Abstract
In yeast, glucose induction of HXT (glucose transporter gene) expression is achieved via the Rgt2 and Snf3 glucose sensing receptor (GSR)-mediated signal transduction pathway. The membrane-associated casein kinases Yck1 and Yck2 (Ycks) are involved in this pathway, but their exact role remains unclear. Previous work suggests that the Ycks are activated by the glucose-bound GSRs and transmit the glucose signal from the plasma membrane to the nucleus. However, here we provide evidence that the YCks are constitutively active and required for the stability of the Rgt2 receptor. Cell surface levels of Rgt2 are significantly decreased in a yck1Δyck2ts mutant, but this is not due to endocytosis-mediated vacuolar degradation of the receptor. Similar observations are made in an akr1Δ mutant, where the Ycks are no longer associated with the membrane, and in a sod1Δ mutant in which the kinases are unstable. Of note, in an akr1Δ mutant, both the Ycks and Rgt2 are mislocalized to the cytoplasm, where Rgt2 is stable and functions as an effective receptor for glucose signaling. We also demonstrate that Rgt2 is phosphorylated on the putative Yck consensus phosphorylation sites in its C-terminal domain (CTD) in a Yck-dependent manner and that this glucose-induced modification is critical for its stability and function. Thus, these results indicate a role for the Ycks in stabilizing Rgt2 and suggest that Rgt2 may use glucose binding as a molecular switch not to activate the Ycks but to promote Yck-dependent interaction and phosphorylation of the CTD that increases its stability.
Similar content being viewed by others
Introduction
Glucose serves as both a fuel for energy and a precursor for the biosynthesis of cellular building blocks such as amino acids, fatty acids, and nucleotides1,2. The budding yeast S. cerevisiae has a remarkable preference for glucose, because regulation of cellular function of glucose determines the organism’s distinctive fermentative metabolism—aerobic fermentation—observed in many kinds of tumor cells2,3,4,5,6. Fermentation is an energy-inefficient process, but it can proceed at a much faster rate, yielding a high glycolytic flux7. The resulting accumulated glycolytic intermediates serve as anabolic precursors required for the biosynthesis of macromolecules, facilitating mass accumulation, and thus accelerating cell proliferation8.
The yeast cells increase their glycolytic capacity, in part, by facilitating glucose uptake through glucose transporter (HXT) genes9,10. This is achieved through a glucose signaling pathway that begins at the cell surface with the glucose sensing receptors (GSRs) Rgt2 and Snf3 and ends in the nucleus with the Rgt1 repressor11,12,13,14. Rgt1 represses expression of the HXT genes in the absence of glucose by recruiting the HXT corepressors Mth1 and Std1 and the general co-repressor complex Ssn6-Tup1 to the HXT promoters in the absence of glucose15,16,17. Glucose induces degradation of Mth1 and Std1, causing Ssn6-Tup1 to dissociate from Rgt1 and allowing its phosphorylation by PKA (Protein Kinase A)15,18,19. Phosphorylated Rgt1 is dissociated from the HXT promoters, resulting in derepression of HXT genes20,21,22. Therefore, glucose-induced degradation of Mth1 and Std1 is the key event that enables induction of HXT gene expression.
Evidence showed that Mth1 and Std1 are ubiquitinated by the SCFGrr1 ubiquitin-protein ligase complex and degraded via the 26S proteasome in response to glucose18 and that this occurs in a GSR-dependent manner15,22. Many SCF substrates are phosphorylated prior to ubiquitination, and the plasma membrane-tethered casein kinases Yck1 and Yck2 (herein referred to as Ycks), the homologs of the casein kinase 1-gamma (CK1γ), were shown to be responsible for the phosphorylation of Mth1 and Std119. These results led to the view that the glucose-activated GSRs activate Ycks, which then catalyze phosphorylation of Mth1 and Std1, priming them for ubiquitination and subsequent degradation4,19,23. In this model of the GSR pathway, the Ycks act as downstream signal transmitters of the glucose receptors. More recently, however, the GSRs have been reported to be epistatic to the Ycks, placing the kinases upstream or at the level of the receptors in the GSR pathway24.
Glucose, as a signaling molecule, appears to play a key role in regulating cell surface levels of GSRs: Rgt2 is expressed in the plasma membrane when glucose is abundant, turning on glucose signaling; it is endocytosed and degraded in the vacuole in response to glucose depletion, turning off signaling25. Thus, the dynamic control of the cell surface levels of the GSRs is of fundamental importance in modulating the activity of the GSR pathway in response to different levels of extracellular glucose. However, the underlying mechanisms remain largely unknown.
Here, we have explored the differences between wild-type and the glucose sensing defective mutants, yck1Δyck2ts, akr1Δ, and sod1Δ, with respect to Rgt2 protein levels at the cell surface. Using the yeast two hybrid assays, we have assessed Rgt2 interaction with the Ycks. Finally, we have performed in vitro kinase assays to assess Yck1 activity in wild-type, rgt2Δsnf3Δ, RGT2-1, and SNF3-1 strains. Our results demonstrate a role for the Ycks in stabilizing Rgt2.
Results
The Ycks are required for the stability of the Rgt2 receptor
The Ycks are involved in the GSR-mediated glucose sensing and signaling, but their exact role remains unclear. To examine the role for the Ycks in the GSR pathway, we first assessed the cell surface levels of Rgt2 in cells lacking Yck activity (yck1Δyck2ts) by Western blot analysis. Rgt2-HA levels are significantly lower in yck1Δyck2ts cells grown on glucose (+) or galactose (−) as compared with wild-type cells (Fig. 1A). However, no significant difference is observed in transcriptional activity of the RGT2 promoter (measured with an RGT2-lacZ reporter), suggesting that the decreased Rgt2 protein levels in yck1Δyck2ts cells may be not due to transcriptional repression of the RGT2 gene (Fig. 1B). This is confirmed by expressing GFP-Rgt2 from the MET25 promoter, which is not regulated by glucose15. The membrane-bound GFP-Rgt2 levels in yck1Δyck2ts cells grown with or without glucose are found to be significantly low compared to those in wild-type cells (Fig. 1C, left). The rgt2 snf3 double mutant grows poorly on glucose-containing media26. This growth defect is complemented by expression of Rgt2-HA25 and GFP-Rgt2, suggesting that both Rgt2-HA and GFP-Rgt2 are functional (Fig. 1C, right).
The Ycks are required for the stability of Rgt2 at the cell surface. (A) Western blot analysis of Rgt2-HA protein levels in WT (LRB939) and yck1Δyck2ts (LRB1613) cells. C-terminally HA-tagged Rgt2-HA was expressed from the RGT2 promoter (PRGT2). Cells were grown in selective SC medium with 2% glucose to mid-log phase (O.D600nm = 1.2–1.5) and equal amounts of cells were shifted to SC medium containing glucose (2%) or galactose (2%) for 1 h. The yck1Δyck2ts cells were incubated at 37 °C for 30 min before the precultures were shifted to fresh glucose medium or galactose medium. Membrane fractions were immunoblotted with anti-HA antibody. Pgk1 was used as loading control (left). WT and yck1Δyck2ts cells were incubated at the permissive temperature 26 °C or the restrictive temperature 37 °C for 2 days (right). (B) RGT2-lacZ expression in WT and yck1Δyck2ts cells. β-Galactosidase activity was assayed in permeabilized cells and expressed in Miller Units. Values are means for at least three independent experiments. (C) Western blot analysis of GFP-Rgt2 protein levels in WT and yck1Δyck2ts cells. GFP-Rgt2 was expressed from the MET25 promoter (PMET25), which is not regulated by glucose15 (left). The growth defect of the rgt2 snf3 double mutant is complemented by expression of GFP-Rgt2 (right). The rgt2Δsnf3Δ mutant (MSY441) transformed with GFP-Rgt2 (a) and an empty vector (b) was scored for growth on SC- medium containing glucose (2%) or galactose (2%). (D) WT and yck1Δyck2ts cells expressing Rgt2-HA were grown as described in (A). Whole cell lysates were immunoprecipitated (IP) with agarose-conjugated anti-HA antibody, and the precipitates were analyzed by Western blotting with anti-HA antibody. PM, membrane fractions. (E) WT and yck1Δyck2ts cells expressing Rgt2-HA were grown in galactose (2%) to mid-log phase (O.D600nm = 1.2–1.5) at 26 °C, and the preculture was shifted to 37 °C for various periods of time as indicated before adding glucose (2%). Then, the cells were grown for 1 h, and Rgt2-HA levels were determined by immunoprecipitation and Western blotting. (F) The cells were grown as described above (E), but the preculture was shifted to 30 °C and 37 °C before adding glucose (2%). Membrane fractions were immunoblotted with anti-HA antibody. (G,H) Western blot analysis of Rgt2-HA protein levels in WT (BY4742) and sod1Δ (KLS62) cells. Membrane fractions were immunoblotted with anti-HA (G). Whole lysates of WT, yck1Δyck2ts, and sod1Δ cells were immunoprecipitated (IP) with agarose-conjugated anti-HA antibody, and the precipitates were analyzed by Western blotting with anti-HA antibody (H). (I) The Ycks are not involved in glucose starvation-induced endocytosis and degradation of Rgt2. Western blot analysis of Rgt2-HA protein levels in WT (LRB939) and yck1Δyck2ts (LRB1613), end3Δ (KFY127), and yck1Δyck2tsend3Δ (KLS95) cells. Membrane fractions were immunoblotted with anti-HA. Pgk1 was used as loading control.
To further explore the possibility of mislocalization of Rgt2 in a yck1Δyck2ts mutant, Rgt2-HA was immunoprecipitated from cell lysates and analyzed by Western blotting. The results reveal two distinct forms of Rgt2-HA from wild-type cells: a major slower-migrating (upper) band, corresponding to the membrane-bound Rgt2-HA (Fig. 1D, lane 1) and a minor faster-migrating (lower) band (Fig. 1D, lane 3). By contrast, Rgt2-HA from yck1Δyck2ts cells shows only the minor, lower band, implicating a role for the Ycks in the stability of Rgt2 at the cell surface.
However, the previous work by Snowdon and Johnston showed that Rgt2 levels are not significantly different between wild type and yck1Δyck2ts strains24. To address this discrepancy, we monitored changes of Rgt2 abundance after shifting yck1Δyck2ts cells from 26 °C to 37 °C for various periods of time. Rgt2-HA was immunoprecipitated from the cell extracts and analyzed by Western blotting. The results show that Rgt2-HA levels are decreased by ~ 50% within 30 min and indicate that Rgt2 stability may directly correlate with Yck activity (Fig. 1E). While we do not know the exact nature of this discrepancy, we shifted yck1Δyck2ts cells to a restrictive temperature (37 °C) before adding glucose to completely inactivate the kinases, whereas they shifted them to 30℃. However, we find that Rgt2 is unstable in yck1Δyck2ts cells at 30 °C (Fig. 1F).
Secondly, we examined Rgt2 levels in cells lacking Sod1, an upstream regulator of the Ycks. Previous work showed that Sod1 (Cu/Zn superoxide dismutase) interacts with and stabilizes the Ycks, and consequently, the kinases are undetectable in a sod1Δ mutant27. Thus, like yck1Δyck2ts cells, sod1Δ cells are unable to properly activate HXT127. Expectedly, we find very low levels of Rgt2-HA in sod1Δ cells, supporting the view that the Ycks may be required for the stability of Rgt2 (Fig. 1G,H).
Glucose starvation-induced Rgt2 endocytosis requires the EH-domain containing protein End325. Our results show that Rgt2 is apparently downregulated at the protein level in a yck1Δyck2ts mutant, but this does not occur through End3-mediated endocytosis (Fig. 1I, yck1Δyck2tsend3Δ). Thus, the Ycks may not be involved in this process.
Cytoplasmic Rgt2 is stable in an akr1Δ strain where Ycks are cytoplasmic
Akr1 is a palmitoyl transferase that tethers proteins to the plasma membrane28. The Ycks are targeted to the plasma membrane through palmitoylation of the C-terminal Cys-Cys sequence by Akr129. Since the yck1∆yck2∆ strain is not viable30, akr1 mutations are often used to model loss of the Ycks27,31. As reported previously31, GFP-Yck1 is found to be localized to the plasma membrane in wild-type cells and uniformly distributed throughout the cytoplasm in akr1Δ cells (Fig. 2A,B). When expressed in an akr1Δ strain, cell surface levels of Rgt2-HA are dramatically decreased (Fig. 2C), without noticeable changes in transcriptional activity of the RGT2 promoter, suggesting that the membrane-bound Rgt2 is downregulated at the protein level in akr1Δ cells (Fig. 2D).
Both Rgt2 and the Ycks are mislocalized to the cytoplasm in an akr1Δ strain, where the Ycks act to stabilize Rgt2. (A) Confocal microscopy of WT (BY474258) and akr1Δ (KLS61) strains expressing GFP-Yck1. Cells were grown in glucose (2%) or galactose (2%), as described in Fig. 1A. Cells were observed under the Zeiss LSM 510 META confocal laser scanning microscope. DIC and GFP fluorescence images are shown. (B,C) Western blot analysis of the membrane fractions prepared from the WT and akr1Δ strains expressing GFP-Yck1 (B) or Rgt2-HA (C). (D) RGT2-lacZ expression in WT and akr1Δ strains was determined as described in Fig. 1B. (E) WT and yck1Δyck2ts strains expressing Rgt2-HA were grown in glucose or galactose as described above. Whole cell lysates prepared from WT, akr1Δ, and yck1Δyck2ts strains were immunoprecipitated with agarose-conjugated anti-HA antibody, and the precipitates were analyzed by Western blotting with anti-HA antibody (IP). (F) WT and akr1Δ strains expressing GFP-Rgt2 were grown in glucose (2%) or galactose (2%) as described above and analyzed by confocal microscopy. Yeast cells were stained with FM4-64 to mark the vacuolar membrane and observed under the Zeiss LSM 510 META confocal laser scanning microscope. DIC and GFP fluorescence images are shown. (G) Membrane fractions prepared from WT and akr1Δ strains expressing GFP-Rgt2 were analyzed by Western blotting using anti-GFP antibody.
Interestingly, Rgt2-HA from akr1Δ cells are detected in immunoprecipitates of cell lysates and its levels are comparable to those of wild-type cell lysates, suggesting that Rgt2-HA is stable in the cytoplasm (Fig. 2E). This is confirmed by confocal microscopy. GFP-Rgt2 is localized in the plasma membrane of glucose-grown wild-type cells, with visible GFP signals in the vacuole, and targeted to the vacuole when the cells were shifted from glucose to galactose (Fig. 2F)25,32. However, GFP-Rgt2 expressed in akr1Δ cells is not properly localized to the plasma membrane but is distributed to the cytoplasm (Fig. 2F,G). Thus, both the Ycks and Rgt2 are accumulated in the cytoplasm of the akr1Δ mutant, where, we believe, the kinases act to protect Rgt2 from degradation, suggesting that the cytoplasmic Rgt2 may be stabilized by interacting with the Ycks.
Cytoplasmic Rgt2 functions as an effective receptor for glucose signaling
Next, we examined whether the cytoplasmic Rgt2 is functional by monitoring glucose signaling markers, including Mth1 degradation and HXT expression. These markers are blocked by yck1Δyck2ts mutations, confirming that the Ycks are required for glucose signaling (Fig. 3A,C). However, Mth1-myc levels and GFP-Mth1 signals are significantly decreased in response to high glucose in akr1Δ cells, indicating that the cytoplasmic Rgt2 is fully functional as a glucose receptor (Fig. 3A,B). Because extracellular glucose is unlikely to bind to the Rgt2 in the cytoplasm, we suspect that the cytoplasmic Rgt2 may directly interact with the cytoplasmic Ycks and that this interaction does not require glucose binding to Rgt2.
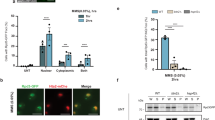
Glucose induces Mth1 degradation when both the Ycks and Rgt2 are present in the same cellular compartment. (A) Western blot analysis of the Mth1-myc protein from WT (LRB939), yck1Δyck2ts (LRB1613), and akr1Δ (KLS61) strains with HA-specific antibody. The indicated yeast strains expressing Mth1-myc were grown to mid-log phase in a selective medium containing 2% glucose. Aliquots were then transferred to 2% glucose medium (Glu) or 2% galactose medium (Gal) and incubated for 1 h. (B) Yeast cells of the indicated genotype expressing GFP-Mth1 were grown as described above. Subcellular localization of GFP-Mth1 was analyzed by confocal microscopy. Cells were observed under the Zeiss LSM 510 META confocal laser scanning microscope. DIC and GFP fluorescence images are shown. (C) Expression of HXT1 and HXT3 genes in yck1Δyck2ts and akr1Δ strains. β-Galactosidase activity was assayed in permeabilized cells and expressed in Miller Units. Values are means for at least three independent experiments.
Of note, glucose-induced Mth1 degradation does not lead to HXT1 expression in akr1Δ cells (Fig. 3C), consistent with the previous report that akr1Δ cells cannot properly activate HXT127. Glucose induces HXT gene expression by ultimately effecting the release of the Rgt1 repressor from the HXT promoters21. Two different glucose-induced events occur for Rgt1 to be released from the HXT promoters via two different glucose sensing pathways: degradation of Mth1 via the GSR pathway and, phosphorylation and inactivation of Rgt1 via the Gpr1-PKA pathway21. Thus, two glucose sensing pathways converge on Rgt1 to regulate expression of HXT genes. Furthermore, PKA phosphorylation of Rgt1 does not occur until Mth1 is degraded22. For this reason, we suspect that in the akr1Δ mutant Mth1 is degraded, but Rgt1 is not phosphorylated.
Rgt2 is phosphorylated in the C-terminal domain in a Yck-dependent manner
The Ycks phosphorylate and regulate the stability of many cell surface receptors and transporters33,34,35,36,37. To address whether the Ycks phosphorylate Rgt2, Rgt2-HA proteins from wild-type and yck1Δyck2ts mutant cells were treated with lambda phosphatase and analyzed by Western blotting. Rgt2 from wild-type cells migrates as two bands: a major slower-migrating (upper) band and a minor faster-migrating (lower) band (Fig. 4A, lanes 1 and 3). Phosphatase treatment causes the upper band to disappear and results in the accumulation of the lower band, indicating that the band shift is due to phosphorylation (Fig. 4A, lanes 2 and 4). However, Rgt2 from yck1Δyck2ts mutant cells does not clearly show the major upper band (Fig. 4A, lane 7); instead, it exhibits faint, smeared bands that are collapsed into a single, lower band upon treatment with phosphatase (Fig. 4A, lane 8), which migrates similarly to phosphatase-treated Rgt2 from wild-type cells (Fig. 4A, lane 2).
Yck-dependent phosphorylation of Rgt2 at its C-terminal domain. (A) The indicated yeast strains expressing Rgt2-HA were grown to mid-log phase in a selective medium containing 2% glucose. Rgt2-HA was immunoprecipitated from cell lysates, treated with lambda phosphatase (400 U) at 30 °C for 30 min, and analyzed by Western blotting with anti-HA antibody. (B) The C-terminal domain of Rgt2 contains two clusters of potential Yck phosphorylation sites, Cluster I and Cluster II (1). Cluster I is partially overlapped with the signaling motif identified26,40. Three Rgt2 mutant proteins lacking Cluster I (3), Cluster II (4), and both (2) were used in this study. (C) The wild type and mutant Rgt2-HA receptors were expressed in WT (LRB939) and yck1Δyck2ts (yck, LRB1613) strains grown on glucose (2%), immunoprecipitated from cell lysates, and analyzed by Western blotting. (D) Rgt2-HA was immunoprecipitated from cell lysates, treated with lambda phosphatase (10 U) at 30 °C for 30 min, and analyzed by Western blotting with anti-HA antibody. (E) The WT strain (BY4742) strain was cotransformed the HXT1-lacZ reporter (pBM3212) with plasmid expressing either the wild type Rgt2 or the indicated mutant Rgt2 proteins. Cells were grown in glucose (2%) or galactose (2%) to mid-log phase and cell extracts were used to assay β-galactosidase activity. (F) The PHXT1-hph reporter strain (KLS76) expressing indicated Rgt2-HA proteins was scored for growth in a SC-2% glucose plate supplemented with 200 µg/ml hygromycin. The first spot of each row represents a count of 5 × 107 cell/ml, which is diluted 1:10 for each spot thereafter.
Previous studies indicate that the C-terminal domains (CTDs) of the GSRs play an important role in glucose signaling26,38,39. The CTDs of the Rgt2 and Snf3 receptors are quite dissimilar, except for a stretch of 25 amino acids (called a signaling motif) that occurs once in the Rgt2-CTD and twice in the Snf3-CTD26,40. The Ycks catalyze phosphorylation of a serine or threonine residue in its consensus sequence (SXXS/T*, where the asterisk indicates the phosphorylated residue)41, and there are two clusters of potential Yck phosphorylation sites in Rgt2-CTD (Fig. 4B, Clusters I and II). To examine the effect of the deletion of these clusters on the stability, phosphorylation, and function of Rgt2, we expressed deletion constructs of individual motifs in wild-type and yck1Δyck2ts strains. Deletion of each cluster individually has minor or no effect on the stability of Rgt2 or its Yck-mediated phosphorylation (Fig. 4C,D). However, a deletion of up to 97-amino-acids from its C-terminus (Δ667–763) containing both clusters significantly reduces the stability of Rgt2-HA, and the resulting mutant Rgt2-HA expressed in wild-type cells migrates similarly to the protein when it is expressed in yck1Δyck2ts cells. Thus, this region may contain the Yck phosphorylation site(s).
Indeed, deletion of both clusters abolishes the ability of Rgt2 to activate the HXT1 promoter (measured by PHXT1-lacZ expression), whereas deletion of either cluster alone partially perturbs Rgt2 function, resulting in ~ 70% (cluster I) and ~ 28% (cluster II) reduced HXT1 expression, respectively (Fig. 4E). Thus, Cluster I may have a more important role than Cluster II in glucose signaling. Similarly, when expressed in the PHXT1-hph reporter strain, Rgt2 with the 97-amino acid deletion (Δ667–763) is unable to activate the HXT1 promoter, confirming an essential role for this region in glucose signaling (Fig. 4F).
Yck1 interacts with the C-terminal domain (CTD) of Rgt2 in the yeast two-hybrid system
To identify the region in Rgt2 that interacts with Yck1, we made deletions in the Rgt2-CTD (BD-Rgt2-CTD) and examined their interaction with palmitoylation–defective AD-Yck1 (lacking the palmitoylation sites 537Cys and 538Cys) in the yeast two-hybrid assay (Fig. 5A). Positive interaction between the two proteins was confirmed by expression of the reporter genes HIS3 and lacZ. We find that Yck1 interacts with the Rgt2-CTD and that this interaction is abolished by deletion of the C-terminal half of the Rgt2-CTD (Δ625–763) or the conserved signaling motif (Δ665–Δ696) (Fig. 5B). These results are interesting because the deletion of this motif has little effect on Rgt2 stability and abolishes the ability of Rgt2 to activate the PHXT1-lacZ reporter (Fig. 5C,D). Furthermore, expression of the Rgt2 receptor lacking this motif (Δ665–Δ696) does not activate the PHXT1-hph reporter (Fig. 5E) and hence cannot restore the growth defect of the rgt2Δsnf3Δ strain on glucose (Fig. 5F). This motif is known to be required for signaling function, but its role remains unknown26. Our results lead us to suggest that Rgt2 may use this motif to interact with the Ycks.
Rgt2 interacts with Yck1 through its C-terminal domain. (A) The yeast strain PJ69-4a was co-transformed with AD-YCK1 (JKP369) and either BD-RGT2 (JKP367) or indicated BD-RGT2 mutant constructs (JKP383, JKP387 and JKP416). (B) Positive interaction between AD-Yck1 and BD-Rgt2 was confirmed by expression of the GAL-HIS3 (− His + 3-AT) and GAL-lacZ reporters (β-galactosidase). (C) Western blot analysis of protein levels of wild-type Rgt2 (JKP253) and a truncated Rgt2 (Δ665–696, JKP408). Cells were grown in glucose (2%) or galactose (2%) and processed from Western blotting as described in Fig. 1A. (D) The WT strain (BY4742) strain was cotransformed the HXT1-lacZ reporter (pBM3212) with plasmid expressing either wild-type Rgt2 (JKP253) or a truncated Rgt2 (Δ665–696, JKP408). β-Galactosidase activity was assayed as described in Fig. 1B. (E,F) The PHXT1-hph reporter strain (KLS76) expressing indicated Rgt2-HA proteins was scored for growth in a SC-2% glucose plate supplemented with 200 µg/ml hygromycin (E). The rgt2 snf3 double mutant (MSY441) expressing indicated Rgt2-HA proteins were spotted on 2% glucose plate supplemented with Antimycin-A (AA, 1 µg/ml) and SC-2% galactose plate (F).
The Ycks are constitutively active and are not regulated by the glucose sensing receptors
Previous work suggested that glucose metabolism is not necessary for glucose signaling by the GSRs: there are dominant mutations in the GSR genes (RGT2-1 and SNF3-1) that cause the receptors to constitutively generate a glucose signal26,42. Based on these observations, it has been postulated that the GSRs undergo a conformational change in them in response to glucose that activates the associated Ycks19. However, whether the catalytic activity of the Ycks has not been experimentally proven. To address this question, we assessed the kinase activity of Yck1 (7His-ProA-Yck1) from wild-type, rgt2Δsnf3Δ, RGT2-1, and SNF3-1 cells using in vitro kinase assay.
If the Ycks are activated by the glucose-bound GSRs, we would expect Mth1 phosphorylation by Yck1 from wild-type cells grown on glucose, but not on galactose, and Yck1 from Rgt2-1 and Snf3-1 mutant cells grown on glucose or galactose. We also would expect Yck1 from rgt2Δsnf3Δ mutant cells not to phosphorylate Mth1. We find, however, that Mth1-myc is phosphorylated by His-ProA-Yck1 from all strains tested, grown on glucose or galactose (Fig. 6A).
The Ycks are constitutively expressed and active and are not regulated by glucose sensing receptors. (A) Mth1-9xmyc (pBM4560) prepared by immunoprecipitation was subjected to in vitro phosphorylation assays using the 7 × His-Protein A-tagged-Yck1(7His-ProA-Yck1, pBM4536) from WT (BY4742), rgt2Δsnf3Δ (YM6370), RGT2-1 (YM6545), and SNF3-1 ((YM6548) yeast strains in the presence of [γ32P] ATP, and the radiolabeled proteins were detected by autoradiography after separating them by SDS-PAGE. The indicated yeast strains expressing 7His-ProA-Yck1 were grown in 2% glucose (+) or 2% galactose (−) as described in Fig. 1A (top). 7His-ProA-Yck1 was immunoprecipitated from cell lysates and analyzed by Western blotting using anti-Protein A antibody as a probe (bottom). (B) Yeast cells (WT and rgt2Δsnf3Δ) expressing 7His-ProA-Yck1 were grown in SC-2% glucose (+) medium to mid-log phase and shifted to 2% galactose (−) medium with or without cycloheximide (CHX, 50 µg/ml) for 1 h. Membrane fractions were immunoblotted with anti-HA antibody. (C) WT (BY4742) and rgt2Δsnf3Δ (YM6370) strains expressing GFP-Yck1 were grown in glucose (2%) or galactose (2%) as described above and analyzed by confocal microscopy. Yeast cells were observed under the Zeiss LSM 510 META confocal laser scanning microscope. DIC and GFP fluorescence images are shown.
To examine whether YCK expression is regulated by the GSRs, we assessed protein levels of Yck1 in wild type and rgt2Δsnf3Δ strains using Western blotting and confocal microscopy. Protein levels of Yck1 (7His-ProA-Yck1) are not significantly different between the wild-type and mutant strains grown with or without glucose, and treatment of the protein synthesis inhibitor cycloheximide (CHX) does not affect Yck1 expression (Fig. 6B). To further explore transcriptional regulation of the YCK1 gene, Yck1 was expressed from the MET25 promoter, which is not regulated by glucose15. We find no significant differences in the protein levels of GFP-Yck1 between wild-type and rgt2Δsnf3Δ strains (Fig. 6C). These results provide evidence that the Ycks are constitutively active and that their catalytic activity is not stimulated by the GSRs.
Discussion
The Ycks are widely known as nutrient sensors that regulate cell surface abundance of many nutrient receptors and transporters33,35,36,37. The Ycks are required for glucose signaling through the GSR pathway, but their role is controversial19,24,31. Early studies suggest that glucose binding to the GSRs induces a conformational change in them that activates the protein kinase activity of the Ycks, which phosphorylates and inactivates the HXT corepressors Mth1 and Std1, direct targets of the GSR pathway19,26,42,43. These results indicate the role of the Yck as downstream kinases of the GSRs that transmit the glucose signal from the cell surface to the nucleus. However, here we provide evidence that the Ycks are not stimulated by the GSRs but are constitutively expressed and active. Mammalian Casein Kinases (CK1 and CK2) including CK1γ (mammalian homolog of Yck1 and Yck2) are constitutively active44, and their functions are regulated via targeting to specific subcellular locations45. Their activity could be modified by second messengers; however, this has not yet been documented in yeast. Instead, we find that Rgt2 is phosphorylated on the putative Yck consensus phosphorylation sites in its CTD in a Yck-dependent manner and that this phosphorylation increases its stability. Thus, the Ycks are likely to act upstream, but not downstream, of the GSRs.
Analysis of constitutively-signaling RGT2 mutations suggests that glucose binding to the GSRs may convert their structures from an outward-facing to an inward-facing, signaling conformation43. We believe that the glucose-bound Rgt2, which is in the signaling state, is phosphorylated and stabilized by Ycks. Interestingly, however, we also find that both Rgt2 and Yck1 expressed in an akr1Δ mutant are mislocalized to the cytoplasm, where Rgt2 remains stable and active as a functional receptor. Since many transporters and receptors are destroyed when removed from the plasma membrane46,47,48,49,50,51, it is plausible that the cytoplasmic Rgt2 is protected from destruction by interacting with the cytoplasmic Ycks. While this intracellular interaction may occur without binding of extracellular glucose to Rgt2, we cannot exclude the possibility of binding of intracellular glucose to the cytoplasmic side of the GSRs, as their glucose binding pocket may be accessible to either the outside or the inside of the cell43.
The Ycks regulate stability of many membrane transporters in different ways; Yck activity is required for membrane trafficking of the multidrug transporter Pdr5 to the cell surface36, whereas Yck phosphorylation of the uracil permease Fur4 facilitates its ubiquitination and internalization52. Rgt2 is endocytosed and degraded when glucose is removed from the medium25, but Yck may not be involved in this process. Rgt2 is found not to be properly localized to the plasma membrane but to be colocalized with Ycks to the cytoplasm in an akr1Δ mutant, suggesting a possible role for the Ycks in membrane targeting of Rgt2. One might argue that CTD phosphorylation of Rgt2 by the Ycks may be required for membrane localization of Rgt2 and that this phosphorylation does not occur in the akr1Δ mutant. However, Rgt2 from the akr1Δ mutant migrates similarly to Rgt2 from wild-type cells, indicating that the cytoplasmic Rgt2 may be fully phosphorylated in the akr1Δ mutant (Fig. 2E).
It is not currently known how the glucose signal generated by the GSRs is transmitted from the cell surface to the nucleus. Previous work suggested that the GSR-CTDs interact with Mth1 and Std1 to bring them to the vicinity of the Ycks19 or that Yck-dependent phosphorylation of the Rgt2-CTD stimulates Mth1 and Std1 interaction with the Rgt2 tail24. In this scenario, Mth1 and Std1 must shuttle between the nucleus and the plasma membrane, because they bind to Rgt1 in the nucleus and to the GSRs at the cell surface19,22. However, GFP-Mth1 is constitutively nuclear, and the glucose-induced Mth1 degradation occurs in the nucleus31. Furthermore, the Ycks are not necessary for glucose signaling in a strain overexpressing RGT2, indicating that the Ycks may not be the kinases responsible for phosphorylation and inhibition of Mth1 and Std124. These observations suggest the involvement of a yet unidentified kinase that catalyzes phosphorylation of Mth1 and Std1 in the nucleus.
Methods
Yeast strains and plasmid construction
The Saccharomyces cerevisiae strains used in this study were listed in Table 1. Yeast strains were grown on YP (2% bacto-peptone, 1% yeast extract) or synthetic yeast nitrogen base medium (0.17% yeast nitrogen base and 0.5% ammonium sulfate) supplemented with appropriate amino acids and carbon sources. Genes were disrupted by homologous recombination using the Hygromycin or KanMX cassette53,54. The plasmids used in this study were listed in Table 2. The plasmids were constructed by using standard molecular biology techniques as described previously25. Plasmids expressing truncated forms of Rgt2-HA were constructed by QuikChange Site-Directed Mutagenesis Kit (Stratagene) according to manufacturer’s protocol.
Yeast membrane preparation
Membrane fractions were essentially prepared, as described previously25,55. Briefly, after washing with phosphate buffer (pH 7.4), the cell pellet was resuspended in ice cold lysis buffer (100 mM Tris–Cl, pH 8, 150 mM NaCl, 5 mM EDTA) containing protease and phosphatase inhibitors and vortexed with acid-washed glass beads. After diluting the samples with the same buffer, membrane enriched fraction was collected by centrifuging the samples at 12,000 rpm for 40 min at 4 °C. The pellet was resuspended in the lysis buffer containing 5 M urea and incubated for 30 min on ice. After centrifuging at 14,000 rpm for 40 min at 4 °C, the pellet was dissolved in SDS buffer (50 mM Tris–HCl (pH, 6.8), 10% glycerol, 2% SDS, 5% β-mercaptoethanol).
Immunoprecipitation and Western blotting
Immunoprecipitation and Western blotting were carried out as described previously13. Briefly, yeast cells were disrupted by vortexing with acid-washed glass beads in ice-cold RIPA buffer (50 mM Tris–HCl, pH 7.5, 140 mM NaCl, 0.1% SDS, 1% NP-40, 0.25% sodium deoxycholate) containing protease and phosphatase inhibitors (10 mM Na-pyrophosphate, 200 µM Na-orthovanadate, 50 mM Na-flouride). The resulting cell lysates were incubated with appropriate antibodies at 4 °C for 3 h and further incubated with protein A/G–conjugated agarose beads at 4 °C for 1 h. The agarose beads were washed three times with RIPA buffer and boiled in SDS–PAGE buffer. The eluted proteins were subjected to Western blot analysis. For Western blotting, proteins were resolved by SDS-PAGE and transferred to polyvinylidene fluoride membrane (Millipore, Billerica, MA). The membranes were incubated with appropriate antibodies in TBST buffer (10 mM Tris–HCl, pH, 7.5, 150 mM NaCl, 1% Tween-20), and proteins were detected by the enhanced chemiluminescence (ECL) system (BioRad, Hercules, CA, USA) (Supplementary Information).
In vitro protein kinase assay
In vitro protein kinase assay was performed as described previously21. Mth1-9xMyc and 7X His-Protein A-tagged Yck1 were affinity-purified using agarose beads as described previously19 and mixed in 50 µl of kinase buffer containing 0.5 µCi of [γ32P] ATP, 100 µM ATP, 10 mM MgCl2 for 30 min. After washing the beads with the kinase buffer containing 0.5 M NaCl, the proteins were eluted by boiling the beads in SDS-sample buffer for 5 min. The eluted proteins were resolved by SDS-PAGE and detected by autoradiography. Each set of in vitro kinase assays was independently repeated twice.
Yeast two-hybrid assay
To construct Gal4 DNA-binding domain hybrids (GAL4-DBD-RGT2), the C-terminal domain of RGT2 (encoding amino acids 546–763) was amplified by PCR using JKP253 as a template, and the PCR products were incorporated into the GAL4-DBD plasmid56. These plasmids were combined with the GAL4 activation domain hybrid (GAL4-AD-YCK1) and used to transform the yeast strain PJ69-4A56 to Leu+Trp+. Cells were grown on selective medium (SC-leu-trp) medium lacking histidine (SC-leu-trp-his + 20 mM 3-AT) to detect expression of the GAL-HIS3 reporter or medium containing X-gal (SC-leu-trp + X-gal) to assay GAL-lacZ report gene expression.
β-Galactosidase assay
β-Galactosidase activity assays were performed using the yeast β-galactosidase assay kit (Pierce) according to the manufacturer’s instructions10. Results were presented in Miller Units ((1000 × A420)/(T × V × A600), where A420 is the optical density at 420 nm, T is the incubation time in minutes, and V is the volume of cells in milliliters). The reported lacZ activities are averages of results from triplicate of usually three different transformants.
Confocal microscopy
GFP-fusion proteins expressed in yeast cells were visualized using a Zeiss LSM 510 META confocal laser scanning microscope with a 63 × Plan-Apochromat 1.4 NA Oil DIC objective lens (Zeiss)57. All images documenting GFP localization were acquired with the Zeiss LSM 510 software version 3.2.
References
Ozcan, S. & Johnston, M. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63, 554–569 (1999).
Towle, H. C. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol. Metab. 16, 489–494. https://doi.org/10.1016/j.tem.2005.10.003 (2005).
Rolland, F., Winderickx, J. & Thevelein, J. M. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci. 26, 310–317 (2001).
Johnston, M. & Kim, J. H. Glucose as a hormone: Receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem. Soc. Trans. 33, 247–252. https://doi.org/10.1042/BST0330247 (2005).
Crabtree, H. G. Observations on the carbohydrate metabolism of tumours. Biochem. J. 23, 536–545 (1929).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Lagunas, R. Energetic irrelevance of aerobiosis for S. cerevisiae growing on sugars. Mol. Cell Biochem. 27, 139–146 (1979).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324, 1029–1033. https://doi.org/10.1126/science.1160809 (2009).
Ozcan, S. & Johnston, M. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol. Cell Biol. 15, 1564–1572 (1995).
Kaniak, A., Xue, Z., Macool, D., Kim, J. H. & Johnston, M. Regulatory network connecting two glucose signal transduction pathways in Saccharomyces cerevisiae. Eukaryot. Cell 3, 221–231 (2004).
Kim, J. H. DNA-binding properties of the yeast Rgt1 repressor. Biochimie 91, 300–303 (2009).
Kim, J. H. Immobilized DNA-binding assay, an approach for in vitro DNA-binding assay. Anal. Biochem. 334, 401–402 (2004).
Kim, J. H., Polish, J. & Johnston, M. Specificity and regulation of DNA binding by the yeast glucose transporter gene repressor Rgt1. Mol. Cell Biol. 23, 5208–5216 (2003).
Ozcan, S., Leong, T. & Johnston, M. Rgt1p of Saccharomyces cerevisiae, a key regulator of glucose-induced genes, is both an activator and a repressor of transcription. Mol. Cell Biol. 16, 6419–6426 (1996).
Kim, J. H., Brachet, V., Moriya, H. & Johnston, M. Integration of transcriptional and posttranslational regulation in a glucose signal transduction pathway in Saccharomyces cerevisiae. Eukaryot. Cell 5, 167–173 (2006).
Lakshmanan, J., Mosley, A. L. & Ozcan, S. Repression of transcription by Rgt1 in the absence of glucose requires Std1 and Mth1. Curr. Genet. 44, 19–25 (2003).
Schmidt, M. C. et al. Std1 and Mth1 proteins interact with the glucose sensors to control glucose-regulated gene expression in Saccharomyces cerevisiae. Mol. Cell Biol. 19, 4561–4571 (1999).
Flick, K. M. et al. Grr1-dependent inactivation of Mth1 mediates glucose-induced dissociation of Rgt1 from HXT gene promoters. Mol. Biol. Cell 14, 3230–3241 (2003).
Moriya, H. & Johnston, M. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. U. S. A. 101, 1572–1577 (2004).
Jouandot, D. 2nd., Roy, A. & Kim, J. H. Functional dissection of the glucose signaling pathways that regulate the yeast glucose transporter gene (HXT) repressor Rgt1. J. Cell Biochem. 112, 3268–3275. https://doi.org/10.1002/jcb.23253 (2011).
Kim, J. H. & Johnston, M. Two glucose-sensing pathways converge on Rgt1 to regulate expression of glucose transporter genes in Saccharomyces cerevisiae. J. Biol. Chem. 281, 26144–26149 (2006).
Roy, A., Shin, Y. J., Cho, K. H. & Kim, J. H. Mth1 regulates the interaction between the Rgt1 repressor and the Ssn6-Tup1 corepressor complex by modulating PKA-dependent phosphorylation of Rgt1. Mol. Biol. Cell https://doi.org/10.1091/mbc.E13-01-0047 (2013).
Kim, J. H., Roy, A., Jouandot, D. 2nd. & Cho, K. H. The glucose signaling network in yeast. Biochim. Biophys. Acta 1830, 5204–5210. https://doi.org/10.1016/j.bbagen.2013.07.025 (2013).
Snowdon, C. & Johnston, M. A novel role for yeast casein kinases in glucose sensing and signaling. Mol. Biol. Cell 27, 3369–3375. https://doi.org/10.1091/mbc.E16-05-0342 (2016).
Kim, J. H. & Rodriguez, R. Glucose regulation of the paralogous glucose sensing receptors Rgt2 and Snf3 of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta Gen. Subj. 1865, 129881. https://doi.org/10.1016/j.bbagen.2021.129881 (2021).
Ozcan, S., Dover, J. & Johnston, M. Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J. 17, 2566–2573 (1998).
Reddi, A. R. & Culotta, V. C. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell 152, 224–235. https://doi.org/10.1016/j.cell.2012.11.046 (2013).
Roth, A. F., Feng, Y., Chen, L. & Davis, N. G. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 159, 23–28 (2002).
Babu, P., Deschenes, R. J. & Robinson, L. C. Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting. J. Biol. Chem. 279, 27138–27147 (2004).
Robinson, L. C. et al. Yeast casein kinase I homologues: An essential gene pair. Proc. Natl. Acad. Sci. U. S. A. 89, 28–32 (1992).
Pasula, S., Chakraborty, S., Choi, J. H. & Kim, J. H. Role of casein kinase 1 in the glucose sensor-mediated signaling pathway in yeast. BMC Cell Biol. 11, 17. https://doi.org/10.1186/1471-2121-11-17 (2010).
Roy, A. et al. The glucose metabolite methylglyoxal inhibits expression of the glucose transport genes by inactivating the cell surface glucose sensors Rgt2 and Snf3 in yeast. Mol. Biol. Cell https://doi.org/10.1091/mbc.E15-11-0789 (2016).
Hicke, L., Zanolari, B. & Riezman, H. Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J. Cell Biol. 141, 349–358 (1998).
Roth, A. F., Sullivan, D. M. & Davis, N. G. A large PEST-like sequence directs the ubiquitination, endocytosis, and vacuolar degradation of the yeast a-factor receptor. J. Cell Biol. 142, 949–961 (1998).
Marchal, C., Haguenauer-Tsapis, R. & Urban-Grimal, D. Casein kinase I-dependent phosphorylation within a PEST sequence and ubiquitination at nearby lysines signal endocytosis of yeast uracil permease. J. Biol. Chem. 275, 23608–23614. https://doi.org/10.1074/jbc.M001735200 (2000).
Decottignies, A., Owsianik, G. & Ghislain, M. Casein kinase I-dependent phosphorylation and stability of the yeast multidrug transporter Pdr5p. J. Biol. Chem. 274, 37139–37146 (1999).
Gadura, N., Robinson, L. C. & Michels, C. A. Glc7-Reg1 phosphatase signals to Yck 1,2 casein kinase 1 to regulate transport activity and glucose-induced inactivation of Saccharomyces maltose permease. Genetics 172, 1427–1439 (2006).
Dlugai, S., Hippler, S., Wieczorke, R. & Boles, E. Glucose-dependent and -independent signalling functions of the yeast glucose sensor Snf3. FEBS Lett. 505, 389–392 (2001).
Coons, D. M., Vagnoli, P. & Bisson, L. F. The C-terminal domain of Snf3p is sufficient to complement the growth defect of snf3 null mutations in Saccharomyces cerevisiae: SNF3 functions in glucose recognition. Yeast 13, 9–20. https://doi.org/10.1002/(SICI)1097-0061(199701)13:1%3c9::AID-YEA51%3e3.0.CO;2-U (1997).
Marshall-Carlson, L., Celenza, J. L., Laurent, B. C. & Carlson, M. Mutational analysis of the SNF3 glucose transporter of Saccharomyces cerevisiae. Mol. Cell Biol. 10, 1105–1115 (1990).
Pearson, R. B. & Kemp, B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: Tabulations. Methods Enzymol. 200, 62–81. https://doi.org/10.1016/0076-6879(91)00127-i (1991).
Ozcan, S., Dover, J., Rosenwald, A. G., Wolfl, S. & Johnston, M. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc. Natl. Acad. Sci. U. S. A. 93, 12428–12432 (1996).
Scharff-Poulsen, P., Moriya, H. & Johnston, M. Genetic analysis of signal generation by the Rgt2 glucose sensor of Saccharomyces cerevisiae. G3 (Bethesda) 8, 2685–2696. https://doi.org/10.1534/g3.118.200338 (2018).
Knippschild, U. et al. The casein kinase 1 family: Participation in multiple cellular processes in eukaryotes. Cell Signal 17, 675–689 (2005).
Kim, Y. B., Shin, Y. J., Roy, A. & Kim, J. H. The role of the pleckstrin homology domain-containing protein CKIP-1 in activation of p21-activated Kinase 1 (PAK1). J. Biol. Chem. 290, 21076–21085. https://doi.org/10.1074/jbc.M115.675124 (2015).
Gitan, R. S., Luo, H., Rodgers, J., Broderius, M. & Eide, D. Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J. Biol. Chem. 273, 28617–28624 (1998).
Liu, J., Sitaram, A. & Burd, C. G. Regulation of copper-dependent endocytosis and vacuolar degradation of the yeast copper transporter, Ctr1p, by the Rsp5 ubiquitin ligase. Traffic 8, 1375–1384. https://doi.org/10.1111/j.1600-0854.2007.00616.x (2007).
Nikko, E., Sullivan, J. A. & Pelham, H. R. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 9, 1216–1221. https://doi.org/10.1038/embor.2008.199 (2008).
Roy, A., Kim, Y. B., Cho, K. H. & Kim, J. H. Glucose starvation-induced turnover of the yeast glucose transporter Hxt1. Biochem. Biophys. Acta. 2878–2885, 2014. https://doi.org/10.1016/j.bbagen.2014.05.004 (1840).
Snowdon, C. & van der Merwe, G. Regulation of Hxt3 and Hxt7 turnover converges on the Vid30 complex and requires inactivation of the Ras/cAMP/PKA pathway in Saccharomyces cerevisiae. PLoS One 7, e50458. https://doi.org/10.1371/journal.pone.0050458 (2012).
Krampe, S., Stamm, O., Hollenberg, C. P. & Boles, E. Catabolite inactivation of the high-affinity hexose transporters Hxt6 and Hxt7 of Saccharomyces cerevisiae occurs in the vacuole after internalization by endocytosis. FEBS Lett. 441, 343–347 (1998).
Marchal, C., Dupre, S. & Urban-Grimal, D. Casein kinase I controls a late step in the endocytic trafficking of yeast uracil permease. J. Cell Sci. 115, 217–226 (2002).
Wach, A., Brachat, A., Pohlmann, R. & Philippsen, P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793–1808 (1994).
Goldstein, A. L. & McCusker, J. H. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541–1553. https://doi.org/10.1002/(SICI)1097-0061(199910)15:14%3c1541::AID-YEA476%3e3.0.CO;2-K (1999).
Galan, J. M., Moreau, V., Andre, B., Volland, C. & Haguenauer-Tsapis, R. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J. Biol. Chem. 271, 10946–10952 (1996).
James, P., Halladay, J. & Craig, E. A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436 (1996).
Pasula, S., Jouandot, D. 2nd. & Kim, J. H. Biochemical evidence for glucose-independent induction of HXT expression in Saccharomyces cerevisiae. FEBS Lett. 581, 3230–3234. https://doi.org/10.1016/j.febslet.2007.06.013 (2007).
Brachmann, C. B. et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132 (1998).
Acknowledgements
This work was supported by the National Institutes of Health Grant R15GM134447 to JHK.
Author information
Authors and Affiliations
Contributions
J.K. designed and performed the research, analyzed data, and wrote the paper and D.B., R.R., E.M., L.M., S.M. and D.J. performed the research. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, JH., Bloor, D., Rodriguez, R. et al. Casein kinases are required for the stability of the glucose-sensing receptor Rgt2 in yeast. Sci Rep 12, 1598 (2022). https://doi.org/10.1038/s41598-022-05569-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05569-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.