Abstract
Gastrointestinal symptoms (GIS) are common in kidney transplant candidates and recipients and may be worsened by HIV. Objective: To determine the frequency and severity of GIS in HIV-positive kidney transplant recipients from HIV-positive donors, and those waiting to receive one. A GIS rating scale (GSRS) was completed by 76 participants at baseline and at 6 months. GIS frequency was defined as having at least one symptom (GSRS > 1). Severity was indicated by the GSRS score. Transplant candidates: GIS frequency was 88.9% and 86.3% at baseline and 6 months respectively. Indigestion was the most frequent (79.6% and 66.7% at baseline and 6 months), and severe GIS (GSRS 2.3). Women reported global mean (p = 0.030) severity significantly more than men. Transplant recipients: GIS frequency was 95.2% and 76.2% at baseline and 6 months respectively. At both assessment points, indigestion occurred most frequently (85.7% and 61.9% respectively). Highest GSRS was reported for indigestion at baseline (2.33) and at 6 months (1.33). Waist circumference (WC) was positively associated with the severity of constipation GSRS. GIS are common in both groups, especially indigestions. WC in transplant recipients should be monitored.
Similar content being viewed by others
Introduction
Patients with impaired kidney function very often experience gastrointestinal symptoms (GIS) at all stages of chronic kidney disease (CKD)1. Symptoms begin early, appearing well before end-stage renal disease (ESRD), at stage 3 (eGFR 45 ml/min/1.73 m2), and become increasingly burdensome as kidney function declines2. Uraemia and dialysis predispose patients to gastrointestinal (GI) mucosal lesions and functional disorders3 that may or may not cause GIS4. In two recent studies, ESRD dialysed and non-dialysed patients reported a prevalence of GIS of 61.6% to 81.0%5,6. Following a transplant, renal function is restored, however, the occurrence of GIS remains frequent and is often an under-estimated problem7. At this point however, GIS is largely attributable to opportunistic infections and immunosuppressant therapy8,9.
CKD often coexists with other illnesses that affect the GIT through the disease process and its treatment. In HIV-positive individuals, replication of the virus in gut-associated lymphoid tissues10, pharmacological side-effects and opportunistic as well as non-opportunistic infections11, are known determinants of GIS. Resultantly, GIS may present at any time, in any area of the GIT12. Despite a paucity of data, in all probability, the prevalence of GIS among HIV-positive patients with ESRD may be higher than among uninfected patients with HIV.
Regardless of aetiology, the severity of GIS range from mild to severe, thereby compromising nutritional status13, psychological health14 and quality of life7. More importantly, GIS could be indicative of high risk complications such as upper gastrointestinal intestinal (UGI) bleeding in dialysed patients15 or graft failure in transplant recipients16.
Individually, both CKD and HIV have a significant impact on the GIT. However, the nature of GIS in ESRD together with HIV is unknown. For this reason, the primary aim of this study was to describe GIS in terms of frequency and severity as experienced by HIV- infected pre- and post-transplant recipients at baseline and 6 month follow-up. In addition, the study investigated the relationship between GIS and selected nutritional and clinical parameters.
Methods
Participants
Groote Schuur Hospital (GSH) in Cape Town, South Africa runs the HIV “positive-to-positive” kidney transplant programme. The programme extends nationally, with candidates and recipients resident across the country. Prospective transplant candidates who meet the inclusion criteria receive dialysis in their home province until a donor becomes available. Candidates then travel to GSH for the transplant, before returning home. For the purposes of this study, the most recent list of transplant recipients and potential candidates was obtained from GSH. The number of candidates and recipients in this programme were still small, but at the time represent 100% of the global population of this unique group. There were 92 prospective participants (68 candidates, 24 recipients).
Figure 1 indicates an overview of participant enrolment. Patients were contacted by phone or in outpatient clinics. Patients did not qualify for participation if they were severely ill, were not contactable, were uncooperative or missed several interview appointments (typically more than two without reason). Seventy-six patients agreed to participation. Written informed consent was obtained after the purpose of the study and practical implications were explained to them. They were assigned to two categories namely (i) HIV-positive transplant recipients who received a kidney from a HIV-positive donor (22 recipients); and (ii) HIV-positive transplant candidates who were on the waiting list to receive a kidney from a HIV-positive donor (54 candidates).
From June 2015, data was collected over one year with participants being followed up across six provinces. Assessments were conducted at two time points namely baseline and at 6 months follow-up.
Anthropometry
Weight (WT), height (Ht) and waist circumference (WC) measurements were taken according to the National Health and Nutrition Examination Survey (NHANES) guidelines17 by a qualified dietitian. The mean of three readings were used for data analysis. Weight was determined post dialysis. BMI was classified according to the World Health Organization (WHO) categories as kg/m2: Underweight (< 18.5), Normal (18.5–24.9), Overweight (25.0–29.9), Obese Class I (30.0–34.9), and Obese Class II (35.0–39.9)18.
Measurement of gastrointestinal symptoms
The Gastrointestinal Symptom Rating Scale (GSRS) was used to determine the frequency and severity of GIS19. Although originally designed for GIS assessment of gastrointestinal diseases, it has been used in all stages of CKD including dialysis14 and transplant recipients20. It consists of 15 items that are collapsed into 5 symptom subscales viz; abdominal pain (abdominal pain, hunger pain and nausea), reflux syndrome (heartburn and acid regurgitation), diarrhoea syndrome (diarrhoea, loose stools and urgent need for defecation), indigestion syndrome (borborygmus, abdominal distension, eructation and increased flatus) and constipation syndrome (constipation, hard stools and a feeling of incomplete evacuation)19,21.
GIS Frequency: The frequency of GIS was defined as having at least one symptom or a GSRS score > 114,21,22.
GIS Severity: To determine the severity of a symptom, each question is rated using a seven-point Likert Scale ranging from one (no discomfort at all) to seven (severe discomfort) to obtain a total score ranging from 15 (minimum) to 105 (maximum) or mean values between one and seven. The combined severity scores of the five subscales, are presented as a global mean score and a mean score per subscale. Higher GSRS scores are indicative of a higher symptom burden. GSRS severity scores were correlated with patients’ clinical, demographic and nutritional parameters.
Statistical analysis
Data was analysed using the Statistical Package for Social Sciences (SPSS®) version 25.0. Means and standard deviation were calculated for all continuous variables, and frequencies with percentages were determined for categorical variables. The means of groups were compared using the independent samples t-test. Cronbach’s alpha was used to determine the internal reliability of the GSRS. Spearman’s correlation was used to determine the relationship between GSRS subscales and clinical and nutritional variables. A p value of < 0.05 was taken as statistically significant.
Ethical approval
Ethical approval for this study was obtained from the Biomedical Research Ethics Committee (BREC) of the University of Kwazulu-Natal. BREC is registered with the following: (i) South Africa’s Department of Health’s National Health Research Ethics Council (http://nhrec.health.gov.za) NHREC REC 290408-009). (ii) The US Office for Human Research Protections (http://www.hhs.gov/ohrp). (iii) Has Federal-Wide Assurance (FWA), Assurance number 678, Institution number IORG 0000923, IRB number 00001293.
Results
Patient characteristics
As all 76 patients completed the GSRS at least once, at either time points, no participants were excluded. At baseline, one patient did not complete the GSRS and four did not complete it at 6 months follow-up for reasons that included hospitalisation, missed appointments and the demise of two participants. Of the 76 participants surveyed, 22 HIV-positive kidney transplant recipients received a kidney from a HIV-positive donor, while 54 HIV-positive patients were on the waiting list to receive a kidney from a HIV-positive donor. The latter group were managed with haemodialysis (HD) (n = 51) or peritoneal dialysis (PD) (n = 3).
Socio-demographic, clinical, and nutritional status characteristics of the study population are given in Tables 1 and 2. The study sample, who were predominantly black (93.4%) and male (60.5%), had a mean age of 43.6 ± 8.1 years. There were significantly more patients with diabetes in the dialysis group compared to the transplant group (29.6% versus 4.5%, p = 0.017). At 6 months WC was significantly larger than that at baseline (p = 0.013).
Gastrointestinal symptoms
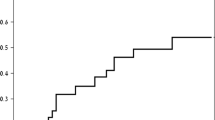
The frequency of at least one GIS (GSRS score of > 1) in the whole study sample is shown in Fig. 2, being 90.7% and 83.3% at baseline and 6 month follow-up respectively.
The final Cronbach’s Alpha for the global mean at baseline and 6 month follow-up, was 0.813 and 0.862 respectively. GSRS for all GIS in the whole group (Fig. 3) was higher at baseline than at 6 month follow-up. At baseline, the global mean GSRS was 1.80 ± 0.76 and lower at 6 months at 1.55 ± 0.74. The individual GIS show a similar order of severity at each assessment time point. Indigestion and diarrhoea had the highest and lowest GSRS respectively.
Frequency and severity of GIS in transplant candidates
Overall, 88.9% of dialysed participants reported at least one GIS at baseline and 81.5% at 6 month follow-up (Fig. 4). At baseline, indigestion (79.6%), abdominal pain (64.8%) and reflux (48.1%) were the most commonly reported GIS, while diarrhoea and constipation were experienced to a lesser extent at 44.4% and 42.6%, respectively (Fig. 4). At 6 month follow-up, indigestion was still the most frequent GIS, albeit to a lesser extent (66.7%). However, more participants complained of constipation, increasing in frequency to 51.0%.
The GSRS scores indicated the severity of symptoms (Table 3) for each treatment group. The most severe GIS for PD patients (n = 3) was diarrhoea at 6 months (GSRS 4). For HD patients, indigestion was slightly more severe than the other GIS at both times (GSRS 1.67). Females had significantly higher median GSRS for several GSRS subscales as well as the global mean at baseline (p = 0.030).
In the transplant candidate group, Spearman’s correlations with GSRS (Table 4) were positive for the global mean score with the length of time on dialysis at baseline and 6 months (baseline rho = 0.287, p = 0.036 and rho = 0.440, p = 0.001). Age correlated negatively with GIS global mean (rho = − 0.338, p = 0.015).
Frequency and severity of GIS in transplant recipients
Over nine out of ten (95.2%) of the transplant group experienced GIS at baseline. However, the prevalence of symptoms decreased by 19.0% to 76.2%. The frequency of symptoms across the five subscales is depicted in Fig. 5. Transplant recipients reported indigestion as the most prevalent symptom at baseline (85.7%), this was followed by abdominal pain (81.0%), reflux (42.9%), with diarrhoea and constipation both occurring at a prevalence of 38.1%. At 6 month follow-up, frequency of GIS symptoms deceased by 19%, from 95.24 to 76.19%. The frequencies in each symptom category also decreased. Indigestion was the most frequently experienced GIS in the transplant group with 85.7% prevalence at baseline and 61.9% at 6 month follow-up. Only one participant reported diarrhoea (4.8%) at 6 month follow-up.
Indigestion was the most severe GIS with the highest median GSRS score of 2.33 at baseline and 1.33 at 6 months (Table 3). All GSRS were lower at 6 month follow-up with the global mean decreasing from 1.86 to 1.15. In the transplant group, WC was positively associated with constipation at baseline (rho = 0.471, p = 0.048).
Discussion
To our knowledge, no data on GIS in a similar patient group exists. In the current study, the occurrence of at a least one GIS (GSRS > 1) in the total group, at baseline and at 6 months was high. At 90.7% and 83.3% respectively, this finding supports previous research that renal non -HIV patients experience a greater frequency of GIS than non-renal patients23 and the general population14,24,25.
Transplant candidates
Frequency and severity of GIS
The frequency of GIS amongst dialysed participants was fairly consistent at both time points (88.9% and 81.5%). These values fell within the 76–90% GIS frequency range experienced by HD and PD populations elsewhere26,27. Across the five subscales indigestion, abdominal pain and, to a lesser extent, reflux occurred at higher freqency than constipation and diarrhoea at baseline. At 6 month follow-up however, more participants suffered from constipation, and with greater severity. These findings are in agreement with a systematic review of GIS in 30 studies conducted among 5161 HD and PD participants. Despite differences in methodology, these studies also reflected constipation, indigestion, abdominal pain and reflux as the most frequently reported GIS3. Constipation in particular, affects up to 71.7% of HD patients28 and is attributed to restrictive diets, medication, inactivity and ignoring the urge to defaecate whilst on dialysis29. Although constipation affected about half of the participants on dialysis, it was not the most bothersome GIS. Indigestion was the GIS of greater frequency and severity.
Indigestion, or dyspepsia is a common occurrance in the HD and PD population, with a frequency ranging between 30.0–72.3% and 31.5–93.1% respectively and is responsible for the regular consumption of acid suppressants in 41.0–76.4% of patients30,31. Endoscopy in dyspeptic patients shows upper gastrointestinal (UGI) pathology in 60.0–68.0% of patients, with erosive and ulcerative changes found in the stomach, oesophagus and duodenum. The causes of UGI morbidity are complex. In addition to risk factors in the general population32, CKD determinants include hypergastrinaemia, inflammation and high levels of ammonia33. Delayed gastric emptying or heparin use in dialysis4,34,35 adds to GIS such that dialysed patients may have a greater symptom burden than non-dialysed ESRD patients14. Within the dialysed group itself, PD participants (albeit only three), had more pronounced GIS than HD participants. This is a common36,37 but inconsistent finding5 related to the effects of the dialysate present in the abdomen37. Between the sexes, females reported significantly higher GSRS, similar to that observed in a Turkish HD group38. However, this is not exclusive to CKD. In the general population, women experience more dyspeptic and irritable bowel syndrome symptoms39,40,41 on account of gender specific psychosocial factors, hormonal activity, as well as anatomical and functional differences in pain transmission pathways affecting sensitivity42,43.
Correlations between GSRS severity scores, clinical and nutritional variables
This study examined the relationships between severity (GSRS scores), rather than frequency of GIS, with selected clinical and nutritional parameters. Although expected, no significant associations were found between GSRS and serum albumin. Lower serum albumin is likely due to underlying illness or inflammation, such as infections rather than nutritional status44, which could worsen the severity of GIS. Abdominal pain and reflux scores decreased with age, possibly due to the disinclination of older individuals to report symptoms. Furthermore, there appears to be an adaptation to the intensity of chronic symptoms as well as symptoms becoming less specific, and more vague with advancing age45,46.
GSRS scores were positively associated with the duration of dialysis. The increasing severity of indigestion, constipation and reflux with a longer period on dialysis, is not a universal finding45, as typically the opposite occurs. More GIS is noted at the start of PD37 and in HD, related to hypotensive episodes at HD initiation47.
Transplant recipients
Frequency and severity of GIS
GI complications are a common occurrence following a solid organ transplant, potentially affecting any area of the GIT9. Severe complications are rare (10.0%), occurring primarily in the first year post transplant48. A transplant is expected to relieve GIS related to uraemia and dialysis, and explains the lower global GSRS scores in this study’s transplant candidates versus transplant recipients. However, for many transplant recipients GIS still persist, albeit with a lower level of severity. The transplant recipients in the current study had a high frequency of GIS at baseline (95.2%), similar to findings in European (88.3–92.0%)7,22 and African (96%) transplant recipients49. In a study by Ponticelli et al., with a cohort of 1130 kidney transplant recipients, patients demonstrated stable GIS throughout the year-long study period7. In contrast, the frequency of GIS dropped by 19.0% to 76.2% in the present study, for reasons that are unclear.
As was the case in the dialysis group, indigestion was a frequent symptom. It was the most severe at baseline and at 6 month follow-up, possibly due to underlying gastropathology. Dyspeptic transplant recipients have shown a high prevalence of erosive changes on endoscopy, mainly gastritis (78.6%), that could be present pre-transplant50 and/or is aggravated by immunosuppressants8. Tacrolimus, which has been linked to duodenitis50, forms part of the anti-rejection regimen in addition to mycophenolate mofetil (MMF), and prednisone51, and could be a contributing factor. It is also interesting to note that indigestion, together with abdominal pain and reflux, were the three most frequent GIS at baseline and 6 month follow-up, similar to a survey of 4232 transplant recipients across four north European countries22. Taken together, these three GIS are typical of gastro-oesophageal reflux disease (GORD)52, for which CKD, transplantation and anti-rejection medication are risk factors53.
Anti-rejection medication also increases the risk of infectious and non-infectious diarrhoea by increasing vulnerability to infectious agents and compromising gut mucosal integrity and function9. In 13 out of 25 (52.0%) transplant recipients with chronic diarrhoea, infections and drug-related colitis associated with MMF were identified via colonoscopy47, while diarrhoea was linked to the toxicity profile of Tacrolimus54. Despite the combination of these two drugs in the current study’s participants treatment regime, diarrhoea was not as bothersome as the other GIS. Diarrhoea affected eight transplant candidates (38.1%) and only one (4.8%) transplant recipient at baseline and 6 month follow-up respectively. Furthermore, the severity scores of diarrhoea were low (GSRS of 1.00). Earlier studies report the frequency of diarrhoea to be between 22.8 and 53.0% and GSRS scores of between 1.44 ± 0.88 and 1.80 ± 1.10 in transplant recipients7,22. In the majority of cases, diarrhoea is transient and resolved with appropriate pharmaceutical and dietary management55. This is probably the reason for the difference in frequency at baseline and then at 6 months.
Correlations between GSRS severity scores, clinical and nutritional variables
The significant increase in WC from baseline to 6 months amongst recipients most likely reflects a combination of greater dietary flexibility, immunosuppressants and lack of exercise. Significant associations between GSRS constipation scores with WC were identified at baseline in the transplant group. In the general population, obesity is a risk factor for GORD and erosive oesophagitis in the long term25, while central obesity is related to non-erosive oesophageal disease56. However, the association of obesity with constipation and functional dyspepsia is less clear57.
It would therefore be sensible to ensure weight maintenance and a WC at optimum values. In 332 non-CKD participants who participated in a weight intervention programme that targeted behaviour, diet, and physical activity, participants reported an 81.0% and 55.0% decrease and resolution of GIS respectively58. In other research, weight management was less likely to improve symptoms in 211 participants for which the BMI – reflux relationship was independent of diet and exercise59. This highlights the contribution of clinical, pharmaceutical and demographic factors to GIS.
Gastrointestinal symptoms and HIV
The contribution of coexisting illness to GIS in ESRD was clearly applicable to this patient group. HIV has always been associated with GIS, and it was not uncommon for HIV-positive individuals to experience regular episodes of diarrhoea60. However, defining research by Mönkemüller et al. has shown a change in the pattern of GI manifestations since the HAART era. The occurrences of opportunistic infections have reduced61, while UGI manifestations have increased, and are associated with improved immunocompetence related to HAART11. HIV-positive Japanese patients, report higher UGIS severity scores than non-HIV infected patients62. Findings of mucosal changes such as gastritis (48%) and gastric erythema (45%)63, and reflux, H pylori infection, and GORD have increased11. In all probability, these would aggravate UGI pathology of ESRD, and could underlie the higher frequency and severity of indigestion compared to the other GIS in the current study sample.
This study has several strengths. It is the first to investigate the frequency and severity of GIS in pre- and post-kidney transplant recipients infected with HIV. Secondly, the GSRS which has been previously validated in South Africa and elsewhere64 encompasses a range of symptoms applicable to the upper and lower GIT. Thirdly, despite the small study sample, the findings of this research are still generalizable as the majority of patients on the transplant lists were included in this study, and as such, are a fair representation of this group. A study limitation in this regard is that the number of PD patients (n = 3) is extremely small. Thus, the power of the statistical analysis using this group is severely limited. Hence, correlations were done using PD and HD combined into a single group (n = 54). For future studies though, PD and HD patients should be considered separately as the former is likely to have a greater influence on GIS. The lack of information on medication used to relieve GIS, as well as detailed renal function parameters, which would have benefited the analysis of the study results, is also a drawback. The study design, which provides a snapshot of GIS at two assessment points is suitable for prevalence studies, but limits the exploration of causal relationships65. Furthermore, conducting the assessments before and after a transplant would have been preferable. However, some patients wait years for a kidney to become available. Unfortunately, for this study, resources and finances were only sufficient for a 6-month data collection period.
Finally, this study did not have a control group to compare GIS with and without the presence of HIV, but should be considered in future research, along with a longer follow-up period to provide better insight into whether the symptoms documented are pervasive or transient.
In conclusion, this research contributes to the body of evidence on GIS experienced by kidney transplant candidates and recipients but extends to an understanding of these symptoms among those infected with HIV. The data confirm a high prevalence, but low severity of GIS in both treatment groups, although similar to that documented for non-HIV infected dialysis and transplant recipients. Indigestion was a bothersome GIS in the whole group at both time points, while those on dialysis experienced a greater frequency of constipation at 6 month follow-up. A comparison of GSRS scores between groups showed higher severity scores in transplant candidates, and Spearman’s correlations with specific GIS were positive for duration of dialysis and negative for age. In the transplant group, specific GIS were positively associated with WC.
Both kidney transplants and dialysis are major medical interventions that are often accompanied by complications, and frequent hospitalisation. However, GIS (especially if they are chronic and low grade), may be discounted by patients and clinicians until they become severe and debilitating. Major gastrointestinal complications are rare, but do occur. The GSRS is a quick, simple, and cost effective monitoring tool that can be used for early identification, or progression, of GI manifestations.
References
Osorio, M. S. & Giraldo, G. C. Gastrointestinal manifestations of chronic kidney disease. Rev. Colomb. Nefrol. 4(1), 3–12 (2017).
Zhang, X., Bansal, N., Go, A. S. & Hsu, C. Gastrointestinal symptoms, inflammation and hypoalbuminemia in chronic kidney disease patients: a cross-sectional study. BMC Nephrol. 16(211), 1–8 (2015).
Zuvela, J. et al. Gastrointestinal symptoms in patients receiving dialysis: a systematic review. Nephrology 23(8), 718–727 (2018).
Sreelatha, M., Kumar, V. S., Shekar, G. C. & Shekar, V. C. Upper gastrointestinal manifestations in chronic renal failure through upper gastrointestinal endoscopy. IJSS. 5(2), 221–225 (2017).
Dong, R., Guo, Z., Ding, J., Zhou, Y. & Wu, H. Gastrointestinal symptoms: A comparison between patients undergoing peritoneal dialysis and hemodialysis. World J. Gastroenterol. 20(32), 11370–11375 (2014).
Ariffin, N. F. M. et al. Appetite and gastrointestinal symptoms in end stage renal disease patients. J. Clin. Exp. Nephrol. https://doi.org/10.21767/2472-5056.100006 (2016).
Ponticelli, C., Colombo, D., Novara, M., Basilisco, G. & CETRA Study Group. Gastrointestinal symptoms impair quality of life in Italian renal transplant recipients but are under-recognized by physicians. Transpl. Int. 23(11), 1126–1134 (2010).
Helderman, J. H. & Goral, S. Gastrointestinal complications of transplant immunosuppression. JASN 13(1), 277–287 (2002).
Lucan, V. C. & Berardinelli, L. Gastrointestinal side effects of post-transplant therapy. J. Gastrointest. Liver Dis. 25(3), 367–373 (2016).
Thompson, C. G., Gay, C. L. & Kashuba, A. D. M. HIV persistence in gut-associated lymphoid tissues: pharmacological challenges and opportunities. AIDS Res. Hum. Retroviruses 33(6), 513–523 (2017).
Nkuize, M., De Wit, S., Muls, V., Arvanitakis, M. & Buset, M. Upper gastrointestinal endoscopic findings in the era of highly active antiretroviral therapy. HIV Med. 11(6), 412–417 (2010).
Thompson, T. et al. Prevalence of gastrointestinal symptoms among ambulatory HIV patients and a control population. Ann. Gastroenterol. 25(3), 243–248 (2012).
Hydarinia-Naieni, Z., Nobahar, M. & Ghorbani, R. Study of nutritional status and gastrointestinal health in patients undergoing hemodialysis and their association with laboratory parameters and dialysis adequacy in Semnan, Iran. Middle East J. Rehabil. Health Stud. 4(3), e12686. https://doi.org/10.5812/mejrh.12686 (2017).
Strid, H. et al. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well being. Nephrol. Dial. Transplant. 17(8), 1434–1439 (2002).
Laeeq, S. M. et al. Upper gastrointestinal bleeding in patients with end stage renal disease: causes, characteristics and factors associated with need for endoscopic therapeutic intervention. J. Transl. Int. Med. 5(2), 106–111 (2017).
Bunnapradist, S. et al. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am. J. Kidney Dis. 51(3), 478–486 (2008).
Centers of Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual. [cited 22/07/2015]. https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf.
World Health Organization. Obesity. Preventing and managing the global epidemic. 2000. [cited 12/03/2018]. Obesity:Preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894). Geneva; 2000.
Svedlund, J. S. I. D. G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 33, 129–134 (1988).
Ponticelli, C. & Passerini, P. Gastrointestinal complications in renal transplant recipients. Transpl. Int. 18(6), 643–650 (2005).
Uehara, R. et al. Characteristics of gastrointestinal symptoms and function following endoscopic submucosal dissection and treatment of the gastrointestinal symptoms using rikkunshito. Exp. Ther. Med. 6(5), 1083–1088 (2013).
Ekberg, H. et al. Increased prevalence of gastrointestinal symptoms associated with impaired quality of life in renal transplant recipients. Transplantation 83(3), 282–289 (2007).
Cano, A. E. et al. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am. J. Gastroenterol. 102(9), 1990–1997 (2007).
Aziz, I. et al. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: A cross-sectional population-based study. Lancet Gastroenterol. Hepatol. 3(4), 252–262 (2018).
Chang, P. & Friedenberg, F. Obesity & GERD. Gastroenterol. Clin. North Am. 43(1), 161–173 (2014).
Dong, R. & Guo, Z. Gastrointestinal symptoms in patients undergoing peritoneal dialysis: Multivariate analysis of correlated factors. World J. Gastroenterol. 14(16), 2812–2817 (2010).
Daniels, G., Robinson, J. R., Walker, C., Pennings, J. S. & Anderson, S. T. Gastrointestinal symptoms among African Americans undergoing hemodialysis. Nephrol. Nurs. J. 42(6), 539–548 (2015).
Zhang, J. et al. Health-related quality of life in dialysis patients with constipation: A cross-sectional study. Patient Prefer. Adherence 7, 589–594 (2013).
Yasuda, G. et al. Prevalence of constipation in continuous ambuatory peritoneal dialysis and comparison with hemodialysis patients. Am. J. Kidney Dis. 39(6), 1292–1299 (2002).
Chong, V. H. & Tan, J. GI and psychosomatic symptoms in Asian ESRD. Nephrology (Carlton). 18(2), 97–103 (2013).
Strid, H., Simrén, M. & Björnsson, E. S. Overuse of acid suppressant drugs in patients with chronic renal failure. Nephrol. Dial. Transplant. 18(3), 570–575 (2003).
Everhart, J. E. & Ruhl, C. E. Burden of digestive diseases in the United States Part I: Overall and upper gastrointestinal diseases. Gastroenterology 136(2), 376–386 (2009).
Krishnan, A., Sigamani, R. & Venkataraman, J. Gastrointestinal evaluation in chronic kidney diseases. J. Nephrol. Ther. 1(3), 110. https://doi.org/10.4172/2161-0959.1000110 (2011).
Junior, L. D. S., Santos, P. R., Santos, A. A. & Ponte de Souza, M. H. L. Dyspepsia and gastric emptying in end-stage renal disease patients on hemodialysis. BMC Nephrol. 14, 275. https://doi.org/10.1186/1471-2369-14-275 (2013).
Brown-Cartwright, D., Smith, H. J. & Feldman, M. Gastric emptying of an indigestible solid in patients with end-stage renal disease on continuous ambulatory peritoneal dialysis. Gastroenterology 95(1), 49–51 (1988).
Salamon, K., Woods, J., Paul, E. & Huggins, C. Peritoneal dialysis patients have higher prevalence of gastrointestinal symptoms than hemodialysis patients. J. Ren. Nutr. 23(2), 114–118 (2013).
Kosmadakis, G., Albaret, J., da Costa Correia, E., Somda, F. & Aguilera, D. Gastrointestinal disorders in peritoneal dialysis patients. Am. J. Nephrol. 48(5), 319–325 (2018).
Gök, E. G., İnci, A., Çoban, M., Kutsal, D. A. & Kürşat, S. Functional bowel disorders and associated risk factors in hemodialysis patients in Turkey. Turk. J. Gastroenterol. 28, 12–19 (2017).
Lee, S. Y. et al. Irritable bowel syndrome is more common in women regardless of the menstrual phase: A Rome II-based survey. J. Korean Med. Sci. 22(5), 851–854 (2007).
Kawakubo, H. et al. Upper gastrointestinal symptoms are more frequent in female than male young healthy Japanese volunteers as evaluated by questionnaire. J. Neurogastroenterol. Motil. 22(2), 48–253 (2016).
Napthali, K., Koloski, N., Walker, M. M. & Talley, N. J. Women and functional dyspepsia. Womens Health (Lond). 12(2), 241–250 (2016).
Mayer, E. A., Naliboff, B., Lee, O., Munakata, J. & Chang, L. Review article: Gender-related differences in functional gastrointestinal disorders. Aliment Pharmacol. Ther. 13(Suppl. 2), 65–69 (1999).
Cain, K. C. et al. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig. Dis. Sci. 54(7), 542–1549 (2008).
Friedman, A. N. & Fadem, S. Z. Reassessment of albumin as a nutritional marker in kidney disease. JASN 21(2), 223–230 (2010).
Carrera-Jiménez, D., Miranda-Alatriste, P., Atilano-Carsi, X., Correa-Rotter, R. & Espinosa-Cuevas, Á. Relationship between nutritional status and gastrointestinal symptoms in geriatric patients with end-stage renal disease on dialysis. Nutrients 10(4), 425. https://doi.org/10.3390/nu10040425 (2018).
Becher, A. & Dent, J. Systematic review: Ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitis. Aliment Pharmacol. Ther. 33, 442–454 (2011).
Thomas, R. et al. Gastrointestinal complications in patients with chronic kidney disease—A 5-year retrospective study from a tertiary referral center. Ren. Fail. 35(1), 49–55 (2013).
Sarkio, S., Halme, L., Kyllönen, L. & Salmela, K. Severe gastrointestinal complications after 1,515 adult kidney transplantations. Transpl. Int. 17(9), 505–510 (2004).
Maru, R. M. Gastrointestinal symptoms and gastrointestinal quality of life in renal transplant patients: a descriptive cross sectional study. Masters. University of Nairobi (2016).
Nazeer, A., Rai, A. A. & Luck, N. H. Factors leading to dyspepsia in renal transplant recipients. Pan. Afr. Med. 28, 120. https://doi.org/10.11604/pamj.2017.28.120.12767 (2017).
Muller, E., Barday, Z., Mendelson, M. & Kahn, D. Renal transplantation between HIV-positive donors and recipients justified. S. Afr. Med. J. 102(6), 497–498 (2012).
Van Rensburg, C. J., Kulich, K. R., Carlsson, J. & Wiklund, I. K. What is the burden of illness in patients with reflux disease in South Africa? SAHARA J: Journal of Social Aspects of HIV/AIDS Research Alliance/SAHARA, Human Sciences Research Council. 2006 [cited 22/09/2018]. https://www.ajol.info/index.php/saharaj/article/view/50461.
Abdulrahman, I. S. & Al-Quorain, A. A. Prevalence of gastroesophageal reflux disease and its association with helicobacter pylori infection in chronic renal failure patients and in renal transplant recipients. Saudi J. Gastroenterol. 14(4), 183–186 (2008).
Ekberg, H. et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrol. Dial. Transplant. 25(6), 2004–2010 (2010).
Maes, B. et al. Severe diarrhea in renal transplant patients: Results of the DIDACT study. Am. J. Transplant. 6, 1466–1472 (2006).
Kim, K. J. & Lee, B. S. Central obesity as a risk factor for non-erosive reflux disease. Yonsei Med. J. 58(4), 743–748 (2017).
Ho, W. & Spiegel, B. M. R. The relationship between obesity and functional gastrointestinal disorders. Causation, association, or neither?. Gastroenterol. Hepatol. (N Y) 4(8), 572–578 (2008).
Singh, M. et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: A prospective intervention trial. Obesity (Silver Spring) 21(2), 284–290 (2013).
Nandurkar, S. et al. Relationship between body mass index, diet, exercise and gastro-oesophageal reflux symptoms in a community. Alimetary Pharmacol. Ther.. 20(5), 497–505 (2008).
Knox, T. A. et al. Assessment of nutrional status, body composition and human immunodeficiency virus-associated morphological changes. Clin. Infect. Dis. 36(S2), S63–S68 (2003).
Mönkemüller, K. E., Call, S. A., Lazenby, A. J. & Wilcox, C. M. Declining prevalence of opportunistic gastrointestinal disease in the era of combination antiretroviral therapy. Am. J. Gastroenterol. 95(2), 457–462 (2000).
Takahashi, Y. et al. Upper gastrointestinal symptoms predictive of candida esophagitis and erosive esophagitis in HIV and non-HIV patients: An endoscopy-based cross-sectional study of 6011 patients. Medicine (Baltimore) 94(47), e2138. https://doi.org/10.1097/MD.0000000000002138 (2015).
Parvin, R., Koll, S., Shah, J., Jhaveri, M. & Reddy, M. Upper and lower gastrointestinal endoscopic findings in HIV-infected patients in the era of highly active antiretroviral therapy. Gastroenterol. Res. 11(2), 95–99 (2018).
Kulich, K. R. et al. Reliability and validity of the gastrointestinal symptom rating scale (GSRS) and quality of life in reflux and dyspepsia (QOLRAD) questionnaire in dyspepsia: A six-country study. Health Qual. Life Outcomes https://doi.org/10.1186/1477-7525-6-12 (2008).
Mann, C. J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg. Med. J. 20, 54–60 (2003).
Acknowledgements
This study was made possible through financial support from the National Research Foundation (NRF) Grants ID: 94193, 104599, The South African Sugar Association (SASA): Project 246 and Haley Stott Grant.
Author information
Authors and Affiliations
Contributions
C.M.: Conceptualization, data collection and writing. E.M.: Project facilitation, reviewing and editing. S.K., D.L., F.V.: Research design, reviewing and editing. Z.E.: Project administration, reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martin, C.J., Veldman, F.J., Labadarios, D. et al. Gastrointestinal symptoms in HIV-positive kidney transplant candidates and recipients from an HIV-positive donor. Sci Rep 11, 12592 (2021). https://doi.org/10.1038/s41598-021-92016-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92016-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.