Abstract
The phase angle is a versatile measurement to assess body composition, frailty and prognosis in patients with chronic diseases. In cirrhosis, patients often present alterations in body composition that are related to adverse outcomes. The phase angle could be useful to evaluate prognosis in these patients, but data are scarce. The aim was to analyse the prognostic value of the phase angle to predict clinically relevant events such as hospitalisation, falls, and mortality in patients with cirrhosis. Outpatients with cirrhosis were consecutively included and the phase angle was determined by electrical bioimpedance. Patients were prospectively followed to determine the incidence of hospitalisations, falls, and mortality. One hundred patients were included. Patients with phase angle ≤ 4.6° (n = 31) showed a higher probability of hospitalisation (35% vs 11%, p = 0.003), falls (41% vs 11%, p = 0.001) and mortality (26% vs 3%, p = 0.001) at 2-year follow-up than patients with PA > 4.6° (n = 69). In the multivariable analysis, the phase angle and MELD-Na were independent predictive factors of hospitalisation and mortality. Phase angle was the only predictive factor for falls. In conclusion, the phase angle showed to be a predictive marker for hospitalisation, falls, and mortality in outpatients with cirrhosis.
Similar content being viewed by others
Introduction
Patients with cirrhosis are predisposed to adverse outcomes, such as admissions to hospitals and long-term care centres, falls and fractures, and premature mortality1,2,3,4. These outcomes are related not only to the degree of liver insufficiency but also to frailty2,5,6. Recent years have seen growing interest in the frailty syndrome in association with chronic diseases, including cirrhosis, regardless of patients’ age5,6,7. Frailty in patients with cirrhosis is understood as a condition of weakness or lability due to physical and psychological deterioration related to the progression of the disease, the development of complications, and associated comorbidities1,2,5,6.
In addition to the adverse outcomes, other findings related to the frailty syndrome that are often present in patients with cirrhosis are functional and cognitive deterioration, impairment in health-related quality of life, immune system disturbances, and alterations in body composition2,5,7,8,9,10,11. Patients with cirrhosis frequently present alterations in body composition related to frailty and prognosis, such as sarcopenia or decreased muscle mass and/or function, decreased bone mass, and excess water and fat12,13,14,15.
Electrical bioimpedance is a simple, non-invasive, and innocuous method to estimate body composition. It is based on the passage of a weak, alternating, electrical current through the body in order to measure the voltage generated. The ratio of voltage to current is the impedance and can be expressed as a complex vector with two components: the electrical resistance (R) and the reactance (Xc). Resistance is linked to the opposition of the electrical current to flow through intra- and extracellular ionic solutions of muscle and fat, and the reactance is linked to the integrity of cell membranes and tissue interfaces. Alternatively, the impedance vector can be expressed as a phase angle (PA)11,14,16,17,18,19. Regression analysis has been used to derive empiric equations with the use of R and Xc together with anthropometric measurements, such as height and weight, to predict body compartment volumes16. The advantage of the PA is that it makes the measurement of any anthropometric parameter unnecessary and is a global indicator of the body composition. Measurement of the PA is fast and portable, and unlike imaging techniques such as CT scan, it does not involve ionizing radiation11,14,20. These characteristics allow it to be easily integrated into daily clinical practice11.
It has been suggested that the PA is a more precise tool than other nutritional, biochemical or anthropometric parameters to assess frailty and prognosis in patients with chronic diseases11,18,21,22,23. Its usefulness as a prognostic factor to predict encephalopathy episodes and mortality in patients with cirrhosis has been demonstrated in previous studies11,14,19,24.
The aim of our study was to analyse the prognostic value of the PA to predict clinically relevant events, such as hospitalisation, falls, and mortality in patients with cirrhosis.
Results
Characteristics of patients according to PA
Between February 2015 and January 2018 we evaluated 123 consecutive outpatients with cirrhosis. Twenty-three were excluded based on exclusion criteria. Characteristics of the remaining 100 patients are shown in the first column of Table 1. During a mean follow-up of 34.2 (14.8) months, two patients were referred to another centre to evaluate the possibility of liver transplantation and seven were lost to follow-up (Fig. 1). Considering the main outcomes, 30% of patients required hospitalisation, 23% presented falls, and 15% died. Using a ROC curve we found the best cut-off value for PA to predict mortality at 2-year follow-up was 4.6° (AUC 0.768, 95%CI 0.574–0.962, p = 0.008) with a sensitivity of 75% and specificity of 78%. Twenty-five per cent of patients were considered frail according to the Fried frailty criteria, and the best cut-off value to identify frail patients was also 4.6° (AUC 0.713, 95%CI 0.582–0.833, p = 0.001) with a sensitivity of 85% and specificity of 56%.
As shown in Table 1, patients with a PA ≤ 4.6° (n = 31) were older and had more advanced liver insufficiency, lower serum albumin and more impaired renal function than patients with PA > 4.6° (n = 69). Moreover, patients with a PA ≤ 4.6° were more frail, as shown by the higher number of Fried frailty criteria, a higher Timed Up&Go test, slower gait speed, and lower handgrip strength. Regarding estimated body compartments, patients with a PA ≤ 4.6° showed higher extracellular water and lower muscle mass and body cell mass than patients with PA > 4.6° (Supplementary Table S1).
Potential biomarkers of frailty are shown in Table 2. Patients with a PA ≤ 4.6° showed lower levels of vitamin D and higher levels of cystatin C, TNF-α and IL-10 than patients with PA > 4.6°.
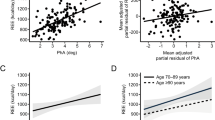
The PA showed a positive correlation with handgrip strength (r = 0.40, p < 0.001) and serum albumin levels (r = 0.32, p = 0.001), and with testosterone concentration in men (r = 0.31, p = 0.02). However, it showed a negative correlation with age (r = −0.25, p = 0.01), the Fried frailty criteria (r = −0.38, p < 0.001), Child–Pugh score (r = −0.30, p = 0.002), MELD-Na score (r = −0.26, p = 0.008), cystatin C levels (r = −0.37, p = 0.001) and TNF-α concentration (r = −0.25, p = 0.02).
The characteristics of patients classified as frail or non-frail according to the Fried frailty criteria are shown in Supplementary Table S2. Frail patients showed a higher body mass index (probably suggesting sarcopenic obesity), a higher degree of comorbidity, more frequent previous falls and present ascites, and more impaired Timed Up&Go test, gait speed, handgrip strength and PA. However, there were no statistical differences between the two groups in age or in the degree of liver insufficiency as assessed by the Child–Pugh and MELD-Na scores. Regarding biomarkers, frail patients showed lower levels of vitamin D [12.4 (9.5) vs 23.7 (23.6) nmol/L, p = 0.04] and higher serum cystatin C [1.5 (0.5) vs 1.2 (0.3) mg/L, p = 0.005] than non-frail patients.
Incidence of composite endpoint, hospitalisation, falls and mortality during follow-up according to PA
At 2-year follow-up (Table 3), patients with PA ≤ 4.6° had a higher incidence of the composite endpoint, hospitalisation and falls. They also had higher mortality throughout follow-up and at 2-year follow-up than patients with PA > 4.6°. Patients with PA ≤ 4.6° also needed more days in hospital, mainly due to complications of cirrhosis, and they presented a higher incidence of new episodes of ascites and hepatic encephalopathy. The incidence of variceal bleeding, infections or hepatocellular carcinoma, however, was similar in both groups. Supplementary Table S3 details the causes of hospitalisation at 2-year follow-up.
Regarding the injuries caused by falls, six patients with PA ≤ 4.6° had contusions, two had wounds and eight had fractures, while among patients with PA > 4.6°, five patients had contusions, one had a wound and seven had fractures (p = 0.06 vs patients with PA ≤ 4.6°). When considering healthcare needs due to falls, two patients with PA ≤ 4.6° were treated at a primary health care centre, three at the emergency room, and six were hospitalised. Among patients with PA > 4.6°, one patient was treated at a primary health care centre, four at an emergency room, and three required hospitalisation (p = 0.02 vs patients with PA ≤ 4.6°). Considering the whole series, comparing patients ≥ 65 years old (n = 48) and those < 65 years old (n = 52), we found that the incidence of falls at 2-year follow-up (20.8% vs 15.4%, respectively, p = 0.60) and throughout follow-up (25% vs 21.2%, p = 0.80) was only slightly higher in the former.
Mortality was higher in patients with a PA ≤ 4.6° than in those with PA > 4.6°. The causes of death in patients with PA ≤ 4.6° were cirrhosis-related in six patients (including one patient with hepatocellular carcinoma) and extrahepatic neoplasia in three patients. The causes of death in patients with PA > 4.6° were cirrhosis-related in five patients (including three patients with hepatocellular carcinoma) and acute myocardial infarction in one patient. Supplementary Table S3 details the causes of death at 2-year follow-up.
At 2-year follow-up, patients with PA ≤ 4.6° showed a higher probability of composite endpoint (56% vs 16%, p < 0.001), hospitalisation (35% vs 11%, p = 0.003) and falls (41% vs 11%, p = 0.001), and lower survival (74% vs 97%, p = 0.001) than patients with PA > 4.6° (Fig. 2).
Multivariable analyses
Univariable and multivariable analyses are shown in Supplementary Table S4 and Table 4, respectively. Among all the factors that reached statistical significance in the univariable analysis, the PA ≤ 4.6° was the only independent predictive factor for the composite end-point at 2-year follow-up. The PA ≤ 4.6° and the MELD-Na score were the independent predictive factors of hospitalisation at 2-year follow-up. The PA ≤ 4.6° was the only predictive factor for falls, and PA ≤ 4.6° and the MELD-Na score were the independent predictive factors of mortality at 2-year follow-up. Due to a potential lack of statistical power an internal validation was made to test possible over-optimistic estimates in discriminative ability by bootstrap technique, and the results confirmed the predictive ability of PA.
Incidence of composite endpoint, hospitalisation, falls and mortality during follow-up according to MELD-Na and PA
Figure 3 shows the probability of the composite endpoint, hospitalisation and falls, and survival classifying patients according to MELD-Na and PA. The cut-off for MELD-Na score was chosen according to the AUC of ROC curve for survival. As seen in this figure, the determination of the PA added predictive value to the MELD-Na score in several endpoints. This finding was confirmed by the results of the integrated discrimination improvement (IDI) and continuous net reclassification improvement (NRI) analyses (Supplementary Table S5).
Discussion
The main finding in this study was the predictive value of the phase angle (PA) for hospitalisation, falls and mortality in outpatients with cirrhosis.
Determining the PA from the relationship between resistance and reactance by means of electrical bioimpedance allows a global assessment of body composition, nutrition and cell membranes integrity11,16,17,18,19,20. It therefore fits well with the holistic concept of frailty, a topic receiving increasing interest in patients with chronic diseases, including cirrhosis, as a prognostic factor and a target for therapeutic and prophylactic interventions2,5,6,7. In the present study patients with a low PA not only showed worse liver and renal function than those with a high PA, but also showed more impaired parameters of frailty according to indicators such as the Timed Up&Go test, gait speed, handgrip strength, and the Fried frailty criteria. The Fried frailty criteria have been widely used to evaluate frailty in several populations, including patients with cirrhosis, where they showed to be a predictive factor for hospitalisation, falls, and mortality2,5,7. However, Fried frailty criteria are time-consuming and non-specific for patients with cirrhosis. It is of note that since the present study was designed, faster and liver-specific indexes, particularly the Liver Frailty Index, have been developed and are now more commonly used than the Fried frailty criteria in this setting1,5,6.
Regarding biomarkers of frailty, patients with a low PA showed lower levels of vitamin D and higher levels of cystatin C, TNF-α and IL-10 than patients with a high PA. Vitamin D deficiency has been related to frailty and falls in the elderly25 and to frailty syndrome2 and mortality26 in patients with cirrhosis. An increase in cystatin C would reflect worse renal function and may be a predictive factor of mortality in these patients27. The increased TNF-α and IL-10 in patients with a low PA in comparison with those with a high PA suggests a more marked proinflammatory state in the former, and inflammation has been associated to frailty in the elderly28 and to prognosis in patients with cirrhosis29.
The PA has been shown to predict mortality in several populations, such as the elderly30 and patients with cancer21, chronic renal failure22 or heart failure23. In patients with cirrhosis, several authors have previously reported the value of PA to detect malnutrition13 and sarcopenia11, and as a predictive factor of hepatic encephalopathy14,24 or mortality11,19,31. However, the cut-off values used vary: 4.4° 32, 4.9° 13,19, 5.1° 31, 5.4° 32, 5.6° in males and 5.4° in females11. The probable explanation for these variations is the differences in the study populations and the different outcomes for which the cut-off was created. We used a cut-off of 4.6° because it gave the best area under the curve to predict mortality during follow-up in our population. Interestingly, 4.6° was also the best cut-off to identify frail patients according to Fried frailty criteria.
In the present study, besides mortality, we assessed other outcomes such as hospitalisations, admissions to a long-term care centre, and falls. These outcomes are relevant to patients with cirrhosis and they are also related to the frailty syndrome and sarcopenia1,2,4,5,6,7,33,34,35. Moreover, we used a composite endpoint including all these outcomes2.
We observed that patients with a low PA presented a higher incidence of the composite endpoint and—in agreement with previous studies—a higher mortality than patients with a high PA11,19,31. In addition, patients with a low PA also showed a higher incidence of hospitalisation and falls during follow-up when we evaluated these two outcomes separately. In the multivariable analysis, the PA was a relevant independent predictive factor of mortality, hospitalisation, and falls. This finding supports the value of a global quantitative evaluation of body composition using electrical bioimpedance in addition to the conventional scores assessing the degree of liver insufficiency, comorbidities and frailty, when determining prognosis in patients with cirrhosis2,5,6,15. Interestingly, PA improved the predictive value of the MELD-Na score to predict the composite endpoint, hospitalisation and mortality, and furthermore, PA was a predictive factor for falls while the MELD-Na score was not.
When analysing the different complications of cirrhosis during follow-up, we observed a higher incidence of ascites and hepatic encephalopathy in patients with PA ≤ 4.6° than in those with PA > 4.6°. This higher incidence of ascites in patients with a a low PA probably reflects a greater hyperhidration in these patients, as suggested by the increase in extracellular water estimated by electrical bioimpedance. Regarding hepatic encephalopathy, a lower PA would reflect a more sarcopenic status and therefore greater predisposition to this complication due to the difficulty in the clearance of ammonia by the muscle9,24. The decrease in muscle mass estimated by electrical bioimpedance and the lower handgrip strength observed in patients with PA ≤ 4.6° in comparison with patients with PA > 4.6° supports this hypothesis. We did not observe differences between the two groups regarding the incidence of variceal bleeding, infections or hepatocellular carcinoma, probably because these complications are less likely to be influenced by body composition.
Falls and fractures are a relevant adverse outcome in patients with cirrhosis4,33,35 and were more frequent in patients with a PA ≤ 4.6° than in those with a PA > 4.6°. Furthermore, fractures were a frequent cause of hospitalisation. Again, a more sarcopenic status in patients with a low PA could contribute to explaining these findings. In effect, patients with a low PA did worse in the Timed Up&Go test and gait speed, both tests evaluating the risk of falls. They also had a non-significant worse performance in the PHES than patients with a high PA that could also have contributed to the higher incidence of falls4,33. Considering the whole series, we found no statistical differences in the incidence of falls between patients ≥ 65 years old and those < 65 years old, and age was not a predictive factor for falls in the univariable analysis. This finding suggests that factors such as neurocognitive abnormalities and sarcopenia play a more relevant role than age in the predisposition to falling in patients with cirrhosis33,35.
Determining the PA by electrical bioimpedance has several advantages over other approaches to assess body composition or frailty, such as anthropometry, dual-energy X-ray absorptiometry, CT scan, Fried frailty criteria and other similar instruments. Electrical bioimpedance is simple, faster (it takes less than 5 min), less intrusive and more objective than anthropometry. Furthermore, it uses a portable, relatively non-expensive device and does not require radiation11,14,20.
Beyond the prognostic value of a single determination of the PA as observed in the present and other studies11,19,32, the sequential determination of the PA could be a simple and useful method to monitor the evolution of patients and the effect of interventions targeting frailty. In a recent longitudinal study, Lai et al36 reported that worsening or improvement of frailty—assessed by the Liver Frailty Index—affected prognosis independently of baseline frailty and MELD-Na. Regarding the effect of therapeutic interventions, it has been observed that the PA can change after therapeutic paracentesis14 or after treatment with a combination of nutritional therapy and supervised exercise37.
Our study has several limitations. First, the main limitation is the small sample size that precludes reliable subanalyses, such as by gender, age, previous decompensation of cirrhosis, or the presence of ascites, and limit the value of the multivariable analyses. Second, as commented earlier, PA can change during follow-up and we did not perform sequential evaluations. Aiming to minimize this limitation we focused our analysis on the first two years of follow-up. It would be interesting to evaluate the changes in the PA during follow-up and their prognostic significance. Third, although the incidence of adverse outcomes in our study was not negligible, particularly in patients with a low PA, the study population consisted of outpatients with well-preserved liver function. Therefore, our results can not necessarily be extrapolated to patients with more impaired liver function. Other authors, however, have demonstrated the prognostic value of the PA in patients with more advanced liver disease19. Fourth, estimating the different body compartments (i.e. extracellular body water and muscle mass) from reactance, resistance and PA using a monofrequency impedance meter in patients with cirrhosis is probably inaccurate and only valid as an approximation11. A multifrequency device could be more accurate to evaluate the specific body compartiments in these patients14,17. Finally, our study lacks a validation cohort. However, bootstrap analysis showed consistent results in internal validation. Further studies with a higher number of patients are necessary to validate the findings of the present study.
We conclude that the PA appears to be a simple and useful tool that can help to predict adverse outcomes such as hospitalisations, falls and mortality in outpatients with cirrhosis and preserved liver function. Identifying high-risk patients allows implementation of preventive strategies with the aim of improving survival and health-related quality of life.
Patients and methods
Design of study
This prospective observational study was performed in consecutive outpatients with cirrhosis visited at Hospital de la Santa Creu i Sant Pau, a tertiary care centre in Barcelona, Spain.
At inclusion to the study, we recorded demographic data and data regarding liver disease, including aetiology, degree of liver insufficiency and previous decompensations, and comorbidities. We assessed PA using electrical bioimpedance11,30, frailty2,7, risk of falls33,34,38, comorbidity2,39, cognitive function40,41, and potential biomarkers of frailty2,25,26,27,28,42,43,44. The incidence of hospitalisation, falls and mortality was prospectively evaluated and predictive factors were analyzed.
Study participants
Participants were selected from among all consecutive outpatients with cirrhosis visited at the nursing outpatient clinic at Hospital de la Santa Creu i Sant Pau. The inclusion criteria were: patients aged ≥ 18 years diagnosed of liver cirrhosis by means of liver biopsy, or by clinical, analytical and ultrasonographic findings. The exclusion criteria were similar to those used by our group in previous studies2,4,33: age < 18 years, severe hepatic insufficiency (Model for end-stage liver disease [MELD] > 25), hospital admission in the previous month, hepatocellular carcinoma or any other active neoplastic disease, expected survival < 6 months, active alcohol intake (< 3 months), functional disability for activities of daily living (Barthel < 80%), moderate or severe cognitive dysfunction (Short portable mental status questionnaire [Pfeiffer test] ≥ 5 points), and overt hepatic encephalopathy.
PA by electrical bioimpedance
Using the total body impedance meter BIA 101 at a signal frequency of 50 kHz and the software BodyGram TM 1.31 (Akern, Florence, Italy) we recorded the electrical resistance (R), reactance (Xc) and PA for each patient, and estimated body compartments after adjusting for age, sex, weight and height. Measurements were made in supine position with 4 conventional electrodes: 2 on the wrist and 2 on the ipsilateral foot. Indications before the test were no food or drink in the previous 4 h, no exercise in the previous 12 h, an empty bladder and removal of jewellery and clothes with metallic elements11,30.
Fried frailty criteria
Frailty was defined on the basis of Fried’s five indicators from the Cardiovascular Health Study2,7: unintentional weight loss, reduced handgrip strength, slow gait speed, self-reported exhaustion, and low physical activity. The assessment of each criterion is detailed in Supplementary information.
Risk of falls
The risk of falling was assessed using the Timed Up&Go test and gait speed33,34. The Timed Up&Go test measures the time the patient needs to stand up from a chair, walk 3 m, turn around, walk back and sit down again in the chair without support33,38. Gait speed was calculated according to the time taken to walk 5 m34.
Comorbidity
The degree of comorbidity was assessed using a modified Charlson index2,39, excluding cirrhosis from the calculation (Supplementary information).
Cognitive function
We assessed cognitive function using the Psychometric hepatic encephalopathy score (PHES) battery, which is widely used to assess cognitive function in patients with cirrhosis. This battery consists of the following neuropsychological tests: the Number Connection Test A and B, the Digit Symbol Test, the Serial Dotting Test and the Line Tracing Test40,41. Patients with a PHES score < − 4 points were considered to have minimal hepatic encephalopathy41.
Potential biomarkers of frailty
At inclusion to the study, blood samples were stored at −80 °C for later determination of potential biomarkers of frailty, consisting of vitamin D, cystatin C, C-reactive protein, interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), interleukin-10 (IL-10), nitrites and nitrates, myostatin and testosterone in men2,25,26,27,28,42,43,44. C-reactive protein and cystatin C were measured by turbidimetric immunoassay (Architect Laboratories, USA), vitamin D was measured by liquid chromatography-tandem mass spectrometry (commercially available kit; Zivak Technologies, Turkey) and testosterone was measured by electrochemiluminescent immunoassay (cobas e601; Roche Diagnostics GmbH, Manheim, Germany). IL-6 (ImmunoTools, Friesoythe, Germany), TNF-α (BD Biosciences Pharmingen, San Diego, CA), IL-10 (BD Biosciences Pharmingen, San Diego, CA) and myostatin (CUSABIO Tech., Houston, TX, USA) were measured by ELISA, and nitrites and nitrates were measured using the Total Nitric Oxide and Nitrate/Nitrite Parameter Assay Kit (R&D Systems, Minneapolis, USA).
Prospective evaluation of composite endpoint
We analyzed a composite endpoint that consisted of the main frailty-related outcomes in the general population: unexpected hospitalisation (we excluded planned hospitalizations for elective procedures), admission to a long-term care centre, falls, and mortality. These outcomes are especially relevant in patients with cirrhosis2,4,5,6,7,33. Patients were considered to present the composite endpoint when any of these four outcomes occurred.
Prospective evaluation of incidence of falls during follow-up
Patients were prospectively evaluated through outpatient visits or phone calls every 3 months. During these evaluations we assessed the incidence of falls, the number of falls per patient, the severity of injuries, and the healthcare needed for falls, using a specific questionnaire2,4,33 (Supplementary information). Fall injuries were classified as contusion, wound or fracture. Healthcare needed was classified as primary care, emergency room care or hospitalisation2,4,33.
Prospective evaluation of hospitalisations, admission to a long-term care centre and mortality during follow-up
Unexpected hospitalisations, admission to a long-term care centre, and mortality were prospectively assessed at each 3-monthly outpatient visit or phone call, and by review of medical records. We also determined the incidence of the main complications of cirrhosis during follow-up.
Statistical analyses
Data are expressed as frequencies, percentages, and mean with SD. To compare categorical variables between two groups we used Fisher's exact test or the Chi2 test. To compare quantitative variables between two groups, we used the Student's "t" test if the variable presented a normal distribution and the Mann–Whitney test if it did not. The normality of the variables was assessed using the Kolmogorov–Smirnov test or the Shapiro–Wilk test. Correlations were assessed by the Pearson test or the Spearman test, according to data presented normal distribution or not, respectively.
We used the area under the Receiver Operating Characteristics (ROC) curves to determine the best cut-off value (maximising sensitivity and specificity) for a given parameter to predict an outcome.
Sample size was calculated according to previous data on mortality during follow-up in patients with cirrhosis and low PA (43%) and patients with high PA (16%)13. With 5% of patients lost during follow-up, an alpha error of 0.05, and a power of 0.80, 45 patients per group were necessary to show statistically significant differences in mortality during follow-up.
The probability of the composite endpoint or transplant-free survival was estimated by Kaplan–Meier curves and compared by log rank test. We used competing risk methods to estimate the probability of hospitalisation or falls during follow-up using the cumulative incidence function approach, in a context of competing risk analyses. The competing event was death during the study period. Patients who were submitted to another centre to evaluate the option of liver transplantation and those who were lost to follow-up were censored at the most recent visit to our center.
Variables with a p-value < 0.05 were included in the multivariable models. A backward elimination method was used to identify independent predictors of events. Factors predicting the composite endpoint or mortality were assessed by the Cox regression model and those predicting hospitalisation or falls were assessed by the Fine-Gray regression model in a competing risk scenario. The competing event for these analyses was death during the study period. The proportional hazard assumption was evaluated by the Schoenfeld residuals test. The discriminative ability of the models was assessed by the C-index. The internal validity of the final models was tested for 500 bootstrap resamples, using the “pec” package in the R Project for Statistical Computing. The integrated discrimination improvement (IDI) and continuous net reclassification improvement (NRI) indexes were implemented to quantify the added predictive value of PA45.
A 2-sided P value < 0.05 was considered statistically significant.
Statistical analyses were performed using the program released in 2017 IBM SPSS Statistics for Windows, Version 25.0; IBM Corp, Armonk, NY, United States; and R package (R Core Group, Version 3.6.1, 2019). Sample size was calculated using the computer program GRANMO version 7.12, released in 2012 and developed by the Institut Municipal d'Investigació Mèdica, Barcelona, Spain.
Ethics declaration
The study conformed to the 1975 Declaration of Helsinki and Guidelines for Good Clinical Practice and was approved by the Clinical Research Ethics Committee at our institution (Comitè Ètic d’Investigació Clínica [CEIC] at Hospital de la Santa Creu i Sant Pau). All patients received information regarding their participation in the study and signed an informed consent form.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Tandon, P. et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: A prospective evaluation of the Clinical Frailty Scale. Am. J. Gastroenterol. 111, 1759–1767 (2016).
Román, E. et al. Frailty in outpatients with cirrhosis: A prospective observational study. Liver Int. 41, 357–368 (2021).
Morales, B. P. et al. Early hospital readmission in decompensated cirrhosis: Incidence, impact on mortality, and predictive factors. Dig Liver Dis. 49, 903–909 (2017).
Román, E. et al. Minimal hepatic encephalopathy is associated with falls. Am. J. Gastroenterol. 106, 476–482 (2011).
Kok, B. & Tandon, P. Frailty in patients with cirrhosis. Curr. Treat Options Gastroenterol. 16, 215–225 (2018).
Lai, J. C. et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology 156, 1675–1682 (2019).
Fried, L. P. et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146-156 (2001).
Román, E. et al. Falls and cognitive dysfunction impair health-related quality of life in patients with cirrhosis. Eur. J. Gastroenterol. Hepatol. 25, 77–84 (2013).
Córdoba, J. & Minguez, B. Hepatic encephalopathy. Semin. Liver Dis. 28, 70–80 (2008).
Montano-Loza, A. J. et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 10, 166–173 (2012).
Ruiz-Margáin, A. et al. Phase angle from bioelectrical impedance for the assessment of sarcopenia in cirrhosis with or without ascites. Clin. Gastroenterol. Hepatol. S1542–3565(20), 31225–31228 (2020).
Montano-Loza, A. J. et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia Sarcopenia Muscle. 7, 126–135 (2016).
Ruiz-Margáin, A. et al. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: A prospective cohort study. Dig. Liver Dis. 47, 309–314 (2015).
Ontanilla-Clavijo, G., Ampuero, J., Borreguero, S., Rosell, J. & Romero-Gómez, M. Usefulness of bioelectrical impedance analysis for monitoring patients with refractory ascites. Rev. Esp. Enferm. Dig. 111, 223–227 (2019).
Tapper, E. B. et al. Body composition predicts mortality and decompensation in compensated cirrhosis patients: A prospective cohort study. JHEP Rep. 2, 100061 (2019).
Piccoli, A., Pillon, L. & Dumler, F. Impedance vector distribution by sex, race, body mass index, and age in the United States: Standard reference intervals as bivariate Z scores. Nutrition 18, 153–167 (2002).
Kyle, U. G. et al. Bioelectrical impedance analysis—part I: Review of principles and methods. Clin. Nutr. 23, 1226–1243 (2004).
Kyle, U. G., Genton, L. & Pichard, C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin. Nutr. 32, 294–299 (2013).
Belarmino, G. et al. Phase angle obtained by bioelectrical impedance analysis independently predicts mortality in patients with cirrhosis. World J. Hepatol. 9, 401–408 (2017).
Molfino, A., Johnson, S. & Medici, V. The challenges of nutritional assessment in cirrhosis. Curr. Nutr. Rep. 6, 274–280 (2017).
Norman, K., Wirth, R., Neubauer, M., Eckardt, R. & Stobäus, N. The bioimpedance phase angle predicts low muscle strength, impaired quality of life, and increased mortality in old patients with cancer. J. Am. Med. Dir. Assoc. 16(173), e17-22 (2015).
Mushnick, R. et al. Relationship of bioelectrical impedance parameters to nutrition and survival in peritoneal dyalisis patients. Kidney Int. 64, S53–S56 (2003).
Colín-Ramírez, E. et al. Bioelectrical impedance phase angle as a prognostic marker in chronic heart failure. Nutrition 28, 901–905 (2012).
Ruiz-Margáin, A. et al. Low phase angle is associated with the development of hepatic encephalopathy in patients with cirrhosis. World J. Gastroenterol. 22, 10064–10070 (2016).
Neelemaat, F. et al. Vitamin D decreases falls in malnourished older adults. J. Am. Geriatr. Soc. 60, 691–699 (2012).
Finkelmeier, F. et al. Low 25-hydroxyvitamin D levels are associated with infections and mortality in patients with cirrhosis. PLoS ONE 10, e0132119 (2015).
Jo, S. K. et al. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci. Rep. 9, 14508 (2019).
Payette, H. et al. Insulin-like growth factor-I and interleukin-6 predict sarcopenian very old community-living men and women: The Framingham Study. J. Am. Geriatr. Soc. 51, 1237–1243 (2003).
Arroyo, V. et al. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol. 74, 670–685 (2021).
Wilhelm-Leen, E. R., Hall, Y. N., Horwitz, R. I. & Chertow, G. M. Phase angle, frailty and mortality in older adults. J. Gen. Intern. Med. 29, 147–154 (2014).
Pagano, A. P. et al. Phase angle as a severity indicator for liver diseases. Nutrition 70, 110607 (2020).
Selberg, O. & Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 86, 509–516 (2002).
Soriano, G. et al. Cognitive dysfunction in cirrhosis is associated with falls: A prospective study. Hepatology 55, 1922–1930 (2012).
Dunn, M. A. et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complication that require hospitalization. Am. J. Gastroenterol. 111, 1768–1775 (2016).
Ezaz, G., Murphy, S. L., Mellinger, J. & Tapper, E. B. Increased morbidity and mortality associated with falls among patients with cirrhosis. Am. J. Med. 131, 645–650 (2018).
Lai, J. C. et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J. Hepatol. 73, 575–581 (2020).
Macías-Rodríguez, R. U. et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: Results of a pilot randomized open clinical trial. Clin. Transl. Gastroenterol. 7, e180 (2016).
Viccaro, L. J., Perera, S. & Studenski, S. Is timed up and go better than gait speed in predicting health, function, and falls in older adults?. J. Am. Geriatr. Soc. 59, 887–892 (2011).
Charlson, M. E., Pompei, P. & Ales, K. L. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383 (1987).
Weissenborn, K., Ennen, J. C., Schomerus, H., Rückert, N. & Hecker, H. Neuropsychological characterization of hepatic encephlopathy. J. Hepatol. 34, 768–773 (2001).
Romero-Gómez, M. et al. Normality tables in the Spanish population for psychometric tests used in the diagnosis of minimal hepatic encephalopathy. Med. Clin. (Barc). 127, 246–249 (2006).
Serviddio, G. et al. Frailty syndrome is associated with altered circulating redox balance and increased markers of oxidative stress. Int. J. Immunopathol. Pharmacol. 22, 819–827 (2009).
McKay, B. R., Ogborn, D. I., Bellamy, L. M., Tarnopolsky, M. A. & Pariseet, G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 26, 2509–2521 (2012).
Sinclair, M., Grossmann, M., Hoermann, R., Angus, P. W. & Gow, P. J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J. Hepatol. 65, 906–913 (2016).
Pencina, M. J., D’Agostino, R. B. Sr., D’Agostino, R. B. Jr. & Vasan, R. S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 27, 157–172 (2008).
Acknowledgements
We thank all the patients participating in this research project and Carolyn Newey for English language revision. The study was supported by grant PI12/00629 from the Instituto de Salud Carlos III, Madrid, Spain.
Author information
Authors and Affiliations
Contributions
E.R., G.S., M.P., E.A., B.C. and C.Gu. were involved in the design of the study and enrolment and follow-up of patients. E.R., C.Ge. and E.H. performed electrical bioimpedance, Fried frailty criteria, cognitive function, risk of falls, blood and urine samples, follow-up and database. J.C.N., E.U. and S.V. performed the analysis of biomarkers. A.F.G. contributed with statistical analysis. G.S., E.R., M.P. and G.A. were involved in the analysis of the results and manuscript writing. J.R., S.V., R.S., C.Gu. and A.E. contributed with a critical review of the manuscript. All the authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Román, E., Poca, M., Amorós-Figueras, G. et al. Phase angle by electrical bioimpedance is a predictive factor of hospitalisation, falls and mortality in patients with cirrhosis. Sci Rep 11, 20415 (2021). https://doi.org/10.1038/s41598-021-99199-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99199-8
This article is cited by
-
Role of phase angle in older adults with focus on the geriatric syndromes sarcopenia and frailty
Reviews in Endocrine and Metabolic Disorders (2023)
-
The use of phase angle in patients with digestive and liver diseases
Reviews in Endocrine and Metabolic Disorders (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.