Abstract
Temporary hypercapnia has been shown to increase cerebral blood flow (CBF) and might be used as a therapeutical tool in patients with severe subarachnoid hemorrhage (SAH). It was the aim of this study was to investigate the optimum duration of hypercapnia. This point is assumed to be the time at which buffer systems become active, cause an adaptation to changes of the arterial partial pressure of carbon dioxide (PaCO2) and annihilate a possible therapeutic effect. In this prospective interventional study in a neurosurgical ICU the arterial partial pressure of carbon dioxide (PaCO2) was increased to a target range of 55 mmHg for 120 min by modification of the respiratory minute volume (RMV) one time a day between day 4 and 14 in 12 mechanically ventilated poor-grade SAH-patients. Arterial blood gases were measured every 15 min. CBF and brain tissue oxygen saturation (StiO2) were the primary and secondary end points. Intracranial pressure (ICP) was controlled by an external ventricular drainage. Under continuous hypercapnia (PaCO2 of 53.17 ± 5.07), CBF was significantly elevated between 15 and 120 min after the start of hypercapnia. During the course of the trial intervention, cardiac output also increased significantly. To assess the direct effect of hypercapnia on brain perfusion, the increase of CBF was corrected by the parallel increase of cardiac output. The maximum direct CBF enhancing effect of hypercapnia of 32% was noted at 45 min after the start of hypercapnia. Thereafter, the CBF enhancing slowly declined. No relevant adverse effects were observed. CBF and StiO2 reproducibly increased by controlled hypercapnia in all patients. After 45 min, the curve of CBF enhancement showed an inflection point when corrected by cardiac output. It is concluded that 45 min might be the optimum duration for a therapeutic use and may provide an optimal balance between the benefits of hypercapnia and risks of a negative rebound effect after return to normal ventilation parameters.
Trial registration: The study was approved by the institutional ethics committee (AZ 230/14) and registered at ClinicalTrials.gov (Trial-ID: NCT01799525). Registered 01/01/2015.
Similar content being viewed by others
Introduction
Cerebral vasospasm and delayed cerebral ischemia (DCI) are much-feared complications and the main reason for prolonged hospitalization and persistent disabilities after aneurysmal subarachnoid hemorrhage (SAH). Cerebral blood flow (CBF) is—under physiological conditions—regulated by autoregulation as reactivity to changes of transmural wall tension, by equilibrium of vessel relaxing and constricting factors and by changes of the arterial partial pressure of carbon dioxide (PaCO2). While autoregulation is deranged and the balance of paracrine factors shift towards vessel contraction, the PaCO2 reactivity may be altered but is, in principle, intact after aneurysmal SAH1,2. Elevated PaCO2 can cause vasodilation of cerebral vessels via the carboanhydrase pathway, which, as a result of increasing pCO2, leads to a lowered pH in the CSF, which in turn leads to relaxation of the vascular smooth muscle cells. Another way is through direct interaction of PaCO2 with the endothelium of the cerebral vascular system3. The degree of reactivity of CBF after SAH has been shown to be an indicator for the course of the disease after SAH with poor reactivity indicating a high risk for delayed cerebral ischemia and poor prognosis. During delayed vasospasm of proximal intracranial vessel trunks, hyperventilation induces ischemia and infarction due to an additional constriction of small distal blood vessels and is associated with poor outcome after SAH4,5. However, previous trials investigated only minor PaCO2 variations around the normal range. Petridis and co-workers have demonstrated that permissive hypercapnia is not hazardous to patients after SAH if they have an external ventricular drainage (EVD) continuously draining cerebrospinal fluid (CSF) during the period of hypercapnia6. In a previous trial, it was demonstrated that more pronounced changes of PaCO2 can reproducibly affect CBF even after severe SAH suggesting a therapeutic potential of temporary hypercapnia7. However, it is well known that alterations of arterial blood gases are followed by adaptation mechanisms. Research data on high altitude adaptation showed that the effect of increased CBF due to hypercapnia is timely limited8,9.
The optimum duration of controlled hypercapnia and its effects on CBF and brain tissue oxygen saturation (StiO2) as a therapeutic tool against DCI are not clarified. In a previous proof-of-principle study7, the CBF reactivity was demonstrated in patients with poor-grade SAH. A stepwise increase of PaCO2 in 15 min intervals up to 60 mmHg resulted in a concordant increase of CBF, which did not result in a negative rebound effect, but a sustained increase of CBF after resetting the respiration volume to baseline parameters. The questions addressed in the present work were at what point the beneficial effects of long-term hypercapnia would be exhausted or reverse to possible negative effects. The measurements of CBF and StiO2 as the primary and secondary endpoints were performed invasively with an intracerebral thermodilution probe and noninvasively by bifrontal near-infrared spectroscopy, respectively. In this prospective trial we aimed to investigate the course of CBF during longer-term hypercapnia and determine the time-point at which physiologic adaptation mechanisms start to mitigate the CBF-increasing effect in order to locate the optimum duration of controlled hypercapnia in poor-grade SAH patients.
Material and methods
The study was approved by the institutional ethics committee (AZ 230/14, Ethics committee of the medical faculty, University of Wuerzburg, Germany) and registered at ClinicalTrials.gov (Trial-ID: NCT04687605, registered 01/01/2015). All methods were performed in accordance with the guidelines and regulations of the institutional ethics committee. Also, the study has been conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written consent of a legal guardian was obtained for each patient prior to inclusion into the study.
Inclusion criteria
Patients were eligible for recruitment if they had suffered severe aneurysmal SAH (Hunt/Hess grade 3–5, Fisher grade 3) no longer than 96 h ago, had received external ventricular drainage (EVD) due to occlusive hydrocephalus and early treatment of the ruptured aneurysm (coiling or clipping) after a 4-vessel angiography of the cerebral vessels. If no other condition like pulmonary complications after aspiration or elevated intracranial pressure prohibited a reduction of analgosedation after occlusion of the aneurysm, an attempt was made to reduce analgosedation. If the patient did not show appropriate reactions, anaesthetics were reapplied and the patient was included in the study.
Exclusion criteria
Age under 18 years, pregnancy, non-aneurysmal SAH and the presence of a not occluded aneurysm were exclusion criteria. Furthermore, patients suffering from chronic obstructive lung disease (COLD) were excluded because their cerebrovascular reactivity to changes of PaCO2 in the target range of this study is unclear. Patients eligible for inclusion who had an ICP over 20 mmHg were not included and were re-evaluated later for a possible start of the study intervention.
Safety measures and criteria for an interruption of the study intervention
Study interventions were performed in 24-h intervals (24 ± 1 h). Prior to the daily study intervention, cardiovascular parameters, blood gases and ICP were assessed. For documentation of ICP, the EVD was closed for 5–6 monitor lengths until a steady plateau was reached, then opened again for continuous drainage. If resting ICP was over 20 mmHg at the desired timepoint, the study intervention was not conducted and the patient re-assessed 2 h later. If ICP was still elevated over 20 mmHg, the study intervention was not performed on that day. Criteria to interrupt the trial intervention were predefined as an increase of ICP over 25 mmHg for more than two minutes, hypoxemia (PaO2 < 70 mmHg or sO2 < 90 mmHg) and severe arterial acidosis with an arterial pH-value under 7.25.
Termination of the study procedures
Study interventions were terminated on day 14. Predefined criteria to terminate the study procedures in an individual patient before day 14 were the cessation of therapy due to a poor prognosis of whatever reason according to the treating physicians’ estimation and if a reduction of analgosedation was possible and the study intervention was no longer feasible due to spontaneous breathing.
Respiratory settings and study intervention
All patients were intubated and mechanically ventilated as defined by the inclusion criteria. If not indicated for ventilatory reasons anyway, the respirator was brought into a volume-controlled ventilation mode for the daily study intervention (Servo-i, Maquet, Rastatt, Germany) maintaining the same respiratory minute volume (RMV). After a first arterial blood gas analysis (RapidLab, Siemens Health Care, Erlangen, Germany), respirator settings were adjusted to reach a PaCO2 within the normal range (36–44 mmHg). This was confirmed by another blood gas analysis. The respiratory rate was then reduced to lower the RMV by 40% in every patient. This value was adopted from a previous study assessing the feasibility and risk profile of the study intervention7 and served as a guide value for further respirator adjustments to reach the target PaCO2 of 55 mmHg. Arterial blood gases were then measured in 5-min intervals and fine adjustment of the respirator settings made until a steady PaCO2 between 50 and 55 mmHg was reached. Thereafter, arterial blood gases were measured in 15-min intervals and further respirator adjustments made in order to maintain a stable PaCO2 of 55 mmHg for 120 min. All other respiratory settings (tidal volume, inspiratory-expiratory ratio, and inspiratory fraction of oxygen) remained unchanged. Respirator settings were returned to pre-trial settings to obtain normal PaCO2 levels after 2 h.
Target parameters
Parameters measuring CBF and brain tissue oxygenation were the predefined study endpoints.
Direct measurement of CBF (primary endpoint): The course of CBF, was measured by an intracerebral thermodilution probe (Q-Flow 500®) located in the right frontal cortex. The probe was placed 1.5 cm anterior to the external ventricular drainage and was monitored by a Bowman Perfusion Monitor® (Hemedex™, Cambridge, MA, USA). To obtain a continuous measurement and avoid recalibration during the study intervention, the automatic calibration was paused during the time of the study intervention10.
Transcranial Doppler sonography (TCD): Prior to the beginning of each study intervention, baseline measurements of TCD were made. At each time-point during the study intervention, TCD measurements were repeated. Measurements were conducted by a well-trained medical technician otherwise not involved in the study procedures with an experience of over 15 years of TCD measurements.
Cerebral tissue oxygen saturation (StiO2) (secondary endpoint): StiO2 was measured by bilateral near-infrared spectroscopy (NIRS) electrodes (INVOS®, Covidien, Neustadt/Donau, Germany), which were attached on both sides of the forehead. A baseline value was set prior to the intervention to which the following measurements (every 15 min for 2 h) were compared.
Physiological parameters
All patients were treated on a neurointensive care unit and received the routine continuous monitoring of cardiovascular parameters including electrocardiography, invasive blood pressure monitoring, monitoring of intracranial pressure via an EVD and central venous pressure (CVP). In patients included into the study, a pulse contour-based measurement of cardiac output (CO) was added to the invasive blood pressure monitoring (proAQT, Pulsion Medical Systems SE, Munich, Germany) in order to follow changes of CO during hypercapnia.
Protocol and statistical analysis
CBF, StiO2, ICP, arterial blood gases, pH and hemodynamic parameters were measured at baseline and at 0, 15, 30, 45, 60, 75, 90, 105 and 120 min of the intervention and documented in a paper-based examination protocol. Acquired data was transferred to Excel®-Worksheets (Microsoft®, Redmont, WA, USA). A Kolmogorov–Smirnov test for data rows was used to decide if a normal distribution can be assumed or has to be rejected. Dynamic changes over time were analysed using a one-way ANOVA for repeated measures. In case of statistical significance, time-points were compared to baseline values using a Dunnett’s post hoc test. Statistical significance was defined if p < 0.05. Statistical analysis was performed using GraphPad Prism 4.0 Statistical Software (GraphPad, La Jolla, CA, USA).
Ethics approval and consent to participate
The study was approved by the institutional ethics committee (AZ 230/14) and registered at ClinicalTrials.gov (Trial-ID: NCT04687605). Written consent of a legal guardian was obtained for each patient prior to inclusion into the study.
Results
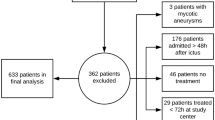
All 12 patients included in this trial suffered from severe aneurysmal SAH. The average and median Hunt/Hess grade was 4 with four patients classified as Hunt/Hess grade 3, four patients as Hunt/Hess grade 4, and four patients as Hunt/Hess grade 5. All patients had thick subarachnoid blood layers and clots according to grade 3 of the Fisher scale, in ten patients additional intracerebral and/or intraventricular blood was detected in the initial CT scan. Cerebral angiogram at admission revealed a total of 15 aneurysms (3 patients with two aneurysms), of which 13 were occluded within 96 h after ictus, 6 by surgical clipping, and 7 by endovascular coiling. The remaining two aneurysms were not considered to be the origin of SAH in patients with more than one aneurysm. Mean age at admission was 48 ± 7.93 years, 9 were female and 3 were male. Patient characteristics, hemorrhage-related features and location and treatment of the ruptured aneurysms are depicted in Table 1. A total of 106 trial interventions were performed from day 4 to day 14 after SAH.
Clinical and radiological course
Mean initial Glasgow Coma Score (GCS) was 8 with deterioration to a GCS of 6 prior to intubation. All patients had developed occlusive hydrocephalus and received an external ventricular drainage (EVD). After occlusion of the aneurysm anesthetics were withdrawn and the patients were allowed to wake up. If regain of consciousness was not appropriate and extubation not possible, anesthetics were readministered and the patients were included in the study after written informed consent was given by a legal guardian. 11 of 12 patients showed a significant increase in daily transcranial Doppler sonography (TCD) and/or Perfusion-CT and underwent follow-up angiography.
Ventilation and blood gas analysis
To reach a target PaCO2 of 55 mmHg, the baseline RMV was reduced by 40% in every patient. After the first arterial blood gas control, the respirator settings were further adjusted (Fig. 1). Under this procedure, PaCO2 changed from a baseline of 38.75 ± 3.75 mmHg to 47.15 ± 4.81 mmHg at the first measurement under hypercapnia. Thereafter, PaCO2 further increased to constantly remain between 50 and 55 mmHg until the end of the trial intervention. Arterial partial pressure of oxygen (PaO2) was not influenced by the alterations of the respirator settings with a mean PaO2 of 112.55 mmHg (± 24.00 mmHg) before trial intervention and a mean PaO2 of 111.67 mmHg (± 13.67 mmHg) after 120 min of hypercapnia. Prolonged hypercapnia led to a decrease in pH as a sign of respiratory acidosis. Nevertheless, no patient reached a pH under 7.25 as a criterion for trial interruption. Mean pH was 7.44 with normocapnia before trial interventions and decreased to 7.28 after 75 min with a slight increase to 7.29 after 90 min, reaching a steady state of 7.29 at 90 to 120 min of hypercapnia (Table 2).
Change of Respiratory Minute Volume (RMV, bars) and concomitant increase of arterial partial pressure of carbon dioxide (PaCO2, line plot). At the beginning of the study intervention, RMV was reduced by 40%. Thereafter, minor corrections were made according to blood gas analyses. Data presented as Mean ± SD; 1-way ANOVA for Repeated Measurements; *p < 0.05, **p < 0.01, ***p < 0.001, plots and error bars presented as Mean ± SD (standard deviations).
Intracranial pressure, arterial blood pressure, cerebral perfusion pressure
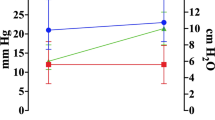
Intracranial pressure (ICP) increased slightly but significantly within the first 45 min. Baseline ICP before the start of the study intervention was 11.7 ± 2.4 mmHg at a PaCO2 of 38.8 ± 3.7 mmHg. The maximum ICP value of 12.7 ± 3.0 mmHg was recorded immediately after induction of hypercapnia at a PaCO2 of 47.1 ± 4.8. Thereafter, a surplus CSF collection resulted in a continuous decline of ICP thereafter to a final value of 11.8 ± 2.6 mmHg after 120 min (Fig. 2). Mean arterial blood pressure (MABP) increased from a baseline of 100.4 ± 9.6 mmHg to a maximum of 102.2 ± 11.3 mmHg immediately after the induction of hypercapnia and continuously decreased thereafter to reach a final value of 96.0 ± 8.0 mmHg after 120 min. Baseline CPP was 88.6 ± 9.9 mmHg to increase to a maximum of 89.5 ± 11.2 mmHg after induction of hypercapnia and continuously decrease to 84.2 ± 7.9 mmHg at the end of the monitoring time. The decreases of MABP and CPP both were significant from 60 min until 120 min after induction of hypercapnia.
Course of intracranial pressure (ICP) during the study intervention. All patients had an external ventricular drainage which was allowed to continuously drain cerebrospinal fluid during the intervention. No relevant increase of ICP was observed during the monitoring period. Data presented as Mean ± SD; 1-way ANOVA for Repeated Measurements; *p < 0.05, **p < 0.01, ***p < 0.001, plots and error bars presented as Mean ± SD (standard deviations).
Cerebral tissue oxygenation
StiO2 measured over the right forebrain increased to a maximum of 109 ± 8.8% of baseline 60 min after induction of hypercapnia at a PaCO2 of 54.22 ± 5.27 mmHg. Over the left forehead, StiO2 increased to a maximum 109 ± 7.9% of baseline after 60 min into the trial intervention, respectively. The course of StiO2 during hypercapnia is depicted in Fig. 3.
Changes of cerebral tissue oxygen saturation (StiO2) was measured by near infrared spectroscopy (NIRS). StiO2 increased over both hemispheres with a peak after 60 min. Thereafter, the beneficial effect of the study intervention seemed to weaken. (*p < 0.05, **p < 0.01, ***p < 0.001). Data presented as Mean ± SD; 1-way ANOVA for Repeated Measurements; *p < 0.05, **p < 0.01, ***p < 0.001, plots and error bars presented as Mean ± SD (standard deviations).
Transcranial Doppler sonography
Mean flow velocities (MFV) in TCD increased in both middle cerebral arteries during the trial intervention. In the right middle cerebral artery (MCA), MFV increased from a baseline of 112 ± 35 cm/s to a steady level of 140–145 cm/s between 45 and 120 min into the trial intervention. In the left MCA, MFV increased from a baseline of 106 ± 51 cm/s to values between 135 and 140 cm/s from 45 to 120 min after induction of hypercapnia. The increase was significant throughout the entire period of monitoring (Fig. 4).
Mean flow velocities (MFV) in transcranial Doppler sonography (TCD) increased during the study intervention in all intracranial vessels. The graph exemplarily depicts the course of MFV in both middle cerebral arteries. The parallel increase of MFV in the high cervical extracranial internal carotid artery (ICA ec) indicates that the increase of flow velocities is due to a global increase of cerebral blood volume and perfusion. (*p < 0.05, **p < 0.01, ***p < 0.001). Data presented as Mean ± SD; 1-way ANOVA for Repeated Measurements; *p < 0.05, **p < 0.01, ***p < 0.001, plots and error bars presented as Mean ± SD (standard deviations).
Cardiac output
During the trial intervention, cardiac output (CO) increased from a baseline of 6.3 ± 2.0 l to 6.6 ± 2.0 l (106%) after induction of hypercapnia, 7.1 ± 2.3 l (114%) after 45 min and 7.5 ± 2.5 l (120%) after 120 min. This increase was caused by an equal 10% increase of stroke volume and heart rate.
Cerebral blood flow
Increasing PaCO2 from a baseline value of 38.77 ± 3.75 mmHg to 53.18 ± 7.16 mmHg after 120 min resulted in a final increase of cerebral blood flow (CBF) to149 ± 93% of baseline. After 30, 45, 60, 75 and 90 min CBF increased to 141 ± 92%, 146 ± 99%, 140 ± 80%, 144 ± 80%, 151 ± 86%, and 147 ± 93% of baseline values, respectively. The peak value was recorded after 75 min (Fig. 5). In order to calculate the intervention’s direct effect upon the brain vasculature, the increase of CBF was corrected by the concomitant increase of CO resulting in a maximum net increase of CBF to 132% of baseline after 45 min and decreased thereafter to reach a final value of 129% of baseline at the end of the monitoring period (Fig. 6).
The course of cerebral blood flow (CBF) during the study intervention was measured by a right frontal intracerebral thermodilution probe. (*p < 0.05, **p < 0.01, ***p < 0.001). Data presented as Mean ± SD; 1-way ANOVA for Repeated Measurements; *p < 0.05, **p < 0.01, ***p < 0.001, plots and error bars presented as Mean ± SD (standard deviations).
Time course of cerebral blood flow (CBF, black dots), cardiac output (CO, white dots) and CBF corrected for CO (grey dots). The correction subtracts the increase of CO from the increase of CBF in order to calculate the intrinsic CBF-increasing effect of the study intervention in the cerebral vasculature. The peak increase was noted after 45 min. Data presented as Mean ± SD; 1-way ANOVA for Repeated Measurements; *p < 0.05, **p < 0.01, ***p < 0.001, plots and error bars presented as Mean ± SD (standard deviations).
Discussion
In a previous phase 1 trial, it was observed that CBF can be reproducibly increased by intermittent controlled hypercapnia in the days following aneurysm rupture in patients with poor grade SAH7,11. In that trial, CBF increased during a sequential increase of arterial PaCO2. After resetting mechanical ventilation to baseline parameters, CBF showed a slow and asymptotic return to starting levels without a negative rebound effect. This observation suggested that a longer duration of hypercapnia might even extend the CBF-increasing effect. The present study was planned as a dose optimization study in order to identify the time-point at which CBF reaches a maximum. It was assumed that after a maximum CBF increase upon elevated PaCO2, buffer mechanisms in blood and CSF may result in adaptation mechanisms that lead first to an attenuation of the CBF-increasing effect and then to a negative rebound effect after the hypercapnic challenge is terminated. For safety reasons, a preliminary termination of the study intervention was included into the study protocol if the CBF-enhancing effect secondarily declined by more than 25%. In order to identify the time-point of the maximum intrinsic effect of controlled hypercapnia upon the cerebral vasculature, the value was corrected by the possible inotropic effect of prolonged hypercapnia which has been described previously12. Indeed, a positive inotropic effect was also noted during the study interventions conducted in this present study, moderate but statistically significant. It resulted in a “net” optimum effect of the hypercapnic challenge on the cerebral vasculature of 45 min. After the proof of principle in a previous study and the present results we suggest that this may be the basis for an evaluation of efficacy in a controlled clinical trial.
Aneurysmal SAH is still a life-threatening incident with poor over-all prognosis. Its course is characterized by serially occurring ischemic events. Immediately after the rupture of the aneurysm, cerebral perfusion pressure (CPP) decreases due to a sudden increase of ICP resulting in a reduction of CBF and global ischemia. Simultaneously or shortly thereafter, diffuse early arterial vasoconstriction has been observed and contributes to the persistent perfusion deficit in the early stage of SAH13,14. Due to developments in emergency care, an increasing number of patients with poor-grade SAH can be adequately resuscitated and find their way to a specialized unit. Patients in a poor clinical condition and large amounts of blood in the subarachnoid space, in turn, are prone to develop vasospasm and delayed cerebral ischemia. To date, no single drug or manipulation has proven undoubtedly effective to prevent or treat the risk for delayed cerebral ischemia (DCI). Even the evidence for nimodipine, the most widely used pharmacological attempt to prevent DCI is based on a single trial which was conducted more than 30 years ago before endovascular aneurysm and vasospasm therapy was invented and without intensive care therapy with contemporary standards15,16.
Delayed vasospasm after a few days, caused by endothelial dysfunction, structural changes of the vessel wall, and inflammatory processes is a decisive factor for delayed ischemic neurological deficits (DIND) and delayed cerebral ischemia (DCI)17,18. A variety of clinical and experimental studies have investigated various approaches to prevent vasospasm15,19,20,21. But even initially promising trials, e.g. with endothelin A receptor antagonists, successful in treating vasospasm, did not prevent DCI or improve clinical outcome22.
Most likely, DCI is a multifactorial phenomenon with delayed cerebral vasospasm, cortical electrical hyperactivity with enhanced energy expenditure, disturbed equilibrium of local vasodilatory and vasoconstrictive paracrine factors and a possible primary metabolic derangement contributing to ischemic damage. However, these factors finally turn into a common finish line, the mismatch of oxygen supply and demand of brain tissue resulting in a breakdown of aerobic metabolism, depletion of energy stores and terminal depolarization of neurons and glial cells23,24.
From a pathophysiological point of view, delayed ischemia after SAH is a multifactorially conditioned slowly arising perfusion deficit rather than a sudden and complete ischemia due to severe vasospasm23,25,26. Energy depletion, therefore, is also likely to be gradual. Thus, the rationale behind intermittent controlled hypercapnia is to temporarily increase a critically reduced CBF so that energy stores can recover.
It has long been shown that cerebral autoregulation of arterial blood pressure is disturbed after severe aneurysmal SAH and that the equilibrium of local factors are shifted towards constriction. However, the reactivity to changes of PaCO2 remains vital2,4,27,28. Increased PaCO2 may not have an effect on CBF and vessel diameter in the early stage of SAH as recently published by Friedrich and co-workers29. For the chronic phase, however, previous work has shown a highly reproducible increase of StiO2 and CBF under staged hypercapnia. Since this effect was still reproducible during angiographically proven vasospasm7 the concept of maximum peripheral vessel dilation downstream of large-trunk vasospasm may have to be rethought. At the same time, it gives space for a therapeutic intervention. A previous phase 1 study has not only shown the proof of principle without noteworthy negative side effects, but also a surprising reduction of the incidence of DCI and relatively favorable outcome as compared to historical controls and data published in literature7,11.
The previous results make it seem worthwhile to evaluate this procedure regarding its therapeutic effect. However, to date no data about the ideal duration of hypercapnia on CBF and cerebral tissue oxygenation exists. From a physiological point of view these positive effects must be temporary since buffer mechanisms in blood and CSF, connected via the carboanhydrase enzyme, trigger an adaptation to long-term changes of CO230.To the best of our knowledge, there is no literature about the long-term effects of hypercarbia upon CBF and the course of its adaptation. From high altitude research it is known that mountaineers start to hyperventilate during ascent, which results in a decrease of CBF. After several hours or days, depending on the altitude, acclimatization processes are leading to a normalization of ventilation and physiological parameters8,9. However, this is not the timeframe of interest for the particular needs of our patient collective. In contrast to our study protocol the effects in high altitude and its impact on CBF do not occur in a range of seconds or minutes but rather within hours and days during or after an ascent. An additional factor is the lower partial pressure of O2 in high altitude leading to hypoxia; therefore, this data is not comparable with normoxic conditions on an intensive care unit. Adaptation mechanisms to long-term hypercapnia, e.g. buffer in the cerebrospinal fluid or blood via carboanhydrase are neither well known nor investigated in trials up to now. Alterations of the pH of the brains’ extracellular space as a regulator of the cerebrovascular response to CO2 were discussed by Lassen et al.31. Yoon et al. suggest that CBF is regulated by pH-changes of the CSF and that possibly both, PaCO2 and CSF-pH “independently and concomitantly regulate cerebrovascular contractility”. According to their findings, an increase of PaCO2 (or a decrease in pH) might directly lead to vasodilatation by hyperpolarization of smooth muscle cells via potassium channels. Indirectly, vasodilatation could occur through endothelium-dependent mechanisms3. Raichle and co-workers examined the effects of transient hyperventilation for several hours on CBF in healthy volunteers covering the period that is of interest for our particular study setting. They showed that CBF decreased with the onset of hyperventilation, but gradually recovered again under continuous hyperventilation and finally exceeded baseline levels in terms of a rebound effect when ventilation was returned to normal. PaCO2 also decreased initially but remained constantly low under hyperventilation and returned to baseline parameters after ending of hyperventilation32.
We assume that the time-course, extent and reaction upon adaptation to hypercapnia is approximately the same as the adaptation to hyperventilation as reported by Raichle et al.32. This implies that in patients with SAH, whose CBF is critically reduced, there is the danger that a negative rebound effect may carry CBF below ischemic thresholds even if there is a beneficial effect during the study intervention. Therefore, the duration of CBF hypercapnia must not exceed a safe time-frame. The additional measurement of StiO2 as a secondary endpoint was therefore mainly because the CBF measurement only covers the right frontal lobe and is therefore more or less a punctual measurement. Bilateral NIRS, on the other hand, covers both MCA and ACA territories, albeit only as a relative indicator for an increase in CBF. NIRS, as well as TCD, however, showed a very good correlation with the right frontal CBF values. Thus, we can conclude that the effect of hypercapnia on CBF is 1. not only selective, and 2. not a steal effect from critically perfused areas into “healthy” territories.
Previous data on the effect of graded hypercapnia after aneurysmal SAH showed a sustained effect on CBF and StiO2 and no rebound effects or adverse events7. In this present trial, under continuous hypercapnia of 50–55 PaCO2 for 120 min, CBF and StiO2 reached peak values after 75 min and 60 min, respectively. Hence, at first glance 60 to 75 min seem to be the ideal duration for the highest treatment benefit. Regarding the parallel increase of cardiac output under prolonged hypercapnia the results have to be corrected in order to calculate the maximum intrinsic effect upon cerebral vessels. As a result of disturbed cerebral autoregulation after severe SAH, systematic increase of cardiac output alone, e.g. via application of catecholamines, could lead to an increase in CBF. In the face of disrupted autoregulation, changes of blood pressure and cardiac output are transferred into brain circulation as well as into other parts of systemic circulation33. Accordingly, we found that blood flow in the high cervical extracranial carotid artery increased during study intervention (Fig. 4). Therefore, we find it necessary to correct the gross increase of CBF for the increase of CO in order to determine the net impact of the hypercapnic challenge on cerebral perfusion. Adjusting CBF by cardiac output, the hypercapnia-induced net increase of CBF was highest as early as 45 min after the start of the trial intervention. Given the statistically significant elevation of cardiac output after 60 min with further increase under prolonged hypercapnia, sustained therapeutic hypercapnia after reaching its maximum effect on CBF seems not advisable; adaptation mechanisms and negative cardiac effects may bear the danger of a negative rebound effect. The latter was not registered in this present study after 120 min of hypercapnia. However, these observations are based on a few cases and interventions. The reason for this is a practical one. This is not an experimental but an ICU setup and the patients are unexceptionally in a critical condition. Thus, they are not available for study purposes in an unlimited extent. Before and after the 2-h study intervention, a variety of medical and nursing measures are necessary which withdraw the patient from specific monitoring measures. For further TCD-monitoring this is a lack of access to the patient. For the primary endpoint it is rather a technical reason since the Hemedex perfusion probe then exceeds the maximum interval the recalibration can be delayed. This interrupts the continuous measurement and may result in incorrect values. Therefore, our conclusion bases on a physiological deliberation. Since a CO2 challenge severely interferes with fundamental physiological regulation mechanism it may potentially cause other unwanted side effects. In this particular kind of intervention safety aspects are of utmost priority. Hence, it is the auxiliary concept that the optimum duration of hypercapnia is the time-span before buffer mechanisms cause a secondary decline of CBF under hypercapnia after a primary increase. Graphically this is the inflection point of the CBF-curve. Since it was the purpose to find the optimum intrinsic effect on brain vessels, the increase of cardiac output has to be subtracted from the gross increase of CBF in the face of deranged autoregulation.
Conclusion
As a result of this study we conclude that 45 min of controlled hypercapnia might be the most suitable duration regarding the CBF-increasing effect, the patients’ safety and the feasibility in an intensive care setting. Considering that a complete normalization of cardiac and physiologic parameters is achieved several hours after returning to baseline ventilation parameters it might be even more beneficial to repeat the CO2-intervention at higher frequency, e.g. 2 or 3 times a day, although this has not been explicitly tested in the current trial. A randomized, controlled trial with 45 min of controlled hypercapnia at 12-h intervals is planned for further evaluation of the therapeutic efficacy of this method in poor-grade aneurysmal SAH.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CBF:
-
Cerebral blood flow
- CO:
-
Cardiac output
- CPP:
-
Cerebral perfusion pressure
- CSF:
-
Cerebrospinal fluid
- CVP:
-
Central venous pressure
- DCI:
-
Delayed cerebral ischemia
- DIND:
-
Delayed ischemic neurological deficits
- EVD:
-
External ventricular drainage
- GCS:
-
Glasgow Coma Score
- ICA ec:
-
Extracranial internal carotid artery
- ICP:
-
Intracranial pressure
- MABP:
-
Mean arterial blood pressure
- MFV:
-
Mean flow velocities
- NIRS:
-
Near infrared spectroscopy
- PaCO2 :
-
Arterial partial pressure of carbon dioxide
- RMV:
-
Respiratory minute volume
- SAH:
-
Subarachnoid hemorrhage
- StiO2 :
-
Brain tissue oxygen saturation
- TCD:
-
Transcranial Doppler sonography
References
Diringer, M. N., Kirsch, J. R., Hanley, D. F. & Traystman, R. J. Altered cerebrovascular CO2 reactivity following subarachnoid hemorrhage in cats. J. Neurosurg. 78, 915–921 (1993).
Schmieder, K., Jarus-Dziedzic, K., Wronski, J. & Harders, A. CO2 reactivity in patients after subarachnoid haemorrhage. Acta Neurochir. (Wien) 139, 1038–1041 (1997).
Yoon, S., Zuccarrello, M. & Rapoport, R. M. pCO2 and pH regulation of cerebral blood flow. Front. Physiol. 3, 365 (2012).
Hassler, W. & Chioffi, F. CO2 reactivity of cerebral vasospasm after aneurysmal subarachnoid haemorrhage. Acta Neurochir. (Wien) 98, 167–175 (1989).
Carrera, E. et al. Cerebrovascular carbon dioxide reactivity and delayed cerebral ischemia after subarachnoid hemorrhage. Arch. Neurol. 67, 434–439 (2010).
Petridis, A. K. et al. The effect of lung-protective permissive hypercapnia in intracerebral pressure in patients with subarachnoid haemorrhage and ARDS. A retrospective study. Acta Neurochir. (Wien) 152, 2143–2145 (2010).
Westermaier, T. et al. Controlled hypercapnia enhances cerebral blood flow and brain tissue oxygenation after aneurysmal subarachnoid haemorrhage: Results of a phase 1 study. Neurocrit. Care 25, 205–214 (2016).
Ainslie, P. N. et al. Influence of sympathoexcitation at high altitude on cerebrovascular function and ventilator control in humans. J. Appl. Physiol. 1985(113), 1058–1067 (2012).
Smith, K. J. et al. Influence of high altitude on cerebral blood flow and fuel utilization during exercise and recovery. J. Physiol. 592, 5507–5527 (2014).
Wolf, S., Vajkoczy, P., Dengler, J., Schürer, L. & Horn, P. Drift of the Bowman Hemedex® cerebral blood flow monitor between calibration cycles. Acta Neurochir. Suppl. 114, 187–190 (2012).
Westermaier, T. et al. Controlled transient hypercapnia: A novel approach for the treatment of delayed cerebral ischemia after subarachnoid hemorrhage?. J. Neurosurg. 121, 1056–1062 (2014).
Kiely, D. G., Cargill, R. I. & Lipworth, B. J. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest 109, 1215–1221 (1996).
Westermaier, T., Stetter, C., Koehler, D., Weiland, J. & Lilla, N. Acute reaction of arterial blood vessels after experimental subarachnoid haemorrhage: An in vivo microscopic study. J. Neurol. Sci. 396, 172–177 (2019).
Uhl, E., Lehmberg, J., Steiger, H. J. & Messmer, K. Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery 52, 1307–1315 (2003).
Rinkel, G. J., Feigin, V. L., Algra, A., Vermeulen, M. & van Gijn, J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD000277 (2002).
van den Bergh, W. M., Mees, S. M. & Rinkel, G. J. Intravenous magnesium versus nimodipine in the treatment of patients with aneurysmal hemorrhage: A randomized study. Neurosurgery 59, E1152 (2006).
Sehba, F. A., Mostafa, G., Knopman, J., Friedrich, V. & Bederson, J. B. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J. Neurosurg. 101, 633–640 (2004).
Hughes, J. T. & Schianchi, P. M. Cerebral artery spasm A histological study at necropsy of the blood vessels in cases of subarachnoid hemorrhage. J. Neurosurg. 48, 515–525 (1978).
Westermaier, T. et al. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: A randomized, placebo-controlled, clinical study. Crit. Care Med. 38, 1284–1290 (2010).
Diringer, M. N. & Zazulia, A. R. Aneurysmal subarachnoid hemorrhage: Strategies for preventing vasospasm in the intensive care unit. Semin. Respir. Crit. Care Med. 38, 760–767 (2017).
Hanggi, D., Beseoglu, K., Turowski, B. & Steiger, H. J. Feasibility and safety of intrathecal nimodipine on posthaemorrhagic cerebral vasospasm refractory to medical and endovascular therapy. Clin. Neurol. Neurosurg. 110, 784–790 (2008).
Macdonald, R. L. et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 39, 3015–3021 (2008).
Macdonald, R. L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 10, 44–58 (2014).
Macdonald, R. L. & Schweizer, T. A. Spontaneous subarachnoid haemorrhage. Lancet 389, 11–17 (2017).
Etminan, N., Vergouwen, M. D., Ilodigwe, D. & Macdonald, R. L. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 31(6), 1443–1451 (2011).
Vergouwen, M. D., Vermeulen, M., Coert, B. A., Stroes, E. S. & Roos, Y. B. Microthrombosis after aneurysmal subarachnoid hemorrhage: An additional explanation for delayed cerebral ischemia. J. Cereb. Blood Flow Metab. 28(11), 1761–1770 (2008).
Diringer, M. N. et al. Cerebrovascular CO2 reactivity during delayed vasospasm in a canine model of subarachnoid hemorrhage. Stroke 22, 367–372 (1991).
Diringer, M. N., Kirsch, J. R. & Traystman, R. J. Reduced cerebral blood flow but intact reactivity to hypercarbia and hypoxia following subarachnoid hemorrhage in rabbits. J. Cereb. Blood Flow Metab. 14, 59–63 (1994).
Friedrich, B. et al. CO2 has no therapeutic effect on early microvasospasm after experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 34, e1-6 (2014).
Raichle, M. E. & Plum, F. Hyperventilation and cerebral blood flow. Stroke 3(5), 566–575 (1972).
Lassen, N. A. Cerebral circulation in anaesthesia. Ann. R. Coll. Surg. Engl. 46, 34–135 (1970).
Raichle, M. E., Posner, J. B. & Plum, F. Cerebral blood flow during and after hyperventilation. Arch. Neurol. 23, 394–403 (1970).
Budohoski, K. P. et al. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat. Rev. Neurol. 9(3), 152–163 (2013).
Funding
Open Access funding enabled and organized by Projekt DEAL. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg. The study was supported by the Else-Kröner-Fresenius Stiftung (2013_A171).
Author information
Authors and Affiliations
Contributions
C.S. together with F.W., N.L., J.W., E.K. and T.W. performed the trial interventions, analyzed and interpreted data; C.S. and T.W. contributed to manuscript writing and drafted the manuscript and figures. C.S., R.M. and T.W. contributed to conception and design of the study, T.W. provided funding. R.M. and R.-I. E. critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stetter, C., Weidner, F., Lilla, N. et al. Therapeutic hypercapnia for prevention of secondary ischemia after severe subarachnoid hemorrhage: physiological responses to continuous hypercapnia. Sci Rep 11, 11715 (2021). https://doi.org/10.1038/s41598-021-91007-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91007-7
This article is cited by
-
Brain Oxygenation Response to Hypercapnia in Patients with Acute Brain Injury
Neurocritical Care (2024)
-
Respiratory challenges and ventilatory management in different types of acute brain-injured patients
Critical Care (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.