Abstract
In this study, an underutilized citrus family fruit named grapefruit was explored for the extraction of lycopene using supercritical carbon dioxide (CO2) extraction technique. An experimental design was developed using response surface methodology to investigate the effect of supercritical carbon dioxide (CO2) operating parameter viz., pressure, temperature, CO2 flow rate, and extraction time on the extraction yield of lycopene yield from grapefruit. A total of 30 sets of experiments were conducted with six central points. The statistical model indicated that extraction pressure and extraction time individually, and their interaction, significantly affected the lycopene yield. The central composite design showed that the polynomial regression models developed were in agreement with the experimental results, with R2 of 0.9885. The optimum conditions for extraction of lycopene from grapefruit were 305 bar pressure, 35 g/min CO2 flow rate, 135 min of extraction time, and 70 °C temperature.
Similar content being viewed by others
Introduction
The overwhelming evidence on the reduced risk of dreadful diseases by consuming vegetable- and fruit-rich diets has triggered humankind to search for new biological resources. These natural resources contain several bioactive phytochemicals, which fight against many human diseases and are used as food preservatives and colorants in the food industry. Many researchers have found that natural food colors are mostly inherited from anthocyanin, carotenoids, chlorophyll, betalains, iridoids, phycobiliproteins, etc.1,2,3,4. Among them, carotenoids have gained more importance and attracted extensive investigation due to its dominating antioxidant properties5,6,7. Around 600 types of carotenoids have been identified on earth. About 40 of these carotenoids have been found in the human diet. However, only 14 types of carotenoids can be absorbed in the human digestive tract, primarily lycopene, α- & β- carotene, zeaxanthin, and lutein others8,9,10. Lycopene is one of the most significant carotenoids due to its dominant antioxidant properties, compared to β-carotene and the others. Lycopene is a red-colored compound and has the identical chemical and molecular structure to the β-carotene. It is a long-chain hydrocarbon (–CH) compound that contains 40 carbons with alternating 13 double bonds and eight isoprene units1. Several studies have investigated the free radical invasion potential of the lycopene at the cellular membrane surface. Studies have shown that it triggers the primary defence mechanism of the human body against several chronic diseases and plays a central role in mitigating oxidative stress as it lessens the inducible nitric oxide synthase activation1,8,11.
The top five lycopene sources among fruits and vegetables are watermelon, pink guava, papaya, tomatoes, and grapefruit, containing the lycopene content of up to 72, 53, 53, 42 34 µg/g (wet weight) respectively1. These products, except grapefruit, are commercially used in our daily lives. Nonetheless, although it contains a relatively fair amount of lycopene, grapefruit is limited by its higher acidity level and significantly bitter taste12. Grapefruit (Citrus paradisi) is a subtropical citrus fruit, also known as “fruit from the paradise” because of its health imparting and disease-preventing properties. It contains several phytochemicals such as lycopene, β-carotene, ascorbic acid, vitamin A and naringin. The presence of naringin causes the consumer’s unacceptability of the grapefruit as it causes a strong bitter taste, which results in very limited or no utilization12,13. Very few attempts have been made for the value addition to grapefruit, such as solvent extraction as well as ultrasound assisted extraction of lycopene14,15, and also by using petroleum ether as extraction solvent16 for successful extraction of this bioactive pigment called lycopene. Apart from this, oil extraction and isolation of polysaccharides has also been carried out from grapefruit17. However, it is necessary to carry out a sustainable and food-grade extraction of lycopene from grapefruit to commercialize as an antioxidant and natural colorant into the food system.
The extraction of lycopene from fruits and vegetables is done using a solvent extraction method, which is the most prevalent and commercial method used in the natural colorant industries. Using standard non-polar solvents like hexane, tetrahydrofuran, and chloroform, is non-sustainable and unsuitable for human consumption15,18. The solvent extraction process has several disadvantages: toxicity, disposal of solvent, a large quantity of organic solvent needed for extraction, high extraction temperatures, prolonged extraction time, less extraction efficiency, and dilution of the extract19,20. Moreover, higher extraction temperatures and prolonged extraction time lead to significant lycopene degradation, resulting in the isomerization of stable trans-isomer to a relatively unstable cis-isomer21,22.
These setbacks of the solvent extraction process necessitate the development of safer and cleaner methods of extraction of lycopene. One such technique is the supercritical fluid extraction (SFE), which utilizes supercritical fluids (the solvent) to separate the desired component (extractant) from the intricate food matrix19,23. The extraction of biotic compounds using a supercritical fluid emerges to be a green technology and produces the purest, cleanest, and safest product among all known extraction techniques available today. Although costlier, it is preferred because of its higher extraction efficiency and absence of residual solvent in the extracted material. Besides, it allows working at moderate temperatures, which invariably reduces degradation caused by thermal effect. Light and oxygen’s negligible presence significantly enhances stability and prevents oxidation reactions19,20. It is also non-toxic and smoothly gets separated from the extract. Carbon dioxide is generally the preferred solvent in this technique because it converts into the supercritical stage at 31 °C under a pressure of 74 bars19,23. Several extraction parameters such as extraction temperature, pressure, time, CO2 flow rate, co-solvent ratio (i.e., ethanol), among others, can be controlled to enhance the extraction efficiency and retain the quality of extracts maximally. So, it is essential to understand that the extraction conditions that maximally influence polyphenolics compounds’ stability and oxidative degradation19,24.
Therefore, this study was investigated to understand the effect of the supercritical extraction parameter such as temperature, pressure, time, and CO2 flow rate on the extraction yield of lycopene from the freeze-dried grapefruit powder. The central composite rotatable design (CCRD) of response surface methodology (RSM) was used to design the experiment. The extraction yield of the lycopene was measured on supercritical fluid chromatography.
Materials and methods
Materials and reagents
The ripe grapefruit was procured from the Division of Fruits and Horticultural Technology, IARI, New Delhi, India. It was harvested at a stage when the fruit exhibited 2/3rd yellow color. Lycopene was purchased from the Sigma Aldrich (St. Louis, MO, USA). Liquid CO2 with the purity of over 99.5% was purchased from the Amit Labs, New Delhi, India. Other solvents used in quantifying extracted lycopene were purchased from Merck Life Science Pvt. Ltd. Mumbai, India.
Supercritical carbon dioxide extraction
The grapefruits were peeled, diced into small pieces, and lyophilized at − 52 °C for four days under dark condition. The lyophilized grapefruits were ground into powder using hammer mill and passed through a 250 µm size SI sieve to restrict the particle size. The powder was packed in LDPE zip lock bag and stored at − 20 °C for supercritical fluid extraction.
A 100 g of the powdered grapefruit was loaded into the extraction vessel of the supercritical carbon dioxide extraction system, Thar Technologies, USA (Model No. 7100). The operating parameters i.e. extraction temperature, pressure, CO2, and time were controlled using the operating software SuperChrom SFC Suite, Thar Technologies, USA (v5.9 version). ethanol @ 5% along with supercritical CO2 @ 95% was used as extraction solvent. Ethanol was used as a co-solvent with CO2 to enhance extraction yield23,25. A schematic of the supercritical carbon dioxide extraction of lycopene is given in Fig. 1. The extracted lycopene was collected in amber colored bottle and stored at − 20 °C before further quantitative study.
Quantification of lycopene
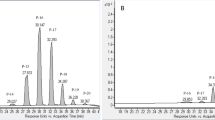
The lycopene’s extraction yield was measured on supercritical fluid chromatography as per the protocol suggested by Runco et al.26, with some modification. The extracted lycopene was vacuum dried and dissolved into hexane. Dissolved lycopene was filtered through 0.22 µm of pore size PVDF membrane (Millipore Ltd., Germany), and 1 mL was loaded into a 1.5 mL amber-colored vial. Quantification was performed on UPC2Acquity System (Waters Technologies, USA) equipped with a BEH 2-EP column (2.1 × 150 mm × 5 µm) and a photodiode array detector. The absorbance was recorded at 452 nm. The mobile phase consisted of CO2 and methanol of 85:15 (v/v) run under isocratic conditions. The chromatographs of the lycopene and n-hexane are given in Fig. 2.
Experimental design and statistical analysis
The experiment was designed using a 24 full factorial central composite design (CCD) with five levels of temperature (50, 60, 70, 80 & 90 °C); pressure (150, 225, 300, 375 & 450 bars); CO2 flow rate (15, 25, 35, 45 & 55 g/min); and extraction time (45, 90, 135, 180 & 225 min). Thirty sets of experiments were conducted (Table 1). Evaluation of the yield (response variable, Y) was done using a full second-order polynomial model of the design considering the yield to be a function of independent variables (x) and their interactions (Eq. 1)
where Y is the response (yield), β0 is the constant coefficient, βi, βj and βij are the linear, quadratic, and interaction coefficients respectively, and xi, xjare the coded values of independent variables.
The design point for the different process variables was obtained using the PROC RSREG procedure of the SAS software (version 9.3, SAS Institute, Cary, NC, USA) with no coding option. It was used for analysis by Response Surface Methodology (RSM) of the experimental data obtained. Five replicates at the center of the design were used to estimate a pure error sum of squares.
Results and discussion
Recovery of lycopene from grapefruit at different experimental runs varied between 13.4% and 77.2% (Table 1). The recovery percentages were calculated by evaluating the lycopene content in the fruit powder before extraction, recovered lycopene using SFE and the lycopene content in the residual powder. Analysis of the data indicated that the lycopene yield from grapefruit could be adequately described using a second order quadratic model as follows:
where Y is the lycopene recovery (%), X1, X2, X3 and X4 represents pressure, flow rate, time and temperature respectively.
Validity of the statistical model is generally adjudged by the lack of fit to check adequacy of the model (Table 2(A)). Significance of the model and a non-significance of the lack of fit at five percent level of significance indicated that the developed model for lycopene yield prediction from grapefruit was a good fit. An R2 value of 0.99 reinforces the same.
ANOVA (Table 2(B)) of the effect of linear, quadratic and interaction effects of the variables indicated that the pressure (X1) and time (X3) individually as well as their interaction effect significantly affected the lycopene yield. However, in its quadratic term, pressure and temperature were found to have significant effect on lycopene yield. All other linear, quadratic and interaction effects were found to be non-significant at 5% level of confidence (P < 0.05).
Effect of independent variables on lycopene extraction was done by analysing the response surface graphs plotted between any two independent variables keeping the third at the central point (Fig. 3a–f).
Response surface plot showing effects of two independent variables on lycopene yield from grapefruit while the remaining were kept at the central point (Pressure—300 bar; Flow rate—35 g/min; Time—135 min; and Temperature—70 °C). [SAS (r) Proprietary Software 9.4 (TS1M1), Copyright (c) 2002–2012 by SAS Institute Inc., Cary, NC, USA].
Effect of independent variables of lycopene extraction
Pressure and time
With an increase in pressure from 150 to 300 bars, it has been observed that lycopene yield increased at a given flow rate of the supercritical CO2and further reduced with increase in pressure up to 450 bars. The rate of increase however gradually reduces with the increase in the flow rate (Fig. 3a). Besides, lycopene yield increased with increase in the flow rate of supercritical CO2 from 15 to 45 g/min and almost got stabilized with further increase in flow rate up to 55 g/min. This increase may be attributed to the fact that the increased pressure enhanced solvent density of the supercritical CO2 resulting in enhanced solubility of lycopene as it has been reported by some researchers too25,31. Nevertheless, beyond a certain limit, pressure plays a negative role by decreasing the diffusion ability of the solvent because of the enhanced compaction of the samples at higher pressure leading to channelling of the supercritical CO2 around it rather than diffusing through it25,30,33.
An increase in pressure decreases lycopene yield significantly at lower extraction time up to about 100 min. The trend, however, reverses to a significant increase with the increase in pressure.The increase of lycopene yield with pressure could have been due to increased solvent density of the supercritical CO2 resulting in enhanced solubility of lycopene25,31. The response surfaces indicated the significant (p < 0.05) effect of both pressure and time individually as well as in conjugation over lycopene yield, which reinforces the significance of these factors found in ANOVA (Table 2). The time of extraction is an indicative measure of the availability of the quantity of supercritical CO2 for the extraction process. The completion of the extraction is adversely affected if the available supercritical CO2 is a limiting factor. Nevertheless, beyond a certain point, any increase in extraction time could have a detrimental effect because of the overpowering of other factors such as temperature24,27,28. There is a significant (p < 0.05) role in both positive and negative terms in the extraction of lycopene from the matrix. The positive effect involves enhancing the extraction rate by solvent densification, whereas the negative effect of sample compaction is higher. Hence, an appropriate balance between the two parameters is of utmost importance for maximizing lycopene yields23,25.
Temperature
Temperature plays a vital role in any extraction process involving supercritical CO2. Increase in lycopene yield is observed with the increase in extraction temperature upto80°C, irrespective of pressure and time of extraction. This is because higher temperatures are known to enhance the solubility of solute, thereby increasing mass transfer of solute in the matrix29,30,31. However, increase in extraction temperature beyond 80 °C causes a significant reduction in the lycopene yieldfrom around 80% to about 30%. This can be explained by the loss of balance between the supercritical CO2 density and solute vapour pressure19,23,31 and lycopene degradation because of isomerization24,27.
Flow rate
An increase in the flow rate of the supercritical CO2 from 15 to 45 g/min was found to increase lycopene yield in the entire range of temperatures considered in the study. This can be attributed to enhanced dissolution rate of lycopene into the supercritical CO2 because of universal concentration gradient phenomenon24,25. However, a further increase in the CO2 flow rate beyond 45 g/min significantly (p > 0.05) reduced the lycopene extraction yield. The reduction in extraction yields may have been because of reduced interaction between the solute (grapefruit powder) and solvent (ethanol + supercritical CO2)28,30. The reduced interaction is because of the solvent quickly passing around attributed to the solvent the sample matrix at a higher flow rate rather than defusing through it. This description is strengthened by the surface plot between extraction and the flow rate of supercritical CO2 (Fig. 3). With the increase in extraction time and the flow rate of supercritical CO2, it was observed that the lycopene yields significantly increased. This increase is primarily because the solvent’s improved dwell time with the solutes triggering the solvent’s penetration into the grapefruit sample matrix which enables maximization of extraction of lycopene32,33.
Taking into account the interaction effect of the independent parameters, up to an extraction time of 100 min, the lycopene yield decreases with increase in pressure. The trend however, reverses to a significant increase with the increase in pressures (Fig. 3b). The surface in fact clearly indicates the significant effect of both pressure and time individually as well as in conjugation over lycopene yieldwhich reinforces the significance of these factors found in ANOVA (Table 2). The time of extraction is an indicative measure of the amount of supercritical CO2 available for the extraction process. In case the available supercritical CO2 is a limiting factor, the completeness of extraction is adversely affected. However, increase in time period beyond a point where the available supercritical CO2 suffices the completeness of extraction, could lead to detrimental effect because of other controlling parameters like temperature24,27,28. Pressure level as described earlier could either play a significant role in aiding extraction by solvent densification or limit it because of sample compaction. Hence, an appropriate balance between the two is essential for maximization of lycopene yields.
Optimization of extraction condition
Second order response surface was fitted to the responses (lycopene extraction) obtained in all the thirty experimental runs and stationary point was obtained through canonical analysis using SAS software. The nature of stationary point was evaluated and found to be the point of maxima and therefore considered the optima. At the optimum conditions i.e. a combination of 305 bars of pressure, 70 °C temperature, flow rate of 35 g/min of supercritical CO2, and 135 min. of extraction time, 93% extraction efficiency of lycopene was achieved. In order to validate the same five additional experimental runs were conducted using the optimum conditions. The experimental yield obtained was 91.03 ± 1.86%indicating a good agreement between the predicted and observed extraction yields. This clearly established the authenticity of the statistical model in predicting lycopene extraction yield at any given experimental combination of independent variables considered for the study.
Conclusion
The results indicated the possibility of successfully achievingupto 93% extraction of lycopene from the grapefruit matrix using the optimum combinations of independent variables considered for the study. Statistical analysis revealed a non-significant effect of temperature, carbon dioxide (CO2), and their interaction on the extraction yield of the lycopene. Whereas, pressure, extraction time, and their interaction, had a significant (p < 0.05) effect on lycopene extraction. Second-order polynomial showed high regression coefficients and a well-fitted model to the obtained experimental data.
References
Delgado-Vargas, F. & Paredes-Lopez, O. Natural Colorants for Food and Nutraceutical Uses (CRC Press, 2002).
Gardner, P. T., White, T. A., McPhail, D. B. & Duthie, G. G. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 68(4), 471–474 (2000).
Murali, S., Kar, A. & Patel, A. S. Storage stability of encapsulated black carrot powder prepared using spray and freeze-drying techniques. Curr. Agric. Res. 7(2), 261–267 (2019).
Mishra, B. B., Patel, A. S. & Kar, A. Storage stability of encapsulated anthocyanin-rich extract from black carrot (Daucuscarota ssp. Sativus) using different coating materials. Cur. Agric. Res. 7(1), 51–63. https://doi.org/10.12944/CARJ.7.1.07 (2019).
Krinsky, N. I. Antioxidant functions of carotenoids. Free Radical Biol. Med. 7(6), 617–635 (1989).
Stahl, W. & Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 24(6), 345–351 (2003).
Sigurdson, G. T., Tang, P. & Giusti, M. M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 8, 261–280 (2017).
Patel, A. S. & Pandey, A. K. Fortification of Limonia acidissima linn fruit powder to develop the phynolic enriched herbal biscuits. J. Bioresource Eng. Technol. 1, 74–85 (2014).
Dhakane, J. P., Kar, A., Patel, A. S. & Khan, I. Effect of soy proteins and emulsification-evaporation process on physical stability of lycopene emulsions. Int. J. Chem. Stud. 5(5), 1354–1358 (2017).
Eggersdorfer, M. & Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 652, 18–26 (2018).
Rafi, M., Yadav, P. & Reyes, M. Lycopene inhibits LPS-induced proinflammatory mediator inducible nitric oxide synthase in mouse macrophage. J. Food Sci. 7, S69–S73 (2007).
Peterson, J. J. et al. Flavanonesin grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 19(Supplement), S74–S80 (2006).
Vanamala, J., Reddivari, L., Yoo, K. S., Pike, L. M. & Patil, B. S. Variation in the content of bioactive flavonoids in different brands of orange and grapefruit juices. J. Food Compos. Anal. 19(2–3), 157–166 (2006).
Xu, Y. & Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf). Ultrasonics Sonochem. 20(4), 1026–1032 (2013).
Salder, G., Davis, J. & Dezman, D. Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 55(5), 1460–1461 (1990).
Xu, Y., Wang, L. F., Xu, X. Y. & Pan, S. Y. Optimization of lycopene extraction from red grapefruit by response surface methodology. Food Sci. 22, 10 (2010).
Ting, S. V. & Deszyck, E. J. The carbohydrates in the peel of oranges and grapefruit. J. Food Sci. 26(2), 146–152 (1961).
Belwal, T. et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 100, 82–102 (2018).
Marsili, R. & Callahan, D. Comparison of a liquid solvent extraction technique and supercritical fluid extraction for the determination of α- and β-carotene in vegetable. J. Chromatogr. Sci. 31, 422–428 (1993).
Vatai, T., Skerget, M. & Knez, Z. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 90, 246–254 (2009).
Wang, C. Y. & Chen, B. H. Tomato pulp as a source for the production of lycopene powder containing high proportion of cis-isomer. Eur. Food Res. Technol. 222, 347–353 (2006).
Lee, M. T. & Chen, B. H. Stability of lycopene during heating and illumination in a model system. Food Chem. 78(4), 425–432 (2002).
Patel, A. S., Kar, A., Dash, S. & Dash, S. K. Supercritical fluid extraction of β-carotene from ripe bitter melon pericarp. Sci. Rep. 9(1), 1–10 (2019).
Gomez-Prieto, M. S., Caja, M. M., Herraiz, M. & Santa-Maria, G. Supercritical fluid extraction of all-trans-lycopene from tomato. J. Agric. Food Chem. 51, 3–7 (2003).
Topal, U., Sasaki, M., Goto, M. & Hayakawa, K. Extraction of lycopene from tomato skin with supercritical carbon dioxide: effect of operating conditions and solubility analysis. J. Agric. Food Chem. 54(15), 5604–5610 (2006).
Runco, J., Subbarao, L. & Chen, R. Qualitative and Quantitative Analysis of β-carotene Using UPC2. APNT134719455, Water, Mississauga, ON (2013).
Nobre, B. P., Palavra, A. F., Pessoa, F. L. P. & Mendes, R. L. Supercritical CO2 extraction of trans-lycopene from Portuguese tomato industrial waste. Food Chem. 116, 680–685 (2009).
Baysal, T., Ersus, S. & Starmans, D. A. J. Supercritical CO2 extraction of β-carotene and lycopene from tomato paste waste. J. Agric. Food Chem. 48, 5507–5511 (2000).
Egydio, J. A., Moraes, A. M. & Rosa, P. T. V. Supercritical fluid extraction of lycopene from tomato juice and characterization of its antioxidant activity. J. Supercrit. Fluids 54, 159–164 (2010).
Reverchon, E. & De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 38, 146–166 (2006).
Shi, J. et al. Solubility of lycopene in supercritical CO2 fluid as affected by temperature and pressure. Sep. Purif. Technol. 66, 322–328 (2009).
Zuknik, M. H., Norulaini, N. N. & Omar, A. M. Supercritical carbon dioxide extraction of lycopene: A review. J. Food Eng. 112(4), 253–262 (2012).
Rozzi, N. L., Singh, R. K., Vierling, R. A. & Watkins, B. A. Supercritical fluid extraction of lycopene from tomato processing byproducts. J. Agric. Food Chem. 50(9), 2638–2643 (2002).
Acknowledgements
Financial and infrastructural help provided under the NASF project entitled “Extraction and micro-encapsulation of nutraceuticals for effective delivery into different food matrices” was duly acknowledged.
Author information
Authors and Affiliations
Contributions
Supriya Priyadarsani initiated the study, executed the experiments, collected, prepared the figures and wrote the manuscript. Avinash Singh Patel assisted Supriya during the experiments and helped to write the manuscript. The experiment was designed, conceived and supervised by Abhijit Kar who also was instrumental in reviewing the manuscript. Analysis of experimental data was done by Sukanta Dash. The final manuscript has been reviewed and approved by all authors for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Certificate
Certified that the use of plants parts in the present study complies with international, national and/or institutional guidelines.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Priyadarsani, S., Patel, A.S., Kar, A. et al. Process optimization for the supercritical carbondioxide extraction of lycopene from ripe grapefruit (Citrus paradisi) endocarp. Sci Rep 11, 10273 (2021). https://doi.org/10.1038/s41598-021-89772-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89772-6
This article is cited by
-
Depolymerization of corn cobs using the CO2/lithium bromide trihydrate system for low molecular weight lignin with high antioxidant activity
Biomass Conversion and Biorefinery (2024)
-
A new green approach for Lavandula stoechas aroma recovery and stabilization coupling supercritical CO2 and natural deep eutectic solvents
Scientific Reports (2023)
-
Optimization of extraction of lycopene from carrot and determination of its antioxidant activity
Journal of Food Measurement and Characterization (2023)
-
Production Ganoderma lucidum extract nanoparticles by expansion of supercritical fluid solution and evaluation of the antioxidant ability
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.