Abstract
The Wnt pathway activates target genes by controlling the β-catenin-T-cell factor (TCF) transcriptional complex during embryonic development and cancer. This pathway can be potentiated by R-spondins, a family of proteins that bind RNF43/ZNRF3 E3 ubiquitin ligases and LGR4/5 receptors to prevent Frizzled degradation. Here we demonstrate that, during Xenopus anteroposterior axis specification, Rspo2 functions as a Wnt antagonist, both morphologically and at the level of gene targets and pathway mediators. Unexpectedly, the binding to RNF43/ZNRF3 and LGR4/5 was not required for the Wnt inhibitory activity. Moreover, Rspo2 did not influence Dishevelled phosphorylation in response to Wnt ligands, suggesting that Frizzled activity is not affected. Further analysis indicated that the Wnt antagonism is due to the inhibitory effect of Rspo2 on TCF3/TCF7L1 phosphorylation that normally leads to target gene activation. Consistent with this mechanism, Rspo2 anteriorizing activity has been rescued in TCF3-depleted embryos. These observations suggest that Rspo2 is a context-specific regulator of TCF3 phosphorylation and Wnt signaling.
Similar content being viewed by others
Introduction
The Wnt pathway is a key conserved developmental pathway that is utilized multiple times during animal development and frequently misregulated in disease1,2. Secreted Wnt proteins associate with Frizzled (Fzd) receptors and LRP5/6 coreceptors to stabilize β-catenin and promote β-catenin/T-cell factor (TCF)-dependent transcription. TCF3, also known as TCF7L1, is a predominant embryonic TCF that functions as a transcriptional repressor during early development3,4,5. In the presence of Wnt ligands, TCF3 is phosphorylated, followed by its dissociation from target promoters and transcriptional activation that can involve other TCF/LEF transcription factors including TCF1/TCF76,7. Whereas many studies of the Wnt pathway mainly focused on the control of β-catenin stability, the regulation of TCF protein activity has been less understood.
R-spondins are prominent extracellular modulators of Wnt signaling in vertebrates8. The R-spondin (Rspo) family consists of four secreted proteins that share high similarity of amino acid sequence and structural organization and play critical roles in development, stem cell biology and cancer9,10. Mice lacking the rspo2 gene die at birth due to lung, limb and craniofacial defects, illustrating its essential functions in embryogenesis11,12,13,14. Additionally, Rspo2 has been implicated in fish skeletogenesis15 and frog muscle development16. The closely related Rspo3 functions in early angiogenesis in mouse and Xenopus embryos17,18. These observations highlight the important functions of R-spondins during embryonic development.
R-spondins are thought to exert their effects by potentiating Wnt/β-catenin signaling9,10,12,16. R-spondins bind LGR4/5 receptors and the E3 ubiquitin ligases ZNRF3/RNF43, thereby stabilizing Frizzled and promoting Wnt signaling19,20,21,22. Recent analysis revealed that the mechanisms used by R-spondins to modulate Wnt signaling are more complex23,24. R-spondins can affect the Wnt pathway independently of LGR4/525,26, indicating the existence of multiple receptors and alternative signaling pathways. Supporting this view, the interaction with heparan sulfate chains is sufficient for R-spondins to modulate Wnt signaling in cells lacking LGR4/5/6 receptors27.
The Wnt pathway plays crucial and specific roles during anteroposterior axis specification and patterning28,29,30. Wnt signals promote posterior structures in the embryo, whereas secreted Wnt antagonists in the anterior region are responsible for head development31,32. One of the reported gain-of-function phenotypes for Rspo2 in Xenopus is the formation of ectopic cement gland16, an anterior mucus-secreting organ33,34. Notably, this phenotype is a common property of Wnt antagonists including GSK331, Axin-related protein35 and is exhibited in embryos with depleted β-catenin36. Since this observation is contrary to what is expected of a Wnt coactivator, we decided to reevaluate a role of Rspo2 in the Wnt pathway during Xenopus anteroposterior patterning. We show that Rspo2 inhibits Wnt signaling in a manner that is independent of the LGR4/5 and ZNRF3/RNF43 interactions. Mechanistically, we find that Rspo2 downregulates TCF3 phosphorylation that is necessary for target gene activation. Our findings indicate that the same R-spondin can function in a context-dependent manner to either stimulate or inhibit the Wnt pathway.
Results
Rspo2 is essential for anterior development
To better characterize the role of Rspo2 in anteroposterior patterning, Rspo2 RNA was injected into early embryos. Confirming earlier findings16,37, the injected embryos developed enlarged cement gland and other head structures (Fig. 1A,B). We next defined early genes induced by Rspo2 by carrying out transcriptome analysis in the ectoderm explants expressing Rspo2 RNA at the onset of gastrulation. We observed the induction of many anterior genes, including otx1, otx2, and otx5, zic3, rax (Supplementary Fig. 1). RT-qPCR validated the induction of otx2 and ag134, whereas the level of krt12.4, epidermal keratin, has decreased (Fig. 1C). Otx genes are required for anterior development and cement gland formation38,39, suggesting that they could be responsible for the observed Rspo2 activity.
Rspo2 function is essential for anterior development. (A,B) Four-cell embryos were injected with 0.5 ng of Rspo2 RNA into both animal-ventral blastomeres and cultured until stage 28. (A) Uninjected control embryo. (B) Representative embryo injected with Rspo2 RNA. The penetrance is indicated as the ratio of the number of embryos with the phenotype and the total number of injected embryos. (C) The effect of Rspo2 on gene marker expression. Animal pole explants were dissected at stage 9 from embryos overexpressing Rspo2 RNA or uninjected controls. RT-qPCR analysis was carried out for otx2, ag1, and krt12.4 at stage 18. (D) Altered gene expression in Rspo2 morphants. RNA was isolated from stage 18 control embryos or embryos depleted of Rspo2. RT-qPCR for ag1 and otx2 was carried out in triplicates. (C,D) Each graph is a single experiment with triplicate samples, representative from at least 3 independent experiments. Means + /− s. d. are shown. Statistical significance has been assessed by Student’s t test, *, p < 0.05. (E–J) In situ hybridization of control and manipulated stage 16 or 25 embryos with krt12.4 (E–G), foxg1 and cdx4 (H–J) probes. (E–G) Width of the anterior neural plate is shown as lack of krt12.4 (arrows). (H–J) The foxg1 domain is indicated by white arrows, the anterior region lacking cdx4—by dashed lines. See Supplementary Table 1 for quantification.
In complementary experiments, Rspo2 has been depleted using previously characterized translation-blocking (RMOATG) and splicing-blocking (RMOSB) morpholino oligonucleotides (MOs)37. Both MOs strongly reduced otx2 and ag1 levels (Fig. 1D), causing severe head defects37. Because RMOATG was more effective for the Rspo2 knockdown, it has been predominantly used in subsequent experiments.
Examination of ectodermal markers in Rspo2-overexpressing embryos by in situ hybridization revealed the expansion of the anterior neural plate at the expense of epidermal keratin krt12.4 in embryos overexpressing Rspo2 (Fig. 1E,F, Supplementary Table 1). By contrast, the anterior neural domain was reduced in Rspo2 morphants (Fig. 1G, Supplementary Table 1). Similarly, the domains of foxg1 and cdx4 expression have been coordinately regulated by Rspo2 manipulation (Fig. 1H–J). Taken together, these observations illustrate an essential role of Rspo2 in anterior development.
We next evaluated whether the observed effect of Rspo2 is mediated by its interaction with ZNRF3/RNF43 and LGR4/519,20,21,22,40,41,42. We generated point mutations in the sequence of the furin-like domains that eliminate the binding of Rspo2 to ZNRF3/RNF43 and LGR4/541. These mutants, made in full-length Rspo2 and in the context of a shorter construct that retained a similar activity (Rspo∆T), were expressed at similar levels and induced enlarged or ectopic cement glands in the majority of the injected embryos (Supplementary Fig. 2). These findings suggest that the binding of Znrf3/Rnf43 and Lgr4/5 is not required for Rspo2 ability to anteriorize the embryo.
Rspo2 is a Wnt antagonist
The anteriorized phenotype caused by Rspo2 is similar to the ones generated by Wnt antagonists31,35,36,43,44,45. We therefore wanted to examine whether Rspo2 could antagonize Wnt signaling.
Rspo2 RNA on its own did not induce a secondary body axis as previously reported16 nor modulated Wnt8 axis-inducing activity in our experiments. During gastrulation, Wnt8 enhances posterior development by inducing a distinct set of target genes30,46,47. To evaluate how Rspo2 affects Wnt signaling, it was co-expressed with Wnt8 in dorsal blastomeres of four-cell embryos. As expected, the majority of embryos injected with wnt8 DNA became headless (Fig. 2A–C). Separate injections of Rspo2 RNA into dorsal blastomeres produced blastopore closure defects (Fig. 2D). When coexpressed with Wnt8, Rspo2 completely rescued the headless phenotype in most of the injected embryos (Fig. 2E,F), revealing its Wnt inhibitory activity.
Rspo2 antagonizes Wnt signaling. (A) Scheme of the experiment. Four-cell embryos were injected animally into both dorsal blastomeres with the indicated constructs and cultured to stage 38. (B) Uninjected control embryo. (C) Headless embryo injected with Wnt8 DNA (50 pg). (D) Embryo injected with Rspo2 RNA (0.5 ng). (E) Embryo coexpressing Wnt8 DNA and Rspo2 mRNA. (F) Quantification of the data in (A–D) representative of 3 independent experiments. Numbers of embryos per group are shown above each bar. ****, p < 0.0001, Fisher's exact test. (G) Target gene expression in Wnt8 and Rspo2-stimulated ectoderm explants. Embryos were injected into four animal blastomeres with Wnt8 DNA (50 pg) and Rspo2 RNA (0.5 ng), as indicated, and ectoderm explants were prepared at stage 9 and cultured until stage 13. (H) Dorsal marginal zones (DMZ) were dissected at stage 10 from the control and Rspo2 RNA- or RMOATG-injected embryos and cultured until stage 12. (G,H) RT-qPCR analysis was carried out in triplicates for axin2 and cdx4, and normalized to eef1a1 levels. Means + /− s.d. are shown. Graphs are representative of three independent experiments. Statistical significance has been assessed by Student’s t test, *, p < 0.05; **, p < 0.01.
This result suggests that Rspo2 prevents the activation of specific Wnt target genes that are involved in posterior development48,49,50,51. In ectoderm explants stimulated with Wnt8, Rspo2 downregulated the known Wnt targets axin252, cdx453, mesogenin1/msgn154,55 and myod156 (Fig. 2G and Supplementary Fig. 3). Importantly, axin2 was also inhibited by Rspo2 overexpression and upregulated after Rspo2 depletion in the marginal zone, where endogenous Wnt signaling takes place (Fig. 2H).
To confirm the specific effect of Rspo on Wnt signaling, we used the transgenic frog line Xla.Tg(WntREs:dEGFP)Vlemx, that contains a multimerized Wnt response element driving the expression of destabilized GFP57. Coinjection of Rspo2 RNA into the transgenic embryos with mRFP RNA as a lineage tracer suppressed GFP fluorescence at the injected side (Fig. 3A–C), demonstrating the Wnt inhibitory activity of Rspo2.
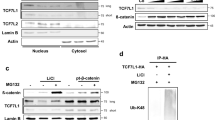
The effects of Rspo2 manipulation on Wnt reporter activity in transgenic embryos. (A) Experimental scheme. Xla.Tg(WntREs:dEGFP)Vlemx embryos were injected into one dorsal blastomere with mRFP RNA (50 pg) with (C) or without (B) Rspo2 RNA (0.5 ng). GFP fluorescence of the injected embryos at stage 18 is shown. Embryo images are representative of 3 different experiments. Asterisk indicates the injected side of the embryo, brackets in C show the comparison of the injected and the control sides. (D,E) Rspo2 modulates Wnt reporter activation. (D) Rspo2 RNA (0.5 ng), RMOATG (10 ng) or RMOSB (20 ng) were injected at two dorsal blastomeres at 4-cell stage. The embryos were lysed at stage 20 and immunoblotted with anti-GFP antibodies. (E) Four-cell stage embryos were injected animally with Wnt3a RNA (50 pg) and Rspo2 RNAs (0.5 ng) or RMOATG (10 ng). Ectoderm explants were dissected at stage 9 and cultured until stage 13, then lysed and immunoblotted with anti-GFP antibodies. Erk1 is a control for loading. In (E) two right lanes were run in the same gel but away from the left lanes (see Supplementary Fig. 5). Five embryos or 10 explants were pooled for each experimental condition in (D,E).
This effect was estimated in a more quantitative way by immunoblotting of the lysates from both Rspo2-overexpressing and Rspo2-depleted embryos. Whole lysates from the embryos injected with Rspo2 RNA contained less GFP, whereas the lysates from the embryos injected with either MO contained more GFP, compared to the control embryos (Fig. 3D). In ectoderm explants, we noticed some variability of transgene expression, nevertheless Wnt3a-stimulated reporter activity was decreased by Rspo2 and upregulated by RMOATG (Fig. 3E).
Together, these findings indicate that Rspo2 antagonizes the Wnt pathway during anteroposterior axis specification.
Rspo2 inhibits TCF3 phosphorylation
R-spondins are composed of two furin-like domains at the N-terminus, one thrombospondin type 1 domain (TSP) and the C-terminus enriched in basic amino acid residues9,10. To examine the mechanism, by which Rspo2 affects Wnt signaling, we assessed the ability of several Rspo2 constructs with specific domain deletions (Fig. 4A) to interfere with Wnt signaling.
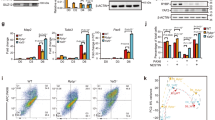
Rspo2 inhibits TCF3 phosphorylation. (A) Schematic of Rspo2 deletion constructs. SP, signal peptide; FU1, furin-like domain 1; FU2, furin-like domain 2; TSP, thrombospondin type 1 domain; BR, the basic amino acid-rich domain. (B,C) Effects of Rspo2 constructs on Wnt-dependent Dvl2 phosphorylation (B) and TCF3 phosphorylation and β-catenin levels (C). Four-cell stage embryos were injected animally with Wnt8 DNA (50 pg or 100 pg) or Wnt8, Wnt3a or Wnt5a RNAs (1 ng each) and Rspo2, Rspo∆F or Rspo∆T RNAs (0.5 ng each) as indicated. Ectoderm explants were dissected at stage 9 and cultured until stage 12 for immunoblotting with antibodies against Dvl2, TCF3, ABC (non-phosphorylated β-catenin). Arrowheads indicate the position of phosphorylated (upshifted) and non-phosphorylated Dvl2 or TCF3 proteins. Erk1 controls for loading. (D) Effects of Rspo2 constructs (0.5 ng each) on TCF3 phosphorylated by endogenous signals. Dorsal marginal zone (D) and ventral marginal zone (V) were dissected from the control and injected embryos at stage 10 and cultured until stage 12.5 for immunoblotting with anti-TCF3 antibodies as shown. Control D and V groups were run in the same gel but separately from the other groups (see Supplementary Fig. 5). (E) Effects of Rspo2 depletion on TCF3 phosphorylation by endogenous signals. DMZ and VMZ explants of embryos injected with control MO (COMO, 20 ng) or RMOATG (20 ng) were dissected and analyzed by immunoblotting as in (B,C).
First, we asked which constructs retain the ability of full length Rspo2 to anteriorize the embryo. Rspo2 lacking the TSP domain (Rspo∆T) had a strong cement gland-inducing activity (Supplementary Fig. 4A,D). Rspo∆F also slightly enhanced head development, but the effect was much weaker than that of Rspo∆T and the wild-type Rspo2 (Supplementary Fig. 4B,C). These observations are consistent with furin-like domains playing an important role in the inhibition of the Wnt pathway that is independent of the known Rspo2 receptors.
To address a specific mechanism of Wnt pathway inhibition by Rspo2, we analyzed Dvl phosphorylation, a common proximal event in Wnt/Frizzled signaling58,59. Phosphorylated Dvl2 migrated slower in ectoderm cells stimulated with Wnt8, Wnt3a and Wnt5a. Rspo2 constructs did not affect Dvl2 mobility on their own or in response to Wnt signals (Fig. 4B), suggesting that Rspo2 does not operate by modulating the activity of Wnt ligands or Frizzled receptors.
We next evaluated the effect of Rspo2 on the downstream signaling intermediates TCF3 and β-catenin. The phosphorylation of TCF3 in response to a Wnt signal leads to TCF3 dissociation from target promoters and transcriptional derepression of Wnt target genes3. TCF3 phosphorylation was visualized by the slower mobility of the TCF3 band from the lysates of ectoderm explants expressing Wnt8 (Fig. 4C). This mobility shift is sensitive to phosphatase treatment3. TCF3 migrated faster in the lysates of cells co-expressing Rspo2. Importantly, both Rspo∆F and Rspo∆T inhibited TCF3 phosphorylation, although Rspo∆F was less effective in this assay. Of note, Rspo∆F negatively regulates FGF signaling37 that acts downstream of the Wnt pathway in anteroposterior patterning60. In the absence of Wnt ligands, we observed that TCF3 levels were consistently higher in cells expressing Rspo2 constructs, suggesting that Rspo2 also influences TCF3 protein stability. In the same experiment, levels of non-phosphorylated β-catenin increased in response to Wnt8, and this effect was reversed by Rspo2 constructs (Fig. 4C).
Our conclusions have been extended to endogenous Wnt signaling that is responsible for TCF3 phosphorylation in the mesoderm (marginal zone) during gastrulation3. Rspo2 and Rspo∆T constructs inhibited TCF3 phosphorylation in ventral marginal zone explants, while Rspo∆F only had a mild effect (Fig. 4D). These observations support our conclusion that Rspo2 antagonizes Wnt signaling by blocking TCF3 phosphorylation.
Furthermore, TCF3 phosphorylation became prominent in the dorsal marginal zone explants isolated from Rspo2 morphants (Fig. 4E). This effect correlated with the accumulation of non-phosphorylated β-catenin. By contrast, no significant changes in Dvl2 levels or mobility have been observed, suggesting that Frizzled receptors are not involved. Based on these results, we propose that Rspo2 enhances anterior development by inhibiting TCF3 phosphorylation.
The Wnt-inhibitory activity of Rspo2 relies on TCF3
If Rspo2 modulates Wnt target genes by inhibiting TCF3 phosphorylation, the depletion of TCF3 should prevent Rspo2 gain-of-function phenotype. For gain-of-function we overexpressed Rspo∆T RNA that consistently produces an ectopic cement gland (Supplementary Fig. 4). Consistent with this prediction, the anteriorized phenotype of Rspo∆T- expressing embryos was suppressed by TCF3 depletion (Fig. 5A,B). Rspo∆T protein levels did not change in TCF3-depleted embryos, supporting knockdown specificity (Fig. 5C). This result suggests that the Wnt antagonistic activity of Rspo2 requires TCF3.
TCF3 is essential for Rspo2 inhibitory effects. (A) TCF3MO rescues the anteriorized phenotype of Rspo∆T RNA overexpressing embryos. Four-cell stage embryos were dorsally injected with TCF3MO (30 ng) and/or Rspo∆T RNA (0.5 ng). Arrowheads indicate the cement gland. (B) Quantification of the data in (A) representative of two independent experiments. Numbers of embryos per group are shown above each bar. (C) Rspo∆T expression levels are not altered by TCF3MO in ectoderm explants (stage 12) in two independent experiments (Exp 1 and Exp 2). ∆T, Rspo∆T; TMO, TCF3MO.
In a converse experiment, Rspo2 depletion is predicted to be rescued by a constitutive TCF3 repressor that does not bind β-catenin (∆βTCF3)3. Supporting this expectation, the effect of Rspo2 depletion on both anterior (otx2 and ag1), and posterior (cdx4 and msgn1) markers were partially rescued in the morphants by ∆βTCF3 (Fig. 6A).
Rspo2 inhibits Wnt signaling through TCF3. (A) ∆βTCF3 RNA (10 pg) rescues ag1, otx2, cdx4, and msgn1 expression in embryos injected with 10 ng of RMOATG. B, C, Rspo2 inhibits axin2 upregulation by Wnt8 (C) but not TCF1 (B) in ectoderm cells. Embryos were injected with Wnt8 (20 pg) or TCF1 (100 pg) RNA without or with Rspo2 RNA (300 pg). Ectoderm explants were prepared at stage 8.5–9 and analyzed at stage 13. RT-qPCR analysis was carried out in triplicates for axin2 and normalized to eef1a1 levels. Means + /− s.d. are shown. Graphs are representative of 2–4 independent experiments. Statistical significance has been assessed by Student’s t test, *, p < 0.05. D, Model for Rspo2-mediated repression. Rspo2 functions via an unknown receptor to inhibit Wnt target gene activation mediated by TCF3 phosphorylation but not TCF1-dependent transcriptional responses.
Based on these observations, we propose that the Wnt-inhibitory function of Rspo2 is mediated by TCF3, a predominant TCF in early embryos that functions as a transcriptional repressor. By contrast, other TCF proteins mediating Wnt signaling, such as TCF1/Tcf7 or Lef1, can activate Wnt targets6,61. Notably, Rspo2 did not downregulate axin2 induction by tcf1 RNA in ectoderm cells, whereas it significantly reduced Wnt8 activity in the same experiment (Fig. 6B,C). This observation suggests a model, in which Rspo2 prevents the ability of Wnt signaling to inhibit TCF3 repressive activity, but does not downregulate TCF1-dependent signaling (Fig. 6D).
Discussion
This study has been focused on Rspo2, a member of the R-spondin family of Wnt pathway modulators. We demonstrate that Rspo2 promotes anterior development by inhibiting TCF3 phosphorylation and Wnt target genes activation, possibly independently of the known interaction with LGR4/5 and ZNRF3/RNF43 receptors. In addition to the Wnt pathway, R-spondins were described to affect TGFβ16,62, BMP63 and FGF37,64 signaling. Although R-spondins are well known to potentiate Wnt signals in various cells and embryonic tissues9,10,16,65,66,67, we demonstrate an alternative role for Rspo2 as a Wnt antagonist during anteroposterior patterning. While surprising, this conclusion is consistent with other reports using zebrafish and cancer cell lines68,69,70. We propose that Rspo2 modulates the Wnt pathway in a context-specific manner.
Similar to other secreted multidomain molecules, Rspo2 is a pleiotropic regulator of signaling. We find that Rspo2 inhibits the Wnt pathway via the Furin-like and the TSP domains, however, it antagonizes the FGF pathway exclusively via the TSP domain37. The effect of the TSP domain could be mediated by its interactions with heparan sulfate proteoglycans27,71. Notably, the TSP domain may inhibit FGF signaling that operates downstream of the Wnt pathway for anteroposterior axis specification in Xenopus embryos60. The binding of LGR4/5 and ZNRF3/RNF43 receptors to the Furin-like domains does not seem to be involved in the Wnt inhibitory activity of Rspo2, although we cannot exclude the possibility that our Rspo2 point mutants retained residual activity. These experiments further illustrate the complexity of the Wnt-FGF crosstalk extending from the extracellular level72 to transcriptional regulation73,74.
The main mechanism for R-spondin signaling in adult stem cells is the modulation of Frizzled degradation by the interaction with LGR4/5 and ZNRF3/RNF43 receptors20,21,75. The phosphorylation of Dishevelled, a proximal marker of Wnt/Frizzled signaling, was not altered in embryonic tissues with manipulated Rspo2 function. This finding suggests that Frizzled signaling is not involved. Moreover, mutations abolishing the binding of LGR4/5 and ZNRF3/RNF43 did not affect the anteriorizing activity of Rspo2, indicating that these interactions are not involved. At present, we cannot exclude a role for LRP5/6 in mediating Rspo2 function, as it was reported to interact with Rspo1, a closely related protein67,76. Consistent with recent reports23,25,26,27, we propose that Rspo2 is a context-dependent Wnt antagonist that may function via yet unknown receptors.
Whereas the Rspo2 receptors mediating its effects on early embryos are not known, we present mechanistic evidence that Rspo2 functions by inhibiting TCF3 phosphorylation. TCF3 is a transcriptional repressor of Wnt targets that is inactivated by Wnt-dependent phosphorylation during anteroposterior patterning3. This phosphorylation is blocked by Rspo2, thereby preventing Wnt target activation. In support of this conclusion, the TCF3 construct, which does not bind β-catenin and is no longer phosphorylated in response to a Wnt signal3, rescued Wnt target gene expression in Rspo2-depleted embryos. It is currently unknown whether Rspo2 modulates the phosphorylation of other TCF proteins, including the ones with a positive effect on transcription, such as TCF1 and LEF16,61. Notably, Rspo2 did not inhibit the activity of TCF1 in our experiments. In a different developmental context, when TCF3 is not expressed7,77, R-spondins might potentiate rather than inhibit Wnt signaling. Several TCF proteins are known to be phosphorylated by HIPK2, Nemo-like kinase and casein kinases 1 and 27,78,79,80, but upstream pathways leading to the activation of these protein kinases remain to be clarified. Additional work is needed to fully understand the molecular basis for the context-dependent activity of Rspo2 in embryonic development.
Methods
Plasmids, in vitro RNA synthesis and morpholino oligonucleotides (MOs)
The DNA clone 6,988,843 encoding X. tropicalis Rspo2 was obtained from Dharmacon. The plasmid encoding full length Rspo2 (pCS2-Rspo2-Flag) was constructed by inserting PCR-amplified coding region of Rspo2 into the EcoRI and BamHI sites of pCS2-Flag. pCS2-Rspo∆F-Flag lacks amino acids 37–134. pCS2-Rspo∆T-Flag lacks amino acids 147–204. Alanine substitutions have been made in pCS2-Rspo2-Flag or pCS2-Rspo∆T-Flag in the furin-like domain 1 (R65A or Q70A), and furin-like domain 2 (F105A or F109A) to generate Rspo2 that does not bind ZNRF3/RNF43 or LGR4/5 as described41. Various Rspo2 constructs containing deletions or alanine substitutions have been generated from pCS2-Rspo2-Flag using single primer-based mutagenesis as described31. Primer sequences are listed in Supplementary Table 2. All constructs have been verified by Sanger sequencing.
Capped mRNAs were synthesized using mMessage mMachine kit (Ambion, Austin, TX). The following linearized plasmids have been used as templates: pSP64T-Wnt3a81, pSP64T-Wnt882, pCS2-Wnt8, and pSP64T-Wnt5a83, ∆βTCF33, pCS2-TCF17, pCS2-mRFP (membrane-targeted), pCS2-Rspo-Flag, pCS2-Rspo∆F, pCS2-Rspo∆T, pCS2-RspoR65A-Flag, pCS2-RspoQ70A-Flag, pCS2-RspoF105A-Flag, and pCS2-RspoF109A-Flag. The following MOs have been purchased from Gene Tools (Philomath, OR): RMOATG, 5′- AAAGAGTTGAAACTGCATTTGG -3′, RMOSB, 5′- GCAGCCTGGATACACAGAAACAAGA-3′, control MO (CoMO), 5′-GCTTCAGCTAGTGACACATGCAT-3′. TCF3MO has been described previously3.
Xenopus embryo culture, microinjections, imaging and statistical analysis
In vitro fertilization and culture of Xenopus laevis embryos were carried out as described84. All manipulations have been carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The experimental protocols have been approved by the IACUC of the Icahn School of Medicine at Mount Sinai. Staging was according to Nieuwkoop and Faber85. The Wnt reporter pbin7LefdGFP transgenic line of Xenopus laevis embryos Xla.Tg(WntREs:dEGFP)Vlemx57 have been obtained from the National Xenopus Resource (Woods Hole, MA). We routinely pooled 5 embryos or 10 explants for each experimental condition to minimize variability of transgene expression. For microinjections, four-cell embryos were transferred into 3% Ficoll in 0.5 × Marc’s Modified Ringer’s (MMR) buffer (50 mM NaCl, 1 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 2.5 mM HEPES pH 7.4)86 and 10 nl of mRNA or MO solution was injected into one or more blastomeres. Amounts of injected mRNA and MOs per embryo, indicated in figure legends, have been optimized in preliminary dose–response experiments. Control MO was injected as at a dose that matched the highest amount of any other MO used in the same experiment.
Embryos were imaged at the indicated stages using Leica Wild M10 stereomicroscope using the OpenLab software. Unless otherwise specified, each experiment has been carried out at least three times. Statistical analyses were performed using GraphPad Prism 6 software. Data are shown as means ± s.d. and statistical significance was assessed using an unpaired two-tailed Student’s t-test or Fisher's exact test. Significant differences are indicated by p values, e. g. *, p < 0.05; **, p < 0.01; ****, p < 0.0001.
Ectoderm and marginal zone explants, RNA sequencing, RT-qPCR
Ectoderm explants were prepared at late blastula stages and cultured until the indicated time to observe morphological changes or lysed for RNA extraction or immunoblotting. Marginal zone explants were dissected at early gastrula stage and cultured until stage 12.5 when they were lysed for immunoblot analysis.
To inhibit FGF receptor activity, ectoderm explants or marginal zone explants have been cultured with SU5402 (100 µM, Calbiochem) from the time of isolation until they were lysed for immunoblot analysis.
For quantitative PCR (RT-qPCR) and RNA sequencing, RNA was extracted from a group of 4–5 embryos, ten animal caps or ten marginal zone explants, at stages 10 or 12.5, using RNeasy kit (Qiagen). RNA sequencing was carried out using the HiSeq PE150 platform (150 b.p., paired end sequencing) and analyzed by Novogene (Sacramento, CA). cDNA was made from 1 µg of total RNA using iScript (Bio-Rad). qPCR reactions were amplified using a CFX96 light cycler (Bio-Rad) with Universal SYBR Green Supermix (Bio-Rad). Primer sequences used for RT-qPCR are listed in Supplementary Table 2. Data represent at least 3 independent experiments each including triplicate samples. All samples were normalized to control embryos. eef1a1 served as an internal control. Means + /− s. d. are shown. Statistical significance was assessed using the Student’s t-test.
Immunoblot analysis
Immunoblot analysis was carried out essentially as described87. Briefly, 10 animal caps or 7 marginal zone explants at stage 12.5 were homogenized in 50 µl of the lysis buffer (50 mM Tris–HCl pH 7.6, 50 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10 mM NaF, 1 mM Na3VO4, 25 mM β-glycerol phosphate, 1 mM PMSF). After centrifugation for 3 min at 16,000 g, the supernatant was subjected to SDS-PAGE and western blot analysis following standard protocols87. The following primary antibodies were used: mouse anti-FLAG (M2, Sigma), mouse anti-non-phosphorylated β-catenin (ABC; Upstate Biotechnology), rabbit anti-XTCF3N88, rabbit anti-Dvl287. Staining with rabbit anti-Erk1 (Cell Signaling) was used as loading control. Chemiluminescence was captured by the ChemiDoc MP imager (BioRad).
In situ hybridization
Whole-mount in situ hybridization with the digoxigenin-labeled antisense RNA probes for krt12.489, foxg1/BF190, and cdx437, was carried out as described91.
References
MacDonald, B. T., Tamai, K. & He, X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
Hikasa, H. et al. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev. Cell 19, 521–532 (2010).
Kim, C. H. et al. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407, 913–916 (2000).
Nguyen, H., Rendl, M. & Fuchs, E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127, 171–183 (2006).
Cadigan, K. M. & Waterman, M. L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 4, a007906 (2012).
Hikasa, H. & Sokol, S. Y. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J. Biol. Chem. 286, 12093–12100 (2011).
Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767–779 (2012).
de Lau, W., Peng, W. C., Gros, P. & Clevers, H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 28, 305–316 (2014).
Raslan, A. A. & Yoon, J. K. R-spondins: Multi-mode WNT signaling regulators in adult stem cells. Int. J. Biochem. Cell Biol. 106, 26–34 (2019).
Aoki, M., Kiyonari, H., Nakamura, H. & Okamoto, H. R-spondin2 expression in the apical ectodermal ridge is essential for outgrowth and patterning in mouse limb development. Dev. Growth Differ. 50, 85–95 (2008).
Bell, S. M. et al. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development 135, 1049–1058 (2008).
Nam, J. S. et al. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev. Biol. 311, 124–135 (2007).
Yamada, W. et al. Craniofacial malformation in R-spondin2 knockout mice. Biochem. Biophys. Res. Commun. 381, 453–458 (2009).
Tatsumi, Y., Takeda, M., Matsuda, M., Suzuki, T. & Yokoi, H. TALEN-mediated mutagenesis in zebrafish reveals a role for r-spondin 2 in fin ray and vertebral development. FEBS Lett. 588, 4543–4550 (2014).
Kazanskaya, O. et al. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev. Cell 7, 525–534 (2004).
Aoki, M. et al. R-spondin3 is required for mouse placental development. Dev. Biol. 301, 218–226 (2007).
Kazanskaya, O. et al. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development 135, 3655–3664 (2008).
Carmon, K. S., Gong, X., Lin, Q., Thomas, A. & Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. U S A 108, 11452–11457 (2011).
de Lau, W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011).
Hao, H. X. et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 (2012).
Koo, B. K. et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 (2012).
Park, S. et al. Differential activities and mechanisms of the four R-spondins in potentiating Wnt/beta-catenin signaling. J. Biol. Chem. 293, 9759–9769 (2018).
Yan, K. S. et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature 545, 238–242 (2017).
Lebensohn, A. M. & Rohatgi, R. R-spondins can potentiate WNT signaling without LGRs. Elife 7, e33126 (2018).
Szenker-Ravi, E. et al. RSPO2 inhibition of RNF43 and ZNRF3 governs limb development independently of LGR4/5/6. Nature 557, 564–569 (2018).
Dubey, R. et al. R-spondins engage heparan sulfate proteoglycans to potentiate WNT signaling. Elife 9, e54469 (2020).
De Robertis, E. M. & Kuroda, H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20, 285–308 (2004).
Heasman, J. Patterning the early Xenopus embryo. Development 133, 1205–1217 (2006).
Hikasa, H. & Sokol, S. Y. Wnt signaling in vertebrate axis specification. Cold Spring Harb. Perspect. Biol. 5, a007955 (2013).
Itoh, K., Tang, T. L., Neel, B. G. & Sokol, S. Y. Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development 121, 3979–3988 (1995).
Kiecker, C. & Niehrs, C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189–4201 (2001).
Picard, J. J. Xenopus laevis cement gland as an experimental model for embryonic differentiation. II. The competence of embryonic cells. J. Embryol. Exp. Morphol. 33, 969–978 (1975).
Sive, H. L., Hattori, K. & Weintraub, H. Progressive detemination during formation of the anteroposterior axis of Xenopus laevis. Cell 58, 171–180 (1989).
Itoh, K., Antipova, A., Ratcliffe, M. J. & Sokol, S. Interaction of dishevelled and Xenopus axin-related protein is required for wnt signal transduction. Mol. Cell Biol. 20, 2228–2238 (2000).
Heasman, J., Kofron, M. & Wylie, C. Beta-catenin signaling activity dissected in the early Xenopus embryo: A novel antisense approach. Dev. Biol. 222, 124–134 (2000).
Reis, A. H. & Sokol, S. Y. Rspo2 antagonizes FGF signaling during vertebrate mesoderm formation and patterning. Development 147 (2020).
Blitz, I. L. & Cho, K. W. Anterior neurectoderm is progressively induced during gastrulation: the role of the Xenopus homeobox gene orthodenticle. Development 121, 993–1004 (1995).
Pannese, M. et al. The Xenopus homologue of Otx2 is a maternal homeobox gene that demarcates and specifies anterior body regions. Development 121, 707–720 (1995).
Wang, D. et al. Structural basis for R-spondin recognition by LGR4/5/6 receptors. Genes Dev. 27, 1339–1344 (2013).
Xie, Y. et al. Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep. 14, 1120–1126 (2013).
Zebisch, M. & Jones, E. Y. Crystal structure of R-spondin 2 in complex with the ectodomains of its receptors LGR5 and ZNRF3. J. Struct. Biol. 191, 149–155 (2015).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 (1998).
Wang, S., Krinks, M., Lin, K., Luyten, F. P. & Moos, M. Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88, 757–766 (1997).
Zhang, X. et al. Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell 149, 1565–1577 (2012).
Christian, J. L. & Moon, R. T. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 7, 13–28 (1993).
Hamilton, F. S., Wheeler, G. N. & Hoppler, S. Difference in XTcf-3 dependency accounts for change in response to beta-catenin-mediated Wnt signalling in Xenopus blastula. Development 128, 2063–2073 (2001).
Ding, Y. et al. Spemann organizer transcriptome induction by early beta-catenin, Wnt, Nodal, and Siamois signals in Xenopus laevis. Proc. Natl. Acad. Sci. U S A 114, E3081–E3090 (2017).
Kjolby, R. A. S. & Harland, R. M. Genome-wide identification of Wnt/beta-catenin transcriptional targets during Xenopus gastrulation. Dev. Biol. 426, 165–175 (2017).
Nakamura, Y., de Paiva Alves, E., Veenstra, G. J. & Hoppler, S. Tissue- and stage-specific Wnt target gene expression is controlled subsequent to beta-catenin recruitment to cis-regulatory modules. Development 143, 1914–1925 (2016).
Nakamura, Y. & Hoppler, S. Genome-wide analysis of canonical Wnt target gene regulation in Xenopus tropicalis challenges beta-catenin paradigm. Genesis 55, e22991 (2017).
Jho, E. H. et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell Biol. 22, 1172–1183 (2002).
Northrop, J. L. & Kimelman, D. Dorsal-ventral differences in Xcad-3 expression in response to FGF-mediated induction in Xenopus. Dev. Biol. 161, 490–503 (1994).
Chalamalasetty, R. B. et al. Mesogenin 1 is a master regulator of paraxial presomitic mesoderm differentiation. Development 141, 4285–4297 (2014).
Wittler, L. et al. Expression of Msgn1 in the presomitic mesoderm is controlled by synergism of WNT signalling and Tbx6. EMBO Rep. 8, 784–789 (2007).
Hoppler, S., Brown, J. D. & Moon, R. T. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 10, 2805–2817 (1996).
Tran, H. T., Sekkali, B., Van Imschoot, G., Janssens, S. & Vleminckx, K. Wnt/beta-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proc. Natl. Acad. Sci. U S A 107, 16160–16165 (2010).
Angers, S. & Moon, R. T. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 (2009).
Yanagawa, S., van Leeuwen, F., Wodarz, A., Klingensmith, J. & Nusse, R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 9, 1087–1097 (1995).
Domingos, P. M. et al. The Wnt/beta-catenin pathway posteriorizes neural tissue in Xenopus by an indirect mechanism requiring FGF signalling. Dev. Biol. 239, 148–160 (2001).
Sokol, S. Y. Wnt signaling through T-cell factor phosphorylation. Cell Res. 21, 1002–1012 (2011).
Zhou, X. et al. R-Spondin1/LGR5 activates TGFbeta signaling and suppresses colon cancer metastasis. Cancer Res. 77, 6589–6602 (2017).
Lee, H., Seidl, C., Sun, R., Glinka, A. & Niehrs, C. R-spondins are BMP receptor antagonists in Xenopus early embryonic development. Nat. Commun. 11, 5570 (2020).
Zhang, M. et al. RSPO3-LGR4 regulates osteogenic differentiation of human adipose-derived stem cells via ERK/FGF signalling. Sci. Rep. 7, 42841 (2017).
Kim, K. A. et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588–2596 (2008).
Nam, J. S., Turcotte, T. J., Smith, P. F., Choi, S. & Yoon, J. K. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 281, 13247–13257 (2006).
Wei, Q. et al. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J. Biol. Chem. 282, 15903–15911 (2007).
Rong, X. et al. R-spondin 3 regulates dorsoventral and anteroposterior patterning by antagonizing Wnt/beta-catenin signaling in zebrafish embryos. PLoS ONE 9, e99514 (2014).
Wang, B. et al. Functional characterization of Cynoglossus semilaevis R-spondin2 and its role in muscle development during embryogenesis. Genes Genet. Syst. 93, 181–190 (2018).
Wu, C. et al. RSPO2-LGR5 signaling has tumour-suppressive activity in colorectal cancer. Nat. Commun. 5, 3149 (2014).
Ohkawara, B., Glinka, A. & Niehrs, C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell 20, 303–314 (2011).
Yamamoto, A., Nagano, T., Takehara, S., Hibi, M. & Aizawa, S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell 120, 223–235 (2005).
Haremaki, T., Tanaka, Y., Hongo, I., Yuge, M. & Okamoto, H. Integration of multiple signal transducing pathways on Fgf response elements of the Xenopus caudal homologue Xcad3. Development 130, 4907–4917 (2003).
Kjolby, R. A. S., Truchado-Garcia, M., Iruvanti, S. & Harland, R. M. Integration of Wnt and FGF signaling in the Xenopus gastrula at TCF and Ets binding sites shows the importance of short-range repression by TCF in patterning the marginal zone. Development 146 (2019).
Glinka, A. et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 (2011).
Binnerts, M. E. et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl. Acad. Sci. U S A 104, 14700–14705 (2007).
Adam, R. C. et al. Temporal layering of signaling effectors drives chromatin remodeling during hair follicle stem cell lineage progression. Cell Stem Cell 22, 398-413 e397 (2018).
Hammerlein, A., Weiske, J. & Huber, O. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/beta-catenin complex. Cell Mol. Life Sci. 62, 606–618 (2005).
Ota, S. et al. NLK positively regulates Wnt/beta-catenin signalling by phosphorylating LEF1 in neural progenitor cells. EMBO J. 31, 1904–1915 (2012).
Smit, L. et al. Wnt activates the Tak1/Nemo-like kinase pathway. J. Biol. Chem. 279, 17232–17240 (2004).
Wolda, S. L., Moody, C. J. & Moon, R. T. Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev. Biol. 155, 46–57 (1993).
Christian, J. L., McMahon, J. A., McMahon, A. P. & Moon, R. T. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 111, 1045–1055 (1991).
Moon, R. T. et al. Xwnt-5A: A maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development 119, 97–111 (1993).
Dollar, G. L., Weber, U., Mlodzik, M. & Sokol, S. Y. Regulation of Lethal giant larvae by Dishevelled. Nature 437, 1376–1380 (2005).
Nieuwkoop, P. D. & Faber, J. Normal Table of Xenopus laevis (North Holland, 1967).
Peng, H. B. Xenopus laevis: Practical uses in cell and molecular biology. Solutions and protocols. Methods Cell Biol. 36, 657–662 (1991).
Itoh, K., Brott, B. K., Bae, G. U., Ratcliffe, M. J. & Sokol, S. Y. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J. Biol. 4, 3 (2005).
Zhang, C., Basta, T., Jensen, E. D. & Klymkowsky, M. W. The beta-catenin/VegT-regulated early zygotic gene Xnr5 is a direct target of SOX3 regulation. Development 130, 5609–5624 (2003).
Winkles, J. A., Sargent, T. D., Parry, D. A., Jonas, E. & Dawid, I. B. Developmentally regulated cytokeratin gene in Xenopus laevis. Mol. Cell Biol. 5, 2575–2581 (1985).
Bourguignon, C., Li, J. & Papalopulu, N. XBF-1, a winged helix transcription factor with dual activity, has a role in positioning neurogenesis in Xenopus competent ectoderm. Development 125, 4889–4900 (1998).
Harland, R. M. In situ hybridization: An improved whole-mount method for Xenopus embryos. In Methods Cell Biol (eds Kay, B. K. & Peng, H. B.) 685–695 (Academic Press Inc., 1991).
Acknowledgements
We thank Miho Matsuda, Keiji Itoh and Jean-Pierre Saint-Jeannet for the comments on the manuscript. We also thank Aurelian Radu for the help with the analysis of RNA sequencing data, Olga Ossipova for qPCR primers, Pamela Mancini for advice on Adobe Illustrator and members of the Sokol laboratory for discussions. This study has been supported by the NIH Grant HD092990 to SYS.
Author information
Authors and Affiliations
Contributions
A.H.R. designed experiments, carried out experiments, analyzed data and wrote the manuscript. S.Y.S. designed experiments, analyzed data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reis, A.H., Sokol, S.Y. Rspo2 inhibits TCF3 phosphorylation to antagonize Wnt signaling during vertebrate anteroposterior axis specification. Sci Rep 11, 13433 (2021). https://doi.org/10.1038/s41598-021-92824-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92824-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.