Abstract
Low serum bilirubin levels have been associated with increased risk of cardiovascular disease (CVD) and metabolic syndrome. Testosterone deficiency could also contribute to increased risk of CVD and metabolic syndrome. Therefore, this study aimed to examine the relationship between serum bilirubin level and testosterone deficiency in 1284 Korean men aged 45 to 70 years. Serum bilirubin level was categorized into quartiles: Q1 ≤ 0.7, Q2 0.8–0.9, Q3 1.0–1.1, and Q4 ≥ 1.2 mg/dL. Testosterone deficiency was defined as level less than 8.0 nmol/L, as suggested by the position statement of International Society of Andrology. The overall prevalence of testosterone deficiency was 5.8% and significantly decreased with the quartiles from Q1 to Q4. Compared with the referent fourth quartile (serum bilirubin ≥ 1.2 mg/dL), the ORs (95% CIs) for testosterone deficiency was 2.29 (1.04–4.94) for the first quartile after adjusting for age, fasting glucose, triglyceride, HDL-cholesterol, leukocyte count, hemoglobin, smoking status, and alcohol intake. We found inversely graded associations of serum bilirubin level with testosterone deficiency. These findings suggest that low bilirubin level may be interpreted as a state of testosterone deficiency in middle-aged and older men.
Similar content being viewed by others
Introduction
Testosterone level in men is well documented to remain stable until around 40 years of age and then to decline at about 1% per year as a natural result of aging1. Male hypogonadism is a condition in middle-aged and older men characterized by low testosterone level with clinical symptoms including sexual dysfunction, depressed mood, lack of muscle mass, abdominal obesity, and deteriorated quality of life2,3. Approximately 2–6% of men aged over 65 years will have symptoms of hypogonadism4. Accumulating epidemiological evidence shows that testosterone deficiency contributes to increase risk of metabolic syndrome and cardiovascular disease (CVD)5, which are highly linked to chronic low-grade inflammation. Hence, early identification of individuals at higher risk for testosterone deficiency is important for male health and quality of life.
Bilirubin is made from biliverdin by the work through heme oxygenase and biliverdin reductase and is an end product of heme catabolism6. Serum bilirubin level has been regarded as an indicator of hepatobiliary dysfunction or hemolysis7, but all the evidence that has piled up suggests that bilirubin is a potent antioxidant and endogenous cytoprotective agent in the progress of cardiometabolic diseases8. Previous studies show that low level of serum bilirubin is relevant to increased risk of metabolic syndrome, carotid atherosclerosis, and coronary artery disease9,10,11.
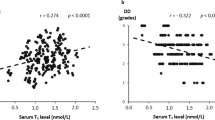
Since low levels of both testosterone and bilirubin are associated with elevated risk of cardiometabolic diseases, we inferred a possible association between testosterone levels and serum bilirubin. Therefore, this study aimed to examine the relationship between serum bilirubin level and testosterone deficiency in 1284 Korean men aged 45–70 years.
Methods
Study population
We reviewed the medical records of 1401 participants aged ≥ 45 years retrospectively who had a medical examination between November 2012 and July 2013 at the Health Promotion Center of Gangnam Severance Hospital in Seoul, Korea. The individuals visited the health promotion center willingly to regularly check their health condition. Written informed consent was obtained from all participants. This study was authorized by the Institutional Review Board of Yonsei University College of Medicine, Seoul, Korea (approval No. 3-2019-0319) and was managed in according to the ethical principles of the Declaration of Helsinki. We excluded men who met at least one of the following criteria: individuals with a history of hepatobiliary disease, positive test for hepatitis C antigens or hepatitis B antibodies, a history of exogenous testosterone therapy, above twice the upper normal limit for aspartate aminotransferase (AST) or alanine aminotransferase (ALT), total bilirubin levels of more than 3.0 mg/dL, hemoglobin level of less than 13 g/dL, not fasting for 12 h before testing or missing data. After applying the exclusion criteria, the final analysis included 1284 participants.
Data collection
Each participant was asked to fill in a questionnaire about his medical history and lifestyle. Self-reported alcohol consumption, physical activity, and cigarette smoking were determined from the questionnaires. Smoking status was classified as current smoker, ex-smoker, and non-smoker. Alcohol drinking was assessed into consumption ≥ twice a week. Regular exercise was considered as more than 30 min of aerobic exercise ≥ three times a week. Body mass and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, in light indoor clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by square of height in meters (kg/m2). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a standard mercury sphygmomanometer (Baumanometer, W.A. Baum Co Inc., Copiague, NY, USA) and the patient’s right arm. After a 12-h overnight fast, all blood samples were taken from the antecubital vein. Testosterone concentrations were determined using an electrochemiluminescence assay with a Modular Analytics E170 system (Roche Diagnostic Systems, Basel, Switzerland). The reproducibility was determined by calculating the intra- and inter-assay coefficients of variation (CV) as follow: [% CV = (SD/mean) * 100]. For intra-assay CV, the same serum was assayed three times in duplicate within the same assay. The inter-assay reproducibility was assessed by analyzing six times the consecutive assays in duplicate12. The intra-assay and inter-assay CVs for testosterone were 3.2% and 3.3%, respectively. Fasting glucose, triglycerides, high density lipoprotein (HDL) cholesterol, AST, ALT, and total bilirubin were quantified with enzymatic methods using a chemistry analyzer (Hitachi 7600, Hitachi Co., Tokyo, Japan). Leukocyte count was measured using an automated blood cell counter (ADVIA 120, Bayer, NY, USA).
Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current use of hypertension medication13. Type 2 diabetes was defined as current use of diabetes medication or fasting plasma glucose ≥ 126 mg/dL14. Dyslipidemia was set as triglyceride ≥ 150 mg/dL, HDL-cholesterol < 40 mg/dL, or use of dyslipidemia medications at present. The modified National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) released in 2001 was applied to designate metabolic syndrome15. According to the position statement of the American College of Endocrinology, we defined obesity as BMI ≥ 25 kg/m2, since waist circumference was not measured16. Thus, metabolic syndrome was defined by the existence of three or more of risk factors as follows: obesity with BMI ≥ 25 kg/m2, elevated fasting plasma glucose ≥ 126 mg/dL or current use of diabetes medication, high systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg, anti-hypertensive drugs, low HDL cholesterol < 40 mg/ dL and high triglycerides ≥ 150 mg/dL.
Statistical analysis
Normal distribution was assessed with determination of skewness by Kolmogorov–Smirnov test. ALT, AST, serum triglyceride levels had skewed distributions, therefore these variables were indicated as median with interquartile range (IQR) in descriptive analysis and log-transformed preceding to simple correlation and multiple logistic regression analysis. Serum bilirubin level was divided into quartiles: Q1 ≤ 0.7, Q2 0.8–0.9, Q3 1.0–1.1, and Q4 ≥ 1.2 mg/dL. The clinical characteristics of the study population according to serum bilirubin quartile were independently compared using one-way analysis of variance (ANOVA) or Kruskal–Wallis test for continuous variables in accordance with the normality of distributions and chi-square test for categorical variables. Continuous data are exhibited as mean (standard deviation [SD]) or median (IQR), and categorical data are marked as frequency. Testosterone deficiency was defined as level less than 8.0 nmol/L, as proposed by the announcement of the International Society of Andrology (ISA)17,18. The odds ratios (ORs) and 95% confidence intervals (95% CIs) for testosterone deficiency were calculated after adjusting for confounding variables over serum bilirubin quartiles using multiple logistic regression analysis. We have included the confounding variables in the logistic regression analysis model considering the commonly performed statistical principles to include clinically established important risk factors and statistically significant variables in the simple regression analysis. All analyses were carried out using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided, and statistical significance was determined at P < 0.05.
Informed consent
Informed consent was obtained from all the participants in the current study.
Results
Table1 shows the clinical characteristics of the study population according to serum bilirubin quartiles. Leukocyte count and fasting plasma glucose level were lowest, while HDL- cholesterol level was highest in the fourth quartile of bilirubin concentration. The proportions of current smoking was highest in the first quartile of serum bilirubin. The prevalence of metabolic syndrome, dyslipidemia, and type 2 diabetes decreased with increasing serum bilirubin quartile.
Figure 1 presents the prevalence of subjects with testosterone deficiency in each bilirubin quartile. The overall prevalence of testosterone deficiency was 5.8% and significantly decreased in accordance with serum bilirubin quartile (Q1 8.1%, Q2 7.0%, Q3 4.9%, and Q4 3.3%) (P = 0.026).
Table 2 indicates the results of multiple logistic regression analysis to assess the association between serum bilirubin level and testosterone deficiency. Compared with the referent fourth quartile (serum bilirubin ≥ 1.2 mg/dL), the ORs (95% CIs) for testosterone deficiency was 2.29 (1.04–4.94) for the first quartile after adjusting for age, fasting glucose, triglyceride, HDL-cholesterol, leukocyte count, hemoglobin, smoking status, and alcohol intake.
Discussion
In this cross-sectional study, serum bilirubin level was independently and inversely associated with testosterone deficiency in a dose–response way after adjusting for potential confounding variables. To date, it has been rarely investigated on the association between serum bilirubin level and testosterone deficiency. The plausible explanations may be offered to explain the inverse relationship between serum bilirubin level and testosterone deficiency.
First, low levels of testosterone and bilirubin are closely interrelated to oxidative stress and chronic low-grade inflammation. In our study, serum bilirubin level was inversely associated with leukocyte count, a nonspecific marker of systemic inflammation. Hwang et al. also reported that CRP level decreased with increasing bilirubin quartiles among apparently healthy adults19. Both animal and human studies have documented that bilirubin could serve as a potent lipid chain-breaking antioxidant under physiological concentrations, inhibiting the formation of atherogenic plaques20,21. Given the antioxidant capacity of bilirubin, epidemiological links between low serum bilirubin level and increased risk for atherosclerotic CVD and the severity of CAD have been shown in cross-sectional and longitudinal studies22. Among 4303 participants aged ≥ 60 years in the NHANES, a low total bilirubin level of 0.1–0.4 mg/dL was associated with higher mortality risk23. English et al. found statistically significant lower levels of testosterone in patients with catheterization-proven CAD compared with control groups24. In addition, mildly elevated unconjugated bilirubin reduces the risk of CVD in individuals with Gilbert’s syndrome25. Likewise, extended evidence also implies that chronic low-grade inflammation is closely involved in the pathogenesis of male hypogonadism26. Pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-6 are released from adipose tissues in testosterone deficiency, which leads to a subclinical inflammatory condition27. A recent study suggests that low testosterone levels may be interpreted as a state of low-grade inflammation. Park et al. revealed an inverse associations of testosterone levels with leukocyte counts in Korean adults26.
Second, low levels of testosterone and bilirubin are closely related to insulin resistance and metabolic abnormalities28,29. Recent epidemiological studies have shown that there is an inverse association of serum bilirubin and testosterone levels with cardiometabolic disturbances, such as obesity, hypertension, type 2 diabetes, and metabolic syndrome. In a prospective study of 468 Taiwanese middle-aged men, a higher bilirubin level was associated with a reduced future risk of metabolic syndrome particularly in nonsmoking men30. Our study also found a significant difference in the prevalence of type 2 diabetes, dyslipidemia, and metabolic syndrome according to serum bilirubin quartiles. A low testosterone level could be a result of insulin resistance through increasing visceral adiposity and decreasing skeletal muscle31. Low testosterone also increases lipoprotein lipase activity, leading to increase triglyceride uptake in central fat depots32, insulin resistance and metabolic syndrome. Thus, increased abdominal visceral fat and low testosterone levels have bidirectional interactions. Previous studies have demonstrated that hypercholesterolemia, glycemic control, visceral adiposity and insulin resistance in hypogonadal men with type 2 diabetes or metabolic syndrome were improved through testosterone replacement therapy33,34. Aslo, Wickramatilake et al. showed a positive association of testosterone with HDL-cholesterol level in angiographically confirmed CAD in men35.
This study had some limitations to its interpretation. First, we used a cross-sectional design and were not able to prove a causal relationship between serum bilirubin and testosterone deficiency. The results may mirror reverse causality and a bidirectional relationship in the association between these variables. Therefore, further large-scale with a long follow-up period, prospective studies are warranted to establish whether there is a cause-and-effect relationship between serum bilirubin and testosterone deficiency. Second, the study population might not represent the general population because the study participants were volunteers visiting a single health promotion center to screen their health condition and seemed to be a little bit healthier than most community-based cohorts. Third, only one measurement of bilirubin and testosterone levels from a baseline examination was included in the analyses. Fourth, the definition of type 2 diabetes does not meet the criteria of American Diabetes Association, since any information about HbA1c and 2-h plasma glucose levels were not collected from the dataset. Finally, the cut-off point for testosterone deficiency in the present study was defined as < 8.0 nmol/L as proposed by the announcement of the ISA. Men with levels between 8–12 nmol/L also may be testosterone deficiency, however testosterone deficiency symptoms and free testosterone levels should also be considered in this range of serum total testosterone. Unfortunately, we could not collect further information on testosterone deficiency symptoms and free testosterone levels from the initial dataset.
In conclusion, we found an inversely graded association of serum bilirubin level with testosterone deficiency. In conclusion, we found an inversely graded association of serum bilirubin level with testosterone deficiency and CVD risk factors. These findings suggest that low serum bilirubin level may be assumed as a state of testosterone deficiency and CVD risk in middle-aged and older men.
Data availability
The datasets in this study are available from the corresponding author on reasonable request.
References
Konaka, H. et al. Effects of long-term androgen replacement therapy on the physical and mental statuses of aging males with late-onset hypogonadism: A multicenter randomized controlled trial in Japan (EARTH Study). Asian J. Androl. 18, 25–34 (2016).
Dudek, P., Kozakowski, J. & Zgliczyński, W. Late-onset hypogonadism. Prz Menopauzalny 16, 66–69 (2017).
Nieschlag, E. Late-onset hypogonadism: A concept comes of age. Andrology 8, 1506–1511 (2019).
Araujo, A. B. et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: Estimates from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 89, 5920–5926 (2004).
Foresta, C., Calogero, A. E., Lombardo, F., Lenzi, A. & Ferlin, A. Late-onset hypogonadism: Beyond testosterone. Asian J. Androl. 17, 236–238 (2015).
Vítek, L. & Ostrow, J. D. Bilirubin chemistry and metabolism; Harmful and protective aspects. Curr. Pharm. Des. 15, 2869–2883 (2009).
Woreta, T. A. & Alqahtani, S. A. Evaluation of abnormal liver tests. Med. Clin. N. Am. 98, 1–16 (2014).
Ziberna, L., Martelanc, M., Franko, M. & Passamonti, S. Bilirubin is an endogenous antioxidant in human vascular endothelial cells. Sci. Rep. 6, 29240 (2016).
Lee, Y. B. et al. Change in serum bilirubin level as a predictor of incident metabolic syndrome. PLoS ONE 11, e0168253 (2016).
Suh, S. et al. Relationship between serum bilirubin levels and cardiovascular disease. PLoS ONE 13, e0193041 (2018).
Tang, L. H., Huang, C. & Feng, Y. Q. Serum total bilirubin concentration is associated with carotid atherosclerosis in patients with prehypertension. Clin. Exp. Hypertens. 41, 682–686 (2019).
Rodbard, D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin. Chem. 20, 1255–1270 (1974).
Kim, H. C. et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: Part I-epidemiology of hypertension. Clin. Hypertens. 25, 16 (2019).
Jung, D. H. et al. Relationship of body composition and C-reactive protein with pulmonary function. Respir. Med. 104, 1197–1203 (2010).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112, 2735–2752 (2005).
Einhorn, D. et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 9, 237–252 (2003).
Bhasin, S. et al. Testosterone therapy in men with hypogonadism: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 103, 1715–1744 (2018).
Wang, C. et al. ISA, ISSAM, EAU, EAA and ASA recommendations: Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 12, 5–12 (2009).
Hwang, H. J., Lee, S. W. & Kim, S. H. Relationship between bilirubin and C-reactive protein. Clin. Chem. Lab. Med. 49, 1823–1828 (2011).
Maruhashi, T., Kihara, Y. & Higashi, Y. Bilirubin and endothelial function. J. Atheroscler. Thromb. 26, 688–696 (2019).
Stocker, R., Yamamoto, Y., McDonagh, A. F., Glazer, A. N. & Ames, B. N. Bilirubin is an antioxidant of possible physiological importance. Science 235, 1043–1046 (1987).
Vítek, L. Bilirubin and atherosclerotic diseases. Physiol. Res. 66, S11-s20 (2017).
Ong, K. L. et al. The relationship between total bilirubin levels and total mortality in older adults: the United States National Health and Nutrition Examination Survey (NHANES) 1999–2004. PLoS ONE 9, e94479 (2014).
English, K., Steeds, R., Jones, T. & Channer, K. Testosterone and coronary heart disease: Is there a link?. QJM Int. J. Med. 90, 787–791 (1997).
Kundur, A. R., Santhakumar, A. B., Bulmer, A. C. & Singh, I. Mildly elevated unconjugated bilirubin is associated with reduced platelet activation-related thrombogenesis and inflammation in Gilbert’s syndrome. Platelets 28, 779–785 (2017).
Park, B. & Lee, Y. J. Inverse association of testosterone and sex hormone binding globulin levels with leukocyte count in middle-aged and elderly men. Aging Male 21, 176–181 (2018).
Zhang, J. et al. Both total testosterone and sex hormone-binding globulin are independent risk factors for metabolic syndrome: Results from Fangchenggang Area Male Health and Examination Survey in China. Diabetes Metab. Res. Rev. 29, 391–397 (2013).
Takei, R. et al. Bilirubin reduces visceral obesity and insulin resistance by suppression of inflammatory cytokines. PLoS ONE 14, e0223302 (2019).
Gevi, F., Fanelli, G. & Zolla, L. Metabolic patterns in insulin-resistant male hypogonadism. Cell Death Dis. 9, 671 (2018).
Huang, S. S. et al. Serum bilirubin levels predict future development of metabolic syndrome in healthy middle-aged nonsmoking men. Am. J. Med. 128(1138), e1135-1141 (2015).
Dhindsa, S. et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care 39, 82–91 (2016).
Kelly, D. M. & Jones, T. H. Testosterone and obesity. Obes. Rev. 16, 581–606 (2015).
Kapoor, D., Goodwin, E., Channer, K. S. & Jones, T. H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 154, 899–906 (2006).
Jones, T. H. et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 34, 828–837 (2011).
Wickramatilake, C. M., Mohideen, M. R. & Pathirana, C. Association of serum testosterone with lipid abnormalities in patients with angiographically proven coronary artery disease. Indian J. Endocrinol. Metab. 17, 1061–1065 (2013).
Acknowledgements
The authors would like to thank to the staffs of Health Promotion Center of Gangnam Severance Hospital.
Author information
Authors and Affiliations
Contributions
H.M.P. contributed to conceptualization, data curation, formal analysis, investigation, methodology, and writing the original draft; H.K. contributed to conceptualization, data curation, investigation, and visualization; H.L. and Y.J.L. helped in conceptualization, methodology, formal analysis, supervision, review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, HM., Kim, H., Lee, H.S. et al. Inverse association between serum bilirubin level and testosterone deficiency in middle-aged and older men. Sci Rep 11, 8026 (2021). https://doi.org/10.1038/s41598-021-87220-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87220-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.