 4) (Table 2; Supplementary Table S2). All microchemistry was congruent, other than one individual that was genetically assigned as sockeye salmon by both 32 SNP TaqMan® assays29 and the 342 SNP GT-seq panel; in this case, microchemistry suggested a resident maternal parent, but anadromous migratory history.
Assignment of 2019 Okanagan river samples
We genotyped 2019 Okanagan River spawners (n = 202) with the 342 SNP GT-seq panel after filtering for 25% missing data across individuals (average read depth = 370.9; genotyping rate = 91.2%). The composition of the 2019 Okanagan River spawners revealed a higher proportion of kokanee (0.57; Table 2; Supplementary Table S3) and lower proportion of sockeye salmon (0.12; Table 2; Supplementary Table S3) than in 2016 and 2017 (kokanee: 0.42–0.44; sockeye salmon: 0.31–0.33; Table 2; Supplementary Table S3). In addition, the 2019 spawners contained substantially more detected backcrosses, including the first B2 backcross-sockeye individuals genetically identified (B2 backcross-kokanee: n = 24, overall proportion = 0.12; B2 backcross-sockeye: n = 19; overall proportion 0.09; Table 2; Supplementary Table S3). We also detected low proportions of F2 hybrids in 2019 (n = 4, overall proportion = 0.02) when compared to other hybrid classes.
We found significant differences in proportions among hybrid classes within a year among all sample years (2016: P < 0.001, χ2 = 45.20, df = 5; 2017: P < 0.001, χ2 = 62.68, df = 5; 2019: P < 0.001, χ2 = 292.61, df = 5). Across sample years, we found that the proportions of kokanee (P = 0.080, χ2 = 5.04, df = 2), F2 (P = 0.779, χ2 = 1.83, df = 2) and B2 backcross-kokanee (P = 0.140, χ2 = 4.35, df = 2) were not significantly different. The proportions of sockeye salmon (P < 0.001, χ2 = 16.98, df = 2), F1 (P < 0.05, χ2 = 10.12, df = 2), and B2 backcross-sockeye (P < 0.05, χ2 = 9.15, df = 2) were significantly different across sample years.
In general, sockeye salmon exhibited larger mean lengths compared with kokanee, with hybrids generally occupying an intermediate range between kokanee and sockeye salmon mean lengths (Supplementary Fig. S2; Supplementary Table S2). However, it is notable that F2 hybrids seem to occupy the same size distribution as B2 backcross-kokanee, signifying smaller body sizes with advanced hybrid classes. The mean lengths between sockeye/kokanee, F1/sockeye, F2/sockeye, B2 backcross-kokanee/sockeye, and B2 backcross-sockeye/sockeye were significantly different (Supplementary Fig. S2; Supplementary Table S2).
Discussion
The accurate identification of advanced hybrid classes is valuable for monitoring the extent of introgression and potential fitness impacts between multiple reproductive forms of a single species or where hybridization between species is possible. SNP panels have been developed for a broad range of taxa with the ability to accurately identify hybridization between closely related species within the same genus up to the third backcross hybrid generation54,55. However, to our knowledge, this is the first GT-seq SNP panel with the resolution to differentiate advanced hybrid classes up to the second backcross hybrid generation across life history forms within-species. Our GT-seq SNP panel represents a valuable tool that may be used to examine the ongoing progression of intra-specific hybridization and potential fitness outcomes associated with an active sockeye salmon reintroduction program in the Okanagan Basin, providing information to guide on-going management strategies and offer a roadmap to other such programs for species with complex life histories.
GT-seq panel development and evaluation
Regarding initial panel development and optimization, we effectively integrated an expected level of primer drop-out within our simulation analyses to inform locus selection and provide preliminary insights on panel performance. To that end, our simulations were consistent with previous work in this system (e.g.29,36), and displayed high assignment accuracy across all hybrid classes (> 94%), as well as comparable panel accuracy, efficiency, and power between the top 300 Fst SNP pool, random 350 SNP subset of the top 600 Fst SNP pool, and ultimately, our optimized 342 SNP panel (Fig. 4, Supplementary Fig. S2). Subsequent error rates (< 0.001% at all levels) exhibited by the optimized 342 SNP panel reflected those of the pilot analyses, effectively streamlining the GT-seq panel preparation process and helping to ensure that panel performance met management relevant benchmarks for accuracy, efficiency, and power.
When applied to 2016 and 2017 Okanagan River spawners previously analyzed using 32 SNP TaqMan® genotyping assays and otolith microchemistry, the optimized 342 SNP panel did result in a small number of shifts in assignment of hybrids to adjacent pure-stock classes or B2 backcross classes. One previously classified pure-stock sockeye salmon had a refined assignment (> 99% probability) to an F1 hybrid. Microchemistry revealed saltwater inhabitance for the both this individual and their maternal parent; the reclassification to F1 hybrid suggests that that the paternal parent was likely a kokanee rather than a sockeye (Supplementary Table S2). Individuals that were refined from hybrid to pure-stock sockeye salmon (n = 2) and kokanee (n = 4) all exhibited a high probability of assignment (> 75%), consistent microchemistry, and had larger body sizes than other fish in the hybrid class (Supplementary Table S2). Reclassification of F1 hybrids to pure-stock sockeye salmon did not display evidence of anadromy, suggesting they may be residual sockeye salmon with anadromous parentage, but did not migrate to sea56. Moreover, all of these newly classified pure-stock sockeye salmon had larger body sizes than the average hybrid individual (Supplementary Table S2).
Management implications
Sockeye salmon have been successfully re-introduced into the Okanagan Basin and comprised 41% of the O. nerka population by 201412. The proportion of sockeye salmon in this system since that peak has significantly varied over time (12–33%), likely due to a combination of factors such as the number of spawners and fry stocked, variable ocean survival, and high water temperatures during upstream migration57. The re-establishment of wild spawning sockeye salmon created the possibility of hybridization with resident kokanee in later years; our results demonstrate that hybrids most recently comprised 31% of the population (2019: 8% F1, 2% F2, 12% B2 backcross-kokanee, 9% B2 backcross-sockeye; Table 2), with advanced hybrid classes in this system present since at least 2016. Given these trends, further hybrid classes (ex: B3 backcross sockeye/kokanee) may develop, while the occurrence of pure-stock sockeye salmon is likely to remain due to on-going stocking programs using broodstock collected downstream from Skaha Lake.
Understanding the fitness outcomes of hybridization is important when considering the overall productivity of the system and long-term management goals. Our results and those of a previous study29 suggest that hybrid O. nerka overwhelmingly exhibit a resident life history, but can express a migratory life history with at least one F1 hybrid spending time in the marine environment (Supplementary Table S1). Progressive hybrid classes may also prefer a resident life history29 with similarities in size between F2 hybrids and B2 backcross-kokanee. However, it is still possible that backcrosses and F2 hybrids expressing anadromy were subject to increased mortality due to smaller size at migration or genomic incompatibilities, limiting their detection within the sampled pool of Okanagan River spawners. These trends are consistent with other salmonids, where cutthroat and steelhead trout hybrids have been found to exhibit intermediate migratory behaviors when compared to parental species that may be maladaptive to their local environment58. Such increased migration mortality in advanced hybrid classes expressing anadromy may signify a decrease of O. nerka productivity in the system. To examine the link between intermediate hybrid behavior and survival moving forward, a combination of identification and tracking methodologies may be appropriate, using passive integrated transponder (PIT) tags to mark juvenile movement, and subsequent comparison of recovered tags and genetic hybridization classes to evaluate survival and age at maturity59.
The population level outcomes arising from an intermediate body size in hybrids may ultimately be detrimental to the fitness of migratory hybrids compared to the larger-bodied sockeye salmon, as body size is often directly correlated with fecundity28. Conversely, the overall increase in body size of the resident population resulting from the presence of hybrids in the system may be advantageous for some management strategies, especially those that target increasing recreational angling quality or harvest opportunities for First Nations, as larger bodied fish generally have increased survival and are often more valued as a food source.
From a stock assessment perspective, our results emphasize that, though morphology and body size have been used historically to determine hybrid class in this system and others, advanced hybrid classes would be indistinguishable from pure-stock, with the B2 backcross-kokanee, F2 and kokanee classes having no significant mean size differences60. Therefore, if stock assessment programs and management goals require an estimate of stock composition, genetic tools such as GT-seq panels can be an effective enumeration method for their ability to accurately detect hybrid classes and expand sample sizes.
Here, we demonstrated the effectiveness of GT-seq for identifying advanced hybrid classes and, in this case, tracking the trajectory of sockeye salmon, kokanee, and hybrid stocks as part of a re-introduction program. Given the uncertainty in the long-term composition of the population and the range of potential outcomes and impacts on management goals, continued genetic monitoring of this system is recommended. More broadly, the GT-seq panel development workflow presented here could be applied to inform other sockeye salmon restoration initiatives in the Columbia and Fraser River systems, or in other species and systems where genetic stock identification and intra-specific hybridization are important management parameters.
Data availability
All probe sequences and SNP genotypic data collected via GT-seq have been deposited in DRYAD (https://doi.org/10.5061/dryad.z34tmpgg4).
References
Winston, M. R. & Taylor, C. M. Upstream extirpation of four minnow species due to damming of a prairie stream. Trans. Am. Fish. Soc. 120, 8 (1991).
Graham, K. Contemporary status of the North American paddlefish, Polyodon spathula. Environ. Biol. Fishes 48, 279–289 (1997).
Kaushal, S. S. et al. Rising stream and river temperatures in the United States. Front. Ecol. Environ. 8, 461–466 (2010).
Vörösmarty, C. J. et al. Global threats to human water security and river biodiversity. Nature 467, 555–561 (2010).
Galbreath, P. F., Bisbee, M. A., Dompier, D. W., Kamphaus, C. M. & Newsome, T. H. Extirpation and tribal reintroduction of coho salmon to the interior columbia river basin. Fisheries 39, 77–87 (2014).
Schmidt, B. A. et al. Determining habitat limitations of Maumee River walleye production to western Lake Erie fish stocks: Documenting a spawning ground barrier. J. Gt. Lakes Res. 46, 1661–1673 (2020).
Kendall, N. W., Marston, G. W. & Klungle, M. M. Declining patterns of Pacific Northwest steelhead trout (Oncorhynchus mykiss) adult abundance and smolt survival in the ocean. Can. J. Fish. Aquat. Sci. 74, 1275–1290 (2017).
Myers, J., Bryant, G. & Lynch, J. Factors Contributing to the Decline of Chinook Salmon: An Addendum to the 1996 West Coast Steelhead Factors for Decline Report (Springer, 1998).
Molony, B. W., Lenanton, R., Jackson, G. & Norriss, J. Stock enhancement as a fisheries management tool. Rev. Fish Biol. Fish. 13, 409–432 (2005).
Merz, J. E. & Setka, J. D. Evaluation of a spawning habitat enhancement site for Chinook salmon in a regulated California river. N. Am. J. Fish. Manag. 24, 397–407 (2004).
Ostberg, C. O., Chase, D. M. & Hauser, L. Hybridization between yellowstone cutthroat trout and rainbow trout alters the expression of muscle growth-related genes and their relationships with growth patterns. PLoS ONE 10, e0141373 (2015).
Veale, A. J. & Russello, M. A. Sockeye salmon repatriation leads to population re-establishment and rapid introgression with native kokanee. Evol. Appl. 9, 1301–1311 (2016).
Fraser, D. J., Cook, A. M., Eddington, J. D., Bentzen, P. & Hutchings, J. A. Mixed evidence for reduced local adaptation in wild salmon resulting from interbreeding with escaped farmed salmon: Complexities in hybrid fitness. Evol. Appl. 1, 501–512 (2008).
Stewart, G. S. et al. The power of evolutionary rescue is constrained by genetic load. Evol. Appl. 10, 731–741 (2017).
Weeks, A. R. et al. Genetic rescue increases fitness and aids rapid recovery of an endangered marsupial population. Nat. Commun. 8, 1071 (2017).
Chan, W. Y., Hoffmann, A. A. & van Oppen, M. J. H. Hybridization as a conservation management tool. Conserv. Lett. 12, e12652 (2019).
Bekkevold, D., Hansen, M. M. & Nielsen, E. E. Genetic impact of gadoid culture on wild fish populations: Predictions, lessons from salmonids, and possibilities for minimizing adverse effects. ICES J. Mar. Sci. 63, 198–208 (2006).
Muhlfeld, C. C. et al. Hybridization rapidly reduces fitness of a native trout in the wild. Biol. Lett. 5, 328–331 (2009).
Harvey, A. C., Glover, K. A., Taylor, M. I., Creer, S. & Carvalho, G. R. A common garden design reveals population-specific variability in potential impacts of hybridization between populations of farmed and wild Atlantic salmon, Salmo salar L. Evol. Appl. 9, 435–449 (2016).
Edmands, S. Does parental divergence predict reproductive compatibility?. Trends Ecol. Evol. 17, 520–527 (2002).
Johnson, B. M., Johnson, M. S. & Thorgaard, G. H. Salmon genetics and management in the Columbia river basin. Northwest Sci. 92, 346–363 (2019).
Hanson, A. J. & Smith, H. D. Mate selection in a population of sockeye salmon (Oncorhynchus nerka) of mixed age-groups. J. Fish. Board Can. 24, 23 (1967).
Wood, C. C. & Foote, C. J. Evidence for sympatric genetic divergence of anadromous and nonanadromous morphs of sockeye salmon (Oncorhynchus nerka). Evolution 50, 1265–1279 (1996).
Foote, C. J. Male mate choice dependent on male size in salmon. Behaviour 106, 63–80 (1988).
Craig, J. K., Foote, C. J. & Wood, C. C. Countergradient variation in carotenoid use between sympatric morphs of sockeye salmon (Oncorhynchus nerka) exposes nonanadromous hybrids in the wild by their mismatched spawning colour. Biol. J. Linn. Soc. 84, 287–305 (2005).
Taylor, E. B. & Foote, C. J. Critical swimming velocities of juvenile sockeye salmon and kokanee, the anadromous and non-anadromous forms of Oncorhynchus nerka (Walbaum). J. Fish Biol. 38, 407–419 (1991).
Foote, C. J., Wood, C. C., Clarke, W. C. & Blackburn, J. Circannual cycle of seawater adaptability in Oncorhynchus nerka: Genetic differences between sympatric sockeye salmon and kokanee. Can. J. Fish. Aquat. Sci. 49, 99–109 (1992).
Wood, C. C. & Foote, C. J. Genetic differences in the early development and growth of sympatric sockeye salmon and kokanee (Oncorhynchus nerka), and their hybrids. Can. J. Fish. Aquat. Sci. 47, 2250–2260 (1990).
Elliott, L. D., Ward, H. G. M. & Russello, M. A. Kokanee–sockeye salmon hybridization leads to intermediate morphology and resident life history: Implications for fisheries management. Can. J. Fish. Aquat. Sci. 77, 355–364 (2020).
Hendry, A. P., Quinn, T. P. & Utter, F. M. Genetic evidence for the persistence and divergence of native and introduced sockeye salmon (Oncorhynchus nerka) within Lake Washington, Washington. Can. J. Fish. Aquat. Sci. 53, 823–832 (1996).
Praebel, K. et al. A diagnostic tool for efficient analysis of the population structure, hybridization and conservation status of European whitefish (Coregonus lavaretus (L.)) and vendace (C. albula (L.)). Adv. Limnol. 64, 247–255 (2013).
Sanz, N., Araguas, R. M., Fernández, R., Vera, M. & García-Marín, J.-L. Efficiency of markers and methods for detecting hybrids and introgression in stocked populations. Conserv. Genet. 10, 225–236 (2009).
Mcfarlane, S. & Pemberton, J. Detecting the true extent of introgression during anthropogenic hybridization. Trends Ecol. Evol. 34, 315–326 (2019).
Vähä, J.-P. & Primmer, C. R. Efficiency of model-based Bayesian methods for detecting hybrid individuals under different hybridization scenarios and with different numbers of loci. Mol. Ecol. 15, 63–72 (2006).
Boecklen, W. J. & Howard, D. J. Genetic analysis of hybrid zones: Numbers of markers and power of resolution. Ecology 78, 2611–2616 (1997).
Elliott, L. & Russello, M. A. SNP panels for differentiating advanced-generation hybrid classes in recently diverged stocks: A sensitivity analysis to inform monitoring of sockeye salmon re-stocking programs. Fish. Res. 208, 339–345 (2018).
Twyford, A. D. & Ennos, R. A. Next-generation hybridization and introgression. Heredity 108, 179–189 (2012).
Campbell, N. R., Harmon, S. A. & Narum, S. R. Genotyping-in-Thousands by sequencing (GT-seq): A cost effective SNP genotyping method based on custom amplicon sequencing. Mol. Ecol. Resour. 15, 855–867 (2015).
Alexander, C. A. & Pickard, D. Skaha Lake Experimental Sockeye Reintroduction: Synthesis of First 4 of 12 Years (2004–2007 Brood Years) (Springer, 2009).
McQueen, D. et al. Evaluation of the Experimental Introduction of Sockeye Salmon (Oncorhynchus nerka) into Skaha Lake and Assessment of Sockeye Rearing in Osoyoos Lake (Springer, 2013).
Hegg, J. C., Kennedy, B. P. & Chittaro, P. What did you say about my mother? The complexities of maternally derived chemical signatures in otoliths. Can. J. Fish. Aquat. Sci. 76, 81–94 (2019).
Veale, A. J. & Russello, M. A. Genomic changes associated with reproductive and migratory ecotypes in sockeye salmon (Oncorhynchus nerka). Genome Biol. Evol. 9, 2921–2939 (2017).
Catchen, J., Hohenlohe, P. A., Bassham, S., Amores, A. & Cresko, W. A. Stacks: An analysis tool set for population genomics. Mol. Ecol. 22, 3124–3140 (2013).
Hohenlohe, P. A., Amish, S. J., Catchen, J. M., Allendorf, F. W. & Luikart, G. Next-generation RAD sequencing identifies thousands of SNPs for assessing hybridization between rainbow and westslope cutthroat trout. Mol. Ecol. Resour. 11, 117–122 (2011).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Weir, B. S. & Cockerham, C. C. Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (1984).
Rousset, F. genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 8, 103–106 (2008).
Anderson, E. C. & Thompson, E. A. A model-based method for identifying species hybrids using multilocus genetic data. Genetics 160, 1217–1229 (2002).
Schmidt, D. A., Campbell, N. R., Govindarajulu, P., Larsen, K. W. & Russello, M. A. Genotyping-in-Thousands by sequencing (GT-seq) panel development and application to minimally invasive DNA samples to support studies in molecular ecology. Mol. Ecol. Resour. 20, 114–124 (2020).
Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Reeves, P. A., Bowker, C. L., Fettig, C. E., Tembrock, L. R. & Richards, C. M. Effect of Error and Missing Data on Population Structure Inference Using Microsatellite Data. (2016) https://doi.org/10.1101/080630.
Wringe, B. F., Stanley, R. R. E., Jeffery, N. W., Anderson, E. C. & Bradbury, I. R. hybriddetective: A workflow and package to facilitate the detection of hybridization using genomic data in r. Mol. Ecol. Resour. 17, e275–e284 (2017).
Walsh, P. S., Metzger, D. A. & Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10, 506–513 (1991).
Russell, T. et al. Development of a novel mule deer genomic assembly and species-diagnostic SNP panel for assessing introgression in mule deer, white-tailed deer, and their interspecific hybrids. Genes Genomes Genet. 9, 911–919 (2019).
Thongda, W. et al. Species-diagnostic SNP markers for the black basses (Micropterus spp.): A new tool for black bass conservation and management. Conserv. Genet. Resour. 12, 319–328 (2020).
Ricker, W. E. ‘Residual’ and kokanee salmon in Cultus lake. J. Fish. Board Can. 27, 192–218 (1938).
Crossin, G. T. et al. Exposure to high temperature influences the behaviour, physiology, and survival of sockeye salmon during spawning migration. Can. J. Zool. 86, 127–140 (2008).
Moore, M. E. et al. Early marine migration patterns of wild coastal cutthroat trout (Oncorhynchus clarkii clarkii), steelhead trout (Oncorhynchus mykiss), and their hybrids. PLoS ONE 5, e12881 (2010).
McCutcheon, C. S., Prentice, E. F. & Park, D. L. Passive monitoring of migrating adult steelhead with PIT tags. N. Am. J. Fish. Manag. 14, 220–223 (1994).
Scribner, K. T., Page, K. S. & Bartron, M. L. Hybridization in freshwater fishes: A review of case studies and cytonuclear methods of biological inference. Rev. Fish Biol. Fish. 10, 293–323 (2001).
Acknowledgements
We would like to acknowledge the support of the Freshwater Fisheries Society of British Columbia and BC’s freshwater anglers for helping fund recreational fisheries research projects, through their license fees. We also thank the staff of the BC Ministry of Forests, Lands, Natural Resource Operations and Rural Development and their contractors for providing samples associated with this project. We are further grateful to Ty Russell (University of Alberta) for providing and assisting with custom code for hybriddetective sensitivity analyses. This work was funded by the Freshwater Fisheries Society of British Columbia.
Author information
Authors and Affiliations
Contributions
Conception: M.A.R. Study design: M.A.R., H.G.M.W., S.L.C. Data collection: S.L.C. Data analysis: S.L.C., L.D.E. Interpretation of the data: S.L.C., M.A.R., H.G.M.W. Drafting of the article: S.L.C., M.A.R., H.G.M.W. Critical revision of the article for important intellectual content: S.L.C., M.A.R., H.G.M.W., L.D.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, S.L., Ward, H.G.M., Elliott, L.D. et al. Genotyping-in-Thousands by sequencing panel development and application for high-resolution monitoring of introgressive hybridization within sockeye salmon. Sci Rep 12, 3441 (2022). https://doi.org/10.1038/s41598-022-07309-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07309-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.