Abstract
This study ascertained the accumulation of polyprenol from four Irish conifer species Picea sitchensis, Cedrus atlantica ‘Glauca’, Pinus sylvestris and Taxus baccata and one flowering tree Cotoneaster hybrida using supercritical fluid extraction with carbon dioxide (SFE-CO2) and solvent extraction. The effects of SFE-CO2 parameters such as temperature (ranged from 40 to 70 \(^\circ{\rm C}\)), pressure (ranged from 100 to 350 bars) and dynamic time (from 70 min to 7 h) were analysed on the extraction efficiency of polyprenol. Qualitative and quantitative analysis of polyprenol was examined using high-performance liquid chromatography. Results showed that P. sylvestris accumulated the highest polyprenol yield of 14.00 ± \(0.4\)mg g−1 DW when extracted with hexane:acetone (1:1 v/v). However, with SFE-CO2 conditions of 200 bars, 70 \(^\circ{\rm C}\), 7 h, with absolute ethanol as a cosolvent with a flow rate of 0.05 ml min−1, P. sitchensis accumulated the highest polyprenol yield of 6.35 ± \(0.4\) mg g−1DW. This study emphasised the potential application of SFE-CO2 in the extraction of polyprenol as an environmentally friendly method to be used in pharmaceutical and food industries.
Similar content being viewed by others
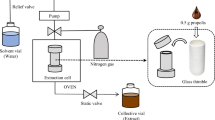
Introduction
Plants are a staple of balanced diet and also an important remedial source for various ailments1,2. Plants are essential for pharmacological research and drug development, where they are sources of direct therapeutic drugs, precursor materials for drug synthesis, and models for pharmacologically active compounds3. One such compound class exploited for the aforementioned reasons are that of polyisoprenoids4,5. Polyisoprenoids (polymers of isoprene unit) are linear hydrophobic, branched chains of fatty alcohol6. Polyisoprenoids are divided into two main subgroups found in nature differing based on the hydrogenation status of α-terminal double bond in the molecule, polyprenols (α-unsaturated) and dolichols (α-saturated)4,7,8 (Supplementary Fig. S17). Polyprenols are long-chain isoprenoid polymers with the formula (C5H8)nOH, that can be found in a wide range of natural sources, including bacteria and plants. In mammals, the mono-saturated dolichols are dominant4,9,10. Plants represent the widest range of polyprenols diversity, containing both poly-cis/trans-prenols6. Polyprenols are always found as a mixture of homologues differing in the number of isoprene units11. The degree of polymerization of polyprenols is specific to plant species, growth stages and cultivation/growth conditions8. In the human body polyprenols are metabolised into dolichols and dolichyl phosphate by enzymatic catalysis11. In humans, dolichols regulate the permeability and stability of membranes, partaking in the biosynthesis of human glycoproteins11,12. Both dolichols and polyprenols are found in the form of esters, carboxylic acids and free alcohol in the cell13, while a small portion is found in the form of phosphates14.
Polyprenols have non-carcinogenic, non-mutagenic, non-teratogenic and non-toxic effects in humans15,16,17, while also providing significant anti-tumour, anti-anaemia, anti-HIV and anti-hepatitis C effects18,19. Polyprenols have also been found to positively affect conditions such as hypertension, high cholesterol, gout, lupus and diabetes, along with other disorders disturbing proper immune functionality13,20,21. Moreover, polyprenols serve as a chemotaxonomic marker for systematic families in botanic taxonomy22, and also act as a scavenger against oxygen species generated by chemicals or accumulated upon ageing or UV light23. Polyprenols extracted from Ginkgo biloba leaves showed anti-bacterial effects against Escherichia coli, Salmonella enterica, Bacillus subtilis24. More studies are required on researching plant sources of polyprenol compounds and their derivatives as suggested in a review by Zhang et al. for developing plant extracts into products for therapeutic use5. Examples of polyprenol-based products available commercially are ROPREN25 and FORTEPREN26, both of which extracted from plants. ROPREN consists of a highly purified mixture of polyprenols (patent No. 001521/07 from 12/07/2007) marketed for the treatment of liver disease. Studies suggest that ROPREN has significantly wider applications than liver disease, such as the ability to normalise immune response, normalise cholesterol levels and alleviate the symptoms of Alzheimer’s disease25,27. FORTEPREN, polyprenyl phosphate extracted from fir needles belongs to the antiviral drug family with immunomodulating activity and has been suggested as a treatment for herpes disease26.
The high cost of extraction and low yield during purification limit the full industrial application of polyprenols. The aim of this study was to screen Irish conifer trees and one flowering tree as a source of polyprenol and utilise SFE-CO2 as a green alternative technology to organic solvent extraction. One of the most valuable characteristic of SFE-CO2 is the highly reduced, often to zero usage of toxic organic solvents making it ideal for use in pharmaceutical and food-based industries. In this study we optimised SFE-CO2 parameters for the maximum polyprenol yield and compared achieved yields against traditional organic solvent extraction. We further aiming to carry out preliminary purification by flash chromatography and profiling of individual isoprene unit by LC–MS.
Materials and methods
Chemical and reagents
HPLC grade 2-propanol (> 99.5%), methanol (> 99.9%) and acetonitrile (> 99.9%) and analytical grade ethanol (≥ 99.8%), hexane (≥ 97%), chloroform (> 99.9%), potassium hydroxide, formic acid and acetone (> 99.9%) were purchased from Lennox (Dublin, Ireland). Polyprenol standard mixes range between (C70–C100 and C80–C125) were purchased from LGC limited (Teddington, England). Carbon-dioxide (> 99.9) were purchased from BOC (Limerick, Ireland).
Preparation of plant material
Needle/leave samples were collected from four conifer Cedrus atlantica ‘Glauca’, Picea sitchensis, Pinus sylvestris and Taxus baccate and one flowering tree Cotoneaster hybrida from urban areas of county Limerick (Ireland) in October and November, 2016 and 2017. The sample timeframe was selected based on the study carried out by Bajda et al.28, which established that the accumulation of polyprenol was the highest at the end of vegetative season. The plant materials were taxonomically authenticated by Professor Trevor Hodkinson in the Botany Department of Trinity Collage Dublin, the University of Dublin. Young and old needles were selected at random from three mature trees of each species and combined. Samples were stored at − 20 \(^\circ{\rm C}\) overnight and subsequently freeze-dried (Thermo Scientific Heto Power Dry LL3000, United Kingdom) at − 50 \(^\circ{\rm C}\) temperature for two days. When the drying process was completed, samples were ground to a fine powder (~ 0.4–0.8 mm) using a mill (MM 400 Retsch, England) and stored at − 20 \(^\circ{\rm C}\) for further analysis.
Extraction with organic solvent
200 mg of dried, homogenised tissue from each species was extracted three times with 5 ml of 4:1 (v/v) hexane:acetone and 1:1 (v/v) hexane:acetone. After each extraction samples were vortexed and sonicated for 15 min followed by incubation at 40 \(^\circ{\rm C}\) for an hour with occasional shaking. The tubes were centrifuged at 3000 rpm for 10 min. The supernatant was collected and placed into new 15 ml plastic tube. The samples were extracted three times, to which 5 ml of fresh solvent was added, incubated at 40 \(^\circ{\rm C}\) for an hour and centrifuged at 3000 rpm for 10 min. The supernatants from the three extracts were combined for a total extract volume of approximately 15 ml. All tubes were fully evaporated under a stream of nitrogen and stored at − 20 \(^\circ{\rm C}\) for further analysis.
Supercritical fluid carbon dioxide extraction (SFE-CO2)
SFE-CO2 was performed on Super Critical Extraction system MV-10 ASCFE which consists of thermocube chiller, fluid delivery module (co-solvent pump and CO2 pump), extraction oven (extraction vessels), automatic backpressure regulator (ABPR) and fraction collection module with makeup pump by Waters (Dublin, Ireland). 3 g of powdered sample was placed into a 3 ml sample vessel and extracted under the following parameters: CO2 flow rate at 10 ml min−1, ethanol co-solvent flow rate at 0.05 ml min−1, and make-up solvent flow rate was 0.6 ml min−1. All flow rates were kept constant throughout the process. A three factors, four levels orthogonal array design of L16 (43)29 was employed for the optimisation of extraction parameters, temperature (\(^\circ{\rm C}\)), pressure (bar), dynamic time (min) (Table 1). Sixteen experiments were performed in order to identify the best parameters for the extraction of polyprenol from selected plant species (Supplementary Table S1).
Further optimisation experiments were carried out to investigate the effect of the extended dynamic time. These extractions were conducted with dynamic time of up to 7 h. The temperature and pressure selections for these further time experiments were 200 bar and 70 \(^\circ{\rm C}\). Those temperature and pressure set-points were selected based on the initial development process being the best parameters which resulted in the highest polyprenol yield.
Saponification of the lipid extracts
All solvent and SFE-CO2 extracts were completely dried under nitrogen gas and saponified by adding to each extract 1500 μl of saponification solution (2.5 M KOH in methanol, 1:4 v/v), prior to being vortexed and incubated at 72 \(^\circ{\rm C}\) for 15 min. Following incubation, 225 μl of formic acid was added, 1725 μl of chloroform and 375 μl of milli-Q water. The final mixture was vortexed and centrifuged for 5 min at 3000 rpm where two layers formed. The organic layer containing free fatty acid and polyprenol was transferred to a clean vial, dried and stored at − 20 \(^\circ{\rm C}\) for further analysis 30.
Sample/standard preparation for HPLC
Solvent and SFE-CO2 extracts were evaporated to dryness under a stream of nitrogen and reconstitute in 5 ml hexane and filtered through 0.20 μm filter before HPLC analysis. Two sets of polyprenol standard mixtures, quantitative mixture of C70–C100 prenologues (P-14 to P-20) and quantitative mixture of C80–C125 prenologues (P-16 to P-25) were used for the HPLC analysis, provided by LGC in liquid form at a concentration of 5 mg/0.5 ml hexane, serial dilutions were prepared.
High performance liquid chromatography (HPLC)
Polyprenol identification and quantification was preformed via HPLC according to a protocol previously described by Yu et al.31 with some modifications. Analysis was performed on an Agilent Technologies HPLC consisting of G1329B auto-sampler, G1316B thermostatted column compartment, G1315C DAD detector, G1312B binary pump and G1379B degasser. Separation was carried out on a ZORBAX Eclipse Plus C18 4.6 × 150 mm, 5 μm column. The mobile phase consisted of 100% acetonitrile (solvent A) and 100% isopropanol (solvent B) using the following gradient: 0–5 min—40%B; 6–35 min—40–75% B; 36–50 min—40% B; flow rate of 0.5 ml min−1; column temperature of 40 \(^\circ{\rm C}\); detection via UV-DAD at 210 nm, and an injection volume of 10 µl 31.
Statistical analysis
Each experiment was carried out in triplicate and the data were expressed as means ± standard deviation (SD). Statistical analysis were prerformed on Microsoft Excel (Version 16.39, Microsoft 365 subscription, data analysis 2019 software). The analysis of variance (ANOVA) with a p-values less than 0.05 were considered to be statistically significant. Regression analysis (t-test) was performed to test the significance of the independent variables and one way ANOVA using STATA (Stata Statistical Software, version 13.0, Collage Station, TX: StataCorp LLC).
Results and discussion
Identification and quantification of polyprenol
Across many cited studies, organic solvents have been investigated for their effects on both the yield and purity of polyprenols extracts including ethyl ether, hexane/acetone (4:1 v/v, 1:1 v/v, and 1:9v/v), and ethyl acetate32,33. Another study investigating the extraction of polyprenols from Ginkgo biloba leaves showed that petroleum ether gave the cleanest extract, with a yield of 0.92 g DW and purity of 18.3%, while hexane/acetone mix (4:1, v/v) gave the highest yield of 1.16 g DW and a purity of 18.3%32. Therefore, in the current study needles from C. atlantica ‘Glauca’, P. sitchensis, P. sylvestris and T. Baccata and leaves from C. hybrida were extracted using SFE-CO2 and a mixture of hexane:acetone at two ratios of (4:1; 1:1 v/v).
The identification of polyprenol compounds from the needles and leaves of conifers/flowering species were based on UV spectral data (210 nm) with the aid of external standards. Polyprenol was naturally present as a mixture of homologues which make it difficult to separate and quantify. Solvent extracts with hexane:acetone (4:1; 1:1 v/v) and 16 different combination of SFE-CO2 parameters (Supplementary Table S1) were optimised for C. atlantica ‘Glauca’. For the purpose of optimisation of the extraction method, only one plant material was chosen at random i.e. C. atlantica ‘Glauca’. Selection was based on availability and consistency within the extraction techniques.
The polyprenol content present in the samples were compared against two sets of standard mixtures, quantitative mixture of C70–C100 prenologues (P-14 to P-20) and quantitative mixture of C80–C125 prenologues (P-16 to P-25) (Fig. 1). Both sets of standards overlapped with regards to the length of the polyprenols tested ensuring adequate identification with a wide range of lengths. As mentioned previously polyprenol from plant material has different cis/trans configuration. These configurations affect the retention time and make it difficult to confirm the exact identity of these polyprenol. However, these variations were small in comparison to the chain length and stereochemistry can affect chromatographic separation.
Evaluation of extraction procedures for polyprenol content of Cedrus atlantica ‘Glauca’
Two solvent extracts and extracts obtained from 16 different SFE-CO2 parameter permutations of C. atlantica ‘Glauca’ were analysed by HPLC for qualitative and quantitative determination of polyprenol. HPLC analysis allowed for the determination of total polyprenol content as well as the range of isoprene units according to chain length. The results of a qualitative evaluation of isoprene chain length in the needles of C. atlantica ‘Glauca’ was shown in Table 2. The quantitative determination of the total polyprenol content in mg g−1dry weight was shown in Fig. 2. Variation in polyprenol chain length was evident between extraction types, indicating differences in selectivity between the methods. These differences were observed in C. atlantica ‘Glauca’ where it accumulated polyprenol with medium to high chain length from C95 extracted by hexane:acetone (4:1) and C75–C100 extracted by hexane:acetone (1:1).
Quantitive analysis of the total polyprenol content (mg g−1DW) extracted from C. atlantica ‘Glauca’ under two extraction methods; solvent using hexane:acetone (4:1; 1:1, v/v) and 16 different combination of SFE-CO2 parameters without saponification. The polyprenol content from SFE-CO2 extractions were based on a dynamic time of 70 min and also without applying saponification on both extraction methods. Each value presents the mean value ± SD. Figure was prepared using Microsoft excel (Version 16.39, Microsoft 365 subscription, data analysis 2019 software).
The polyprenol chain length identified in SFE-CO2 of C. atlantica ‘Glauca’ extracts ranged from C70 to C100 (P-14 to P-20). Treatment number 8, with conditions of 200 bar, 70 \(^\circ{\rm C}\), 70 min and ethanol modifier (0.05 ml min−1), were identified as the best parameters at extracting the longer chains of polyprenol. A wide range of isoprene unit lengths were detectable across the SFE-CO2 conditions investigated with lengths of P-20 dominating, followed by P-19 and P-18 isoprene unit.
The identification and quantification of the total polyprenol content concluded that hexane:acetone (1:1; v/v) and SFE-CO2 treatment 8 had the highest polyprenol content of 1.94 \(\pm\) 0.03 mg g−1DW and 1.14 ± 0.004 mg g−1DW, respectively. The effect of pressure (bar) and temperature (\(^\circ{\rm C}\)) were analysed see Fig. 3A. An increase in polyprenol content was observed (p < 0.05) from 0.28 ± 0.001 to 2.78 ± 0.02 mg g−1DW as the dynamic time was increased from 70 min to 7 h, as depicted in Fig. 3B. These results correlate with data presented by Jozwiak et al.13; as extraction time increases so does the content of polyprenol. During their extraction of Sorbus spp., the amount of polyprenol increased slowly, in proportion to time and was observed to require at least 56 h to achieve the maximum yield. Extraction times were much quicker from Spruce spp., for which maximum yield was achieved in 7 h13. The effect of time would also depend on the compositional nature of the needles/leaves of the species selected. Wang et al. showed that extraction time inversely affects the extract composition; compounds of low molecular mass and which were less polar tend to be extracted faster, since the extraction mechanism was controlled by internal diffusion34.
The effect of SFE-CO2 parameters (A) effect of pressure and temperature and (B) dynamic time (C) effect of saponification on the total polyprenol content in mg/g DW at a constant temperature of 70 \(^\circ{\rm C}\) and pressure of 200 bar with a presence of ethanol as co-solvent. During the time course of the SFE-CO2 of C. atlantica ‘Glauca’ extracts were collected at different time intervals and samples were analysed for total polyprenol using HPLC. Each value presents the mean value ± SD. Figures prepared using Microsoft excel (Version 16.39, Microsoft 365 subscription, data analysis 2019 software).
In the SFE process, CO2 is small and linear in structure molecule which increases its permeability yet, one of its limitation include non-polar and its capacity to form specific interaction between solvent and solute. Adding polar co-solvent improves the CO2 polarity; this will enhance the solubility power and extract more polyprenol content. In this study ethanol as a co-solvent at a flow rate of 0.05 ml/min and kept constant however the volume of ethanol varies across different extraction time, for example at 40 min a total volume of 2 ml ethanol was used in comparison to 3 ml at 70 min extraction time. Other types of co-solvent with higher polarity than ethanol can be applied such as acetone. However, the aim was to use an eco-friendly process for the extraction of polyprenol hence applying SFE and moreover ethanol as a modifier. Ethanol is less toxic and of lower risk to human health and normally accepted in pharmaceutical as per FDA regulation. In addition, avoiding the use of toxic chemicals as a modifier (such as acetone), eliminates the purification step after SFE extraction.
The optimisation of the SFE-CO2 process depends on multiple parameters which affect the solubility of polyprenol in supercritical fluid (SF). Based on the literature, there are data available regarding the solubility of solid-supercritical fluid equilibrium system. However, no solubility data has been reported on polyprenol-supercritical carbon dioxide system. There are different models to study and estimate the solubility of solute-supercritical fluid including, solubility parameter model, semiempirical methods, equation of state and molecular dynamic simulation35. In the current study the solubility of polyprenol in carbon dioxide was correlated with other parameters such as pressure, temperature, CO2 density and co-solvent. Other factors, which affect the solubility are the solute molar mass, polarity and vapour pressures. At a constant temperature, an increase in pressure enhances the solvating power; and at a constant pressure, an increase in the temperature reduces the solvating power and the solvent density. High solvent flow rate increases the capacity of extraction of solute from plant, however in some cases it causes a decrease in the extraction yield, due to less time allowed for solvent–solute interaction36.
Supercritical fluids possess thermal and chemical properties between those of a pure gas and a liquid which makes them capable of dissolving other materials. CO2 has a low viscosity as a gas, high density as liquid and intermediate level of diffusion between those of gases and liquids at which densities are similar. Addition of a highly polar cosolvent, will increase its solvating power and improve its polarity. In this study, ethanol is used as a cosolvent as it is less toxic and of low risk to human health and mostly used in pharmaceutical as per FDA.
Solvent density plays a critical role on the solute solubility in supercritical fluid due to its solvating power at critical conditions rather than solute-vapour pressure hence, density decreases with an increase in temperature as seen in Supplementary Table S2. At a higher pressure, CO2 density increases which increases solubility and the intermolecular distances between CO2 molecules reduced, this will increase solute–solvent interaction. Based on the crossover behaviour of solubility isotherms, at a pressure below the lower crossover pressure and above the upper crossover pressure, the solubility increases with increase in temperature hence higher solid-vapour pressure. At pressure between lower and upper crossover pressure, solubility increases as a result of rapid decrease in solvent density; known as ‘retrograde vaporisation’. At the lower and upper crossover pressure the effect of solute vapour pressure and solvent density on solid solubility is equal. The crossover pressure is at the point where the slope of the solubility versus temperature change37.
In Fig. 3A, at the pressures of 100, 300 and 350 bars across the temperature studied, with exception to 70 \(^\circ{\rm C}\), as the pressure range between lower crossover pressure and upper crossover pressure. Where, as temperature increase, solubility of solute in solvent decrease hence lower content of polyprenol. This is a result of interaction effect of CO2 density and solute vapour pressure. However an exception to the above phenomenon observed at 200 bar where the solubility of solute in solvent increase as the temperature increased to 70 \(^\circ{\rm C}\). To account for this anomaly, at a pressure above crossover pressure, the solute vapour pressure effect became dominant and solubility increase when temperature increase.
In this study, the effect of saponification on the quantification of the total polyprenol content was also analysed, as indicated in Fig. 3C. Saponification in this case is a pre-treatment of the samples to release the compound of interest or remove some interfering components and, therefore, enhance the HPLC analysis of polyprenol. A significant increase (p < 0.05) in polyprenol content was observed with approximately 21% increase in both extraction techniques. Regression analysis was performed to test the significance of the independent variables using t-test and p-values (See Supplementary Tables S3 and S4). According to the p-values the variables pressure (bar) and standard deviation are significant according to the 95% confidence intervals where p < 0.05.
Screening other conifer and flowering trees for the accumulation of polyprenol
Based on the identification of polyprenol from both solvent and SFE-CO2 extraction, the best solvent and optimum SFE-CO2 conditions were defined to be hexane:acetone (1:1 v/v), and 200 bars, 70 \(^\circ{\rm C}\), 7 h and 100% ethanol (0.5 ml min −1). The above extractions conditions were further applied to the needles of T. bacatta, P. sylvestris, and P. sitchensis and leaves of C. hybrida.
The results of qualitative analysis of polyprenol chain length identified in the needles/leaves of the species studied were shown in Table 3. Among all plants, the polyprenols composed of P-14 to P-20 of isoprene units were detectable with P-19 dominating followed by P-20. The extraction techniques effect the isoprene identified within the same plant species. For example T. baccata extracted with hexane:acetone (1:1, v/v) comprised more isoprene unit in comparison to SFE-CO2 which only comprised P-19. All HPLC chromatograms of the plants species extracted by both techniques and the composition isoprene chain length can be detected are presented in Table 4.
Quantification of the total polyprenol content in mg g−1 DW was calculated based on the total peak areas of all polyprenol isoprene chain length identified. SFE-CO2 resulted in the extraction of a wider variety of isoprene chain lengths when compared to solvent extraction, but resulted in lower polyprenol yields in some of the plant species studied as depicted in Fig. 4. The total polyprenol content in the needles/leaves of plants studied was shown to be as high as 1.2% of the dry weight.
The total polyprenol content extracted by both extraction method (mg/g DW) calculated for C. atlantica ‘Glauca’, P. sitchensis, P. sylvestris, C. hybrida and T. baccata Each value presents the mean value ± SD. Figure prepared using Microsoft excel (Version 16.39, Microsoft 365 subscription, data analysis 2019 software).
Polyprenol content in the total SFE-CO2 extracts were 3.07 ± 0.3 mg g−1DW, 6.35 ± 0.4 mg g−1DW, 6.00 ± 0.4 mg g−1DW, 2.47 ± 0.2 mg g−1DW and 0.06 ± 0.01 mg g−1DW for C. atlantica ‘Glauca’, P. sitchensis, P. sylvestris, C. hybrida and T. baccata respectively. For the organic solvent, polyprenol content in the respective extracts were 3.35 ± 0.24 mg g−1DW, 5.34 ± 0.2 mg g−1DW, 14.00 \(\hspace{0.17em}\pm \hspace{0.17em}0.4\) mg g−1DW, 1.37 ± 0.1 mg g−1DW and 0.16 ± 0.02 mg g−1DW, respectively.
General discussion
Based on the results of the current study, the plant species screened proved to be rich sources of polyprenols. The polyprenols in species such as P. sylvestris38,39 and T. baccata40 have been characterised previously; however, to the best of the author’s knowledge, this was the first record of P. sitchensis, C. hybrida and C. atlantica ‘Glauca’ screened for the accumulation of polyprenols. This is also the first record investigating the use of SFE-CO2 as an extraction technique for these polyprenols from the listed plant species.
In the current study, the highest yield obtained was 14.00 ± 0.4 mg g−1 DW from P. sylvestris using hexane:acetone (1:1, v/v). P. sylvestris accumulated polyprenol with medium to high chain lengths ranging from C70–C100. In previous literature, petroleum ether was used to extract polyprenol from the old needles of Taxus chinensis var. mairei, resulting in 30 mg g−1DW yield31. Ethyl acetate was used to optimize the extraction yield of polyprenol from Cunninghamia lanceolate needles in terms of extraction time, temperature, and liquid—solid ratio. The percentage yield was 1.22 ± 0.04% under the following condition (71.4 \(^\circ{\rm C}\), 5.96 h and 9.3 mg ml-1)33. Hexane:acetone (1:1, v/v) was used to extract Sorbus intermedia, Picea abies and Nicotiana tabacum and resulted in 6.7 ± 0.5, 10 ± 0.6 and 2.1 ± 0.2 mg g−1DW13. Various species from the Lauraceae family were subjected to hexane:acetone (1:1, v/v) for the extraction of polyprenol with resulting yields ranging from 0.06 to 3%41.
Research presented by Jozwiak et al.13 illustrated optimum conditions were 210 bar and 60 \(^\circ{\rm C}\) giving a CO2 density of 736.4 kg/m3, whereas the optimum conditions of current study were 200 bar and 70 \(^\circ{\rm C}\) giving a CO2 density of 736.7 kg/m3. However, due to the differences in the extraction time applied and the plant species screened by both studies, direct comparison was not applicable since these differences effect the accumulation of polyprenol.
Conclusion
In this study, the extraction, identification and quantification of polyprenol as a health promoting bioactive ingredient from plant needles/leaves was investigated. Two solvent extraction methods (hexane:acetone 4:1; 1:1 v/v) and 16 different parameters of SFE-CO2 were applied to C. atlantica ‘Glauca’ and the optimal conditions were identified. SFE-CO2 extraction was three time less efficient based on polyprenol total yield content, however gave a wider range of isoprene chain lengths. P. sylvestris was identified to be the best source of polyprenol among the plant species studied showing a resultant yield of 14.00 ± \(0.4\) mg g−1DW by hexane:acetone 1:1 v/v. However, the highest resultant yield of 6.35 ± 0.4 mg g−1DW by SFE-CO2 was obtained from P. sitchensis. Due to the wide applications for polyprenol from natural sources, environmentally friendly extraction technique such as SFE-CO2 will be an appropriate method for the food, pharmaceutical and nutraceuticals industries.
References
Kim, E. J., Seo, H. B. & Gu, M. B. Prescreening of natural products in drug discovery using recombinant bioluminescent bacteria. Biotechnol. Bioprocess Eng. 24, 264–271 (2019).
Fridlender, M., Kapulnik, Y. & Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 6, 1–9 (2015).
Mendonça-Filho, R. R. Bioactive phytocompounds: New approaches in the phytosciences. in Modern Phytomedicine 1–24 (Wiley-VCH Verlag GmbH & Co. KGaA, 2006). https://doi.org/10.1002/9783527609987.ch1.
Sagami, H., Swiezewska, E. & Shidoji, Y. The history and recent advances in research of polyprenol and its derivatives. Biosci. Biotechnol. Biochem. 82, 947–955 (2018).
Zhang, Q. et al. Synthesis and biological activity of polyprenols. Fitoterapia 106, 184–193 (2015).
Salvador-Castell, M., Tourte, M. & Oger, P. M. Molecular sciences in search for the membrane regulators of archaea. Int. J. Mol. Sci. 20, 1–26 (2019).
Surowiecki, P., Onysk, A., Manko, K., Swiezewska, E. & Surmacz, L. Long-chain polyisoprenoids are synthesized by AtCPT1 in Arabidopsis thaliana. Molecules 24, 1–14 (2019).
Akhtar, T. A. et al. Polyprenols are synthesized by a plastidial cis-prenyltransferase and influence photosynthetic performance. Plant Cell 29, 1709–1725 (2017).
Khodanovich, M. Y. et al. Plant polyprenols reduce demyelination and recover impaired oligodendrogenesis and neurogenesis in the cuprizone murine model of multiple sclerosis. Phyther. Res. 33, 1363–1373 (2019).
Hofmann, N. R. The who, what, and where of plant polyprenol biosynthesis point to thylakoid membranes and photosynthetic performance. Plant Cell 29, 1552–1553 (2017).
Zimbres, F. M. et al. Metabolomics profiling reveals new aspects of dolichol biosynthesis in Plasmodium falciparum. Sci. Rep. 10, 1–17 (2020).
Naseri, R. et al. Targeting glycoproteins as a therapeutic strategy for diabetes mellitus and its complications. DARU J. Pharm. Sci. 28, 333–358 (2020).
Jozwiak, A., Brzozowski, R., Bujnowski, Z., Chojnacki, T. & Swiezewska, E. Application of supercritical CO2for extraction of polyisoprenoid alcohols and their esters from plant tissues. J. Lipid Res. 54, 2023–2028 (2013).
Eichler, J. & Imperiali, B. Stereochemical divergence of polyprenol phosphate glycosyltransferases HHS Public Access. Trends Biochem. Sci. 43, 10–17 (2018).
Guan, Z., Meyer, B. H., Albers, S.-V. & Eichler, J. The thermoacidophilic archaeon Sulfolobus acidocaldarius contains an unsually short, highly reduced dolichyl phosphate. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1811, 607–616 (2011).
Kukina, T. The Siberian flora as a source of polyisoprenoids. Materials, Methods and Technologies 11, 63–75 (2017).
Basyuni, M. et al. Changes to the polyisoprenoid composition in aging leaves of mangrove plants. AIP Conf. Proc. 2021, 030007 (1–7) (2018).
Khidyrova, N. K., Zokirova, U. T., Koroleva, A. A., Kuchin, A. V. & Shakhidoyatov, K. M. Alkylation of o-cresol by polyprenols. Chem. Nat. Compd. 47, 983–984 (2012).
Wang, C.-Z., Li, W.-J., Tao, R., Ye, J.-Z. & Zhang, H.-Y. Antiviral activity of a nanoemulsion of polyprenols from ginkgo leaves against influenza A H3N2 and hepatitis B virus in vitro. Molecules 20, 5137–5151 (2015).
Scalais, E. et al. Early myoclonic epilepsy, hypertrophic cardiomyopathy and subsequently a nephrotic syndrome in a patient with CoQ10 deficiency caused by mutations in para-hydroxybenzoate-polyprenyl transferase (COQ2). Eur. J. Paediatr. Neurol. 17, 625–630 (2013).
Tao, R. et al. Characterization, cytotoxicity, and genotoxicity of TiO2 and folate-coupled chitosan nanoparticles loading polyprenol-based nanoemulsion. Biol. Trace Elem. Res. 184, 60–74 (2018).
Basyuni, M. et al. Detection of polyisoprenoids in the roots and stems of coastal grasses using a two-dimensional thin layer chromatography. J. Phys. Conf. Ser. 1116, 1–5 (2018).
Zhang, C.-W. et al. Physiochemical property and antibacterial activity of formulation containing polyprenol extracted from Ginkgo biloba leaves. Ind. Crops Prod. 147, 112213 (1–9) (2020).
Tao, R., Wang, C., Ye, J., Zhou, H. & Chen, H. Polyprenols of Ginkgo biloba enhance antibacterial activity of five classes of antibiotics. Biomed. Res. Int. 2016, 1–18 (2016).
Fedotova, J. et al. Ropren®treatment reverses anxiety-like behavior and monoamines levels in gonadectomized rat model of Alzheimer’s disease. Biomed. Pharmacother. 83, 1444–1455 (2016).
Pronin, A. V., Narovlyansky, A. N., Shulzhenko, A. E., Sanin, A. V. & Sedov, A. M. New polyprenyl phosphate based preparation Fortepren® as promising cytokine regulationg antiviral remedy. Cytokine Growth Factor Rev. 30, 119–126 (2016).
Fedotova, J., Soultanov, V., Nikitina, T., Roschin, V. & Ordayn, N. Ropren® is a polyprenol preparation from coniferous plants that ameliorates cognitive deficiency in a rat model of beta-amyloid peptide-(25–35)-induced amnesia. Phytomedicine 19, 451–456 (2012).
Bajda, A. et al. Light conditions alter accumulation of long chain polyprenols in leaves of trees and shrubs throughout the vegetation season. Acta. Biochim. Pol. 52, 233–241 (2005).
Wang, Y. et al. Supercritical carbon dioxide extraction of bioactive compounds from Ampelopsis grossedentata stems: Process optimization and antioxidant activity. Int. J. Mol. Sci. 12, 6856–6870 (2011).
Otero, P. et al. Identification of optimum fatty acid extraction methods for two different microalgae Phaeodactylum tricornutum and Haematococcus pluvialis for food and biodiesel applications. Anal. Bioanal. Chem. 409, 4659–4667 (2017).
Yu, J. et al. Polyprenols from the needles of Taxus chinensis var. mairei. Fitoterapia 83, 831–837 (2012).
van Beek, T. A. & Montoro, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 1216, 2002–2032 (2009).
Jiang, P., Zhang, Y., Shan, Z. & Zheng, Q. Optimizing the extraction yield of polyprenols from needles of Cunninghamia lanceolata (Lamb.) hook using response surface methodology and its antioxidative activities. BioResources 8, 545–556 (2012).
Wang, L. & Weller, C. L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 17, 300–312 (2006).
Rad, H. B., Sabet, J. K. & Varaminian, F. Study of solubility in supercritical fluids: Thermodynamic concepts and measurement methods—A review. Braz. J. Chem. Eng. 36, 1367–1392 (2019).
Pereira, C. G. & Meireles, M. A. A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 3, 340–372 (2010).
Foster, N. R. et al. Significance of the crossover pressure in solid-supercritical fluid phase equilibria. Ind. Eng. Chem. Res. 30, 1955–1964 (1991).
Tateyama, S., Wititsuwannakul, R., Wititsuwannakul, D., Sagami, H. & Ogura, K. Dolichols of rubber plant, ginkgo and pine. Phytochemistry 51, 11–15 (1999).
Kukina, T. The siberian flora as a source of polyisoprenoids. J. Int. Sci. Publ. 11, 63–73 (2017).
Chouda, M. & Jankowski, W. The occurrence of polyprenols in seeds and leaves of woody plants. Acta Biochim. Pol. 52, 243–253 (2005).
Rosalinska, M., Walinska, K., Swiezewska, E. & Chojnacki, T. Plant long-chain polyprenols as chemotaxonomic markers. Cell. Mol. Biol. Lett. 6, 228 (2001).
Acknowledgements
Authors would like to thank Professor Trevor Hodkinson at the Botany Department, School of Natural Sciences at Trinity Collage Dublin, the University of Dublin for taxonomically authenticating the plant species. Financial support was received by GRO bursary awards from Limerick Institute of Technology. The authors would like to thank Shannon applied biotechnology centre and CELLS group for the help and support.
Author information
Authors and Affiliations
Contributions
H.A. performed the research work, collected and analysed the data, and prepared the manuscript. T.B., P.D. and M.M. supervised the research work as well as helped in editing of the manuscript. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alaydi, H., Downey, P., McKeon-Bennett, M. et al. Supercritical-CO2 extraction, identification and quantification of polyprenol as a bioactive ingredient from Irish trees species. Sci Rep 11, 7461 (2021). https://doi.org/10.1038/s41598-021-86393-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86393-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.






