Abstract
It has been suggested that the relationship between cognitive function and functional outcome in schizophrenia is mediated by clinical symptoms, while functional outcome is assessed by the Quality of Life Scale (QLS) and the Global Assessment of Functioning (GAF) Scale. To determine the outcome assessed by QLS and GAF, we established a bagging ensemble framework with a feature selection algorithm resulting from the analysis of factors such as 3 clinical symptom scales and 11 cognitive function scores of 302 patients with schizophrenia in the Taiwanese population. We compared our bagging ensemble framework with other state-of-the-art algorithms such as multilayer feedforward neural networks, support vector machine, linear regression, and random forests. The analysis revealed that the bagging ensemble model with feature selection performed best among predictive models in predicting the QLS functional outcome by using 20-item Scale for the Assessment of Negative Symptoms (SANS20) and 17-item Hamilton Depression Rating Scale (HAMD17). Moreover, to predict the GAF outcome, the bagging ensemble model with feature selection performed best among predictive models by using SANS20 and the Positive and Negative Syndrome Scale-Positive (PANSS-Positive) subscale. The study indicates that there are synergistic effects between negative (SANS20) and depressive (HAMD17) symptoms as well as between negative and positive (PANSS-Positive) symptoms in influencing functional outcome of schizophrenia using the bagging ensemble framework with feature selection.
Similar content being viewed by others
Introduction
Functional outcome of schizophrenia, which is commonly assessed by the tools such as Quality of Life Scale (QLS)1 and the Global Assessment of Functioning (GAF) Scale2, has an impact on psychiatric diagnosis and treatment. In patients with schizophrenia, multiple functional domains, including work activities, social relationships, and independent living, of everyday life are usually impaired3, 4. Thereby, it is crucial to identify probable factors that influence functional outcome of schizophrenia5. Several potential predictors of its functional outcome include negative symptoms, verbal learning, visual learning, working memory, and social cognition, to name a few3, 4, 6, 7. GAF is recognized as an important objective measure to assess global psychological, social, and occupational functioning in patients with schizophrenia2. On the other hand, QLS is also useful in evaluating their functional outcome8. Thus, QLS and GAF have been used together for the assessment of longitudinal outcome of schizophrenia9. While some studies showed the limited predictive effect of clinical symptoms, such as positive symptoms5, 10, on functional outcome of schizophrenia, other studies indicated that clinical symptoms, particularly negative symptoms11, 12, were associated with its functional outcome. Moreover, it has been suggested that numerous cognitive functions such as neuro- and social-cognitions are also linked to its functional outcome13, 14. On another note, precision psychiatry is an emerging multidisciplinary arena of psychiatry and precision medicine15, 16, where state-of-the-art artificial intelligence and machine learning algorithms are incorporated with multiple data types such as genetic and clinical data to facilitate appropriate individual-tailored decisions during all stages of patient care17,18,19,20. For example, various applications in precision psychiatry encompass the prediction of patients with schizophrenia21, 22 and the prediction of antidepressant treatment outcome in patients with major depressive disorder23, 24 using machine learning models. We therefore proposed that machine learning models may be able to predict potential factors that affect functional outcomes of schizophrenia by using various clinical data (namely clinical symptoms and cognitive functions).
In a previous study, Lin et al.5 indicated that clinical symptoms mediated the relationship between cognitive impairment and functional outcome of schizophrenia by using the structural equation modeling method. Here, we utilized the same cohort of 302 patients with schizophrenia and carried out the first study on the QLS and GAF functional outcome prediction in schizophrenia patients with 3 clinical symptom scales and 11 cognitive function tests by using a bagging ensemble machine learning approach25. In addition, in order to forecast functional outcomes, we employed the M5 Prime feature selection algorithm26 to pinpoint a small subset of feasible factors from 3 clinical symptom scales and 11 cognitive function tests. We hypothesized that our bagging ensemble machine learning method would be able to predict the QLS- and GAF-related outcome in patients with schizophrenia by using a small subset of selected clinical symptom scales and/or cognitive function assessments. While no previous studies have evaluated predictive models for functional outcome of schizophrenia by using the bagging ensemble machine learning method with the M5 Prime feature selection algorithm, there have been studies that utilized the bagging and feature selection approaches generally for the prediction of functional outcome for individuals with psychosis27, 28. The bagging approach, which was created for simple bootstrapping in 1994, has been frequently utilized for experiments that employ a resampling scheme. We selected the bagging ensemble machine learning method since this method had been frequently applied to solve complex prediction and classification problems because of its advantages in reduction of variance and overfitting25, 26. This study directly compared the bagging ensemble machine learning model with widely-used machine learning algorithms, including multi-layer feedforward neural networks (MFNNs), support vector machine (SVM), linear regression, and random forests. We hypothesized that our bagging ensemble machine learning approach with the M5 Prime feature selection algorithm could lead to better performance.
Results
The clinical symptoms, cognitive manifestations, and functional outcome of the study cohort
The participants included 302 patients with schizophrenia in the Taiwanese population. Study measures relevant to their demographic characteristics, 3 clinical symptoms, 11 cognitive functions, QLS and GAF were detailed before5.
Feature selection using clinical symptom scales
We performed a series of different feature combinations (Table 1; the Feature-A, Feature-B, and Feature-C sets) to predict the QLS and GAF scores using the 3 clinical symptom scales. Note that the Feature-A set includes the 3 clinical symptom scales, namely 17-item Hamilton Depression Rating Scale (HAMD17), 20-item Scale for the Assessment of Negative Symptoms (SANS20), and the Positive and Negative Syndrome Scale-Positive subscale (PANSS-Positive).
For predicting the QLS score, we used the M5 Prime feature selection algorithm (see Methods) to identify 2 features (including SANS20 and HAMD17) from the 3 clinical symptom scales, where these 2 chosen features comprised the Feature-B dataset.
For predicting the GAF score, we used the M5 Prime feature selection algorithm to find 2 features (including PANSS-Positive and SANS20) from the 3 clinical symptom scales, where these 2 chosen features comprised the Feature-C dataset.
Prediction of QLS and GAF using clinical symptom scales
We combined clinical symptom scales (namely the Feature-A, Feature-B, and Feature-C datasets) to construct the predictive models for the QLS and GAF scores by employing the bagging ensemble framework, respectively. Table 1 summarizes the results of repeated tenfold cross-validation experiments for the predictive models using clinical symptom scales by the bagging ensemble model with feature selection, the bagging ensemble model, MFNNs, SVM, linear regression, and random forests. Moreover, we used the root mean square error (RMSE) values to measure the performance of the predictive models.
As indicated in Table 1, to predict the QLS, the bagging ensemble model with feature selection performed best in terms of the RMSE value of 6.4293 ± 1.1332 using the Feature-B dataset (namely SANS20 and HAMD17) among the predictive models. In other words, the combination of SANS20 and HAMD17 best predicted the QLS outcome among all combinations of clinical symptom scales.
In addition, to predict the GAF, the bagging ensemble model with feature selection performed best in terms of the RMSE value of 7.7806 ± 1.1595 using the Feature-C dataset (namely PANSS-Positive and SANS20) among the predictive models (Table 1). In other words, the combination of PANSS-Positive and SANS20 best predicted the GAF among all combinations of clinical symptom scales.
Feature selection using cognitive function scores
We performed various feature combinations (Table 2; the Feature-D, Feature-E, and Feature-F datasets) to predict the QLS and GAF of schizophrenia using the cognitive function scores. Note that the Feature-D set included the 11 cognitive function scores.
For predicting the QLS, we used the M5 Prime feature selection algorithm (see Methods) to identify 5 features (including category fluency, WAIS-III digit symbol-coding, verbal working memory, nonverbal working memory, and social cognition) from the 11 cognitive function scores, where these 5 chosen features comprised the Feature-E dataset.
For predicting the GAF, we used the M5 Prime feature selection algorithm to find 5 features (including category fluency, WAIS-III digit symbol-coding, d-Prime of blurred version, verbal working memory, and reasoning and problem solving) from the 11 cognitive function scores, where these 5 selected features comprised the Feature-F dataset.
Prediction of the QLS and GAF of schizophrenia using cognitive function scores
We used cognitive function scores (namely the Feature-D, Feature-E, and Feature-F datasets) to construct the predictive models for the QLS and GAF scores by employing the bagging ensemble framework, respectively. Table 2 summarizes the results of repeated tenfold cross-validation experiments for the predictive models using cognitive function scores by the bagging ensemble model with feature selection, the bagging ensemble model, MFNNs, SVM, linear regression, and random forests.
As shown in Table 2, to predict the QLS, the bagging ensemble model with feature selection performed best in terms of the RMSE value of 7.7717 ± 1.0024 using the Feature-E dataset (including category fluency, WAIS-III digit symbol-coding, verbal working memory, nonverbal working memory, and social cognition) among the predictive models. In other words, among all combinations of cognitive tests, the combination of category fluency, WAIS-III digit symbol-coding, verbal working memory, nonverbal working memory, and social cognition best predicted the QLS score.
In addition, to predict the GAF, the bagging ensemble model with feature selection performed best in terms of the RMSE value of 8.6050 ± 1.1101 using the Feature-F dataset (including category fluency, WAIS-III digit symbol-coding, d-Prime of blurred version, verbal working memory, and reasoning and problem solving) among the predictive models (Table 2). In other words, among all combinations of cognitive tests, the combination of category fluency, WAIS-III digit symbol-coding, d-Prime of blurred version, verbal working memory, and reasoning and problem solving best predicted the GAF score.
Benchmarking
By comparing the results (Tables 1 and 2) for predicting the QLS of schizophrenia patients among machine learning predictive algorithms (including the bagging ensemble model with feature selection, the bagging ensemble model, MFNNs, SVM, linear regression, and random forests) using 4 feature datasets (including Feature-A, Feature-B, Feature-D, and Feature-E), the bagging ensemble model with feature selection (using Feature-B) performed best. The best RMSE value for predicting the QLS was 6.4293 ± 1.1332 (Table 1). In other words, the combination of SANS20 and HAMD17 best predicted the QLS performance among all clinical combinations and cognitive combinations.
By comparing the results (Tables 1 and 2) for predicting the GAF of schizophrenia among machine learning predictive algorithms (including the bagging ensemble model with feature selection, the bagging ensemble model, MFNNs, SVM, linear regression, and random forests) using 4 feature datasets (including Feature-A, Feature-C, Feature-D, and Feature-F), the bagging ensemble model with feature selection (using Feature-C) performed best. The best RMSE value for predicting the GAF was 7.7806 ± 1.1595 (Table 1). In other words, the combination of PANSS-Positive and SANS20 best predicted the GAF score among all clinical combinations and cognitive combinations.
Here, we found that the bagging ensemble model with feature selection using the selected features from clinical symptom scales performed best in predicting the QLS or GAF outcome when compared with other state-of-the-art algorithms, including MFNNs, SVM, linear regression, and random forests. Our analysis indicated that the bagging ensemble model with feature selection was well-suited for predictive models in the functional outcome of schizophrenia.
Discussion
To our knowledge, this is the first study to date to identify synergistic effects between SANS20 and HAMD17 as well as between PANSS-Positive and SANS20 in influencing functional outcomes in schizophrenia among Taiwanese individuals using a bagging ensemble machine learning approach with the M5 Prime feature selection algorithm. Moreover, we performed the first study to predict potential factors affecting functional outcome of schizophrenia by utilizing various clinical data (that is, clinical symptoms and cognitive functions). The findings pinpointed that the bagging ensemble model with feature selection using 2 factors excelled other state-of-the-art predictive models in terms of RMSE for predicting the QLS outcome of schizophrenia, where these 2 factors encompassed SANS20 and HAMD17. Moreover, for predicting the GAF of schizophrenia patients, we found that the bagging ensemble model with feature selection using 2 factors outperformed other state-of-the-art predictive models in terms of RMSE, where these 2 factors encompassed PANSS-Positive and SANS20.
Interestingly, our analysis revealed that the combination of SANS20 (for measuring negative symptoms) and HAMD17 (for measuring depressive symptoms) was the best predictor for the QLS functional outcome of schizophrenia among all clinical symptom combinations and cognitive function combinations. In addition, the combination of SANS20 (for negative symptoms) and PANSS-Positive (for positive symptoms) was the best predictor for the GAF functional outcome of schizophrenia among all clinical symptom combinations and cognitive function combinations. In other words, there are synergistic effects between negative and depressive symptoms as well as between negative and positive symptoms in influencing functional outcome of schizophrenia. To the best of our knowledge, no previous studies have been conducted to identify a synergistic benefit beyond that of either clinical symptom scale standing alone. The interaction effects of clinical symptoms remain to be elucidated. It has been suggested that negative symptoms may act as a key predictor for functional outcome of schizophrenia5, 29, 30; moreover, positive symptoms may contribute to the GAF functional outcome5, 31. In addition, it has been suggested that depressive symptoms were related to the QLS performance5, 30. In consideration with the previous results5, 29,30,31, we speculated that SAN20 (for measuring negative symptoms) may likely incorporate with other factors such as HAMD17 (for depressive symptoms) or PANSS-Positive (for positive symptoms) to influence functional outcome of schizophrenia since all clinical symptoms are important predictors for functional outcome of schizophrenia.
By leveraging the clinical data, we established the predictive models of functional outcome of schizophrenia by using the bagging ensemble machine learning approach with the M5 Prime feature selection algorithm. Our analysis also suggests that the bagging ensemble model with feature selection may offer a feasible solution to construct predictive models for forecasting functional outcome of schizophrenia with purposeful accuracy. Therefore, the bagging ensemble approach with feature selection in this study is a proof-of-concept machine learning tool for predicting functional outcome of schizophrenia.
Furthermore, it is worthwhile to bring the discussion on the M5 Prime feature selection algorithm for dealing with potential factors affecting functional outcome of schizophrenia in our study. We observed that the bagging ensemble model with the chosen factors of the M5 Prime feature selection algorithm always surpassed the bagging ensemble model without using feature selection. For instance, the bagging ensemble model with the Feature-B dataset outperformed the bagging ensemble model with the Feature-A in predicting the QLS. Similarly, the bagging ensemble model with the Feature-C dataset outperformed the bagging ensemble model with the Feature-A dataset in predicting the GAF. That is, the bagging ensemble models with feature selection tended to have lower RMSE values. In terms of the predictive performance, the lower the RMSE value, the better the performance. We speculated that it may be due to the advantage of the M5 Prime feature selection algorithm to pinpoint probable factors influencing functional outcome of schizophrenia. In line with our analysis, previous studies indicated that machine learning algorithms with feature selection performed better than the ones without feature selection in forecasting disease status or treatment response for psychiatric disorders24, 32, 33.
Remarkably, an intriguing finding was that any of the machine learning models with clinical symptom scales always surpassed any of the machine learning models with cognitive function scores. For example, the predictive models with the Feature-A and Feature-B datasets always excelled the predictive models with the Feature-D and Feature-E datasets in predicting the QLS. Similarly, the predictive models with the Feature-A and Feature-C datasets always outperformed the predictive models with the Feature-D and Feature-F datasets in predicting the GAF. We hypothesized that it may be due to the advantage of clinical symptom scales over cognitive function scores on affecting functional outcomes in schizophrenia. In accordance with our analysis, it has been reported that clinical symptoms and cognitive functions accounted for 89% and 44% of the variance in the functional outcome of schizophrenia, respectively5.
This study had some limitations. The first weakness was that the cross-sectional methodology limited the predictive value. Second, both QLS and GAF scores were related to clinical symptoms, thereby causing an overlap between the predictors and the outcome.
In conclusion, we constructed a bagging ensemble machine learning framework with feature selection for estimating functional outcomes in schizophrenia in Taiwanese subjects by using clinical data. The analysis indicates that our bagging ensemble machine learning framework with feature selection detects synergistic effects between negative and depressive symptoms as well as between negative and positive symptoms in influencing functional outcomes in schizophrenia. In the long run, we would expect that the discoveries of the present study may be generalized for precision psychiatry studies to forecast functional outcome and disease status for psychiatric disorders. Moreover, the discoveries may be likely utilized to contribute to prognostic and diagnostic applications in the near future. All in all, it is indispensable to investigate in independent studies with replication samples and further explore the role of the bagging ensemble machine learning framework created in the present study.
Materials and methods
This study was approved by the institutional review board of the China Medical University Hospital in Taiwan and was conducted in accordance with the Declaration of Helsinki.
Study population
The study cohort consisted of 302 patients with schizophrenia, who were recruited from the China Medical University Hospital and affiliated Taichung Chin-Ho Hospital in Taiwan5. In this study, patients with schizophrenia were aged 18–65 years and were healthy in the physical conditions. After presenting a complete description of this study to the subjects, we obtained written informed consents from a parent and/or legal guardian in line with the institutional review board guidelines. Details of the diagnosis of schizophrenia were published previously5.
Clinical symptom scales
In this study, we employed 3 clinical symptom scales to assess positive, negative and depressive symptoms5, including the PANSS-Positive subscale34, SANS2035, and HAMD1736.
Cognitive function scores
We employed 11 cognitive function scores to assess cognitive functions5, including category fluency, trail making A, digit symbol-coding (Wechsler Adult Intelligence Scale, third edition (WAIS-III)), d-Prime of clear version, d-Prime of blurred version, verbal working memory, nonverbal working memory, verbal learning and memory, visual learning and memory, reasoning and problem solving, and social cognition. In brief, these 11 cognitive function scores were used to assess 7 cognitive domains such as speed of processing, sustained attention, working memory, verbal learning and memory, visual learning and memory, reasoning and problem solving, and social cognition5. The speed of processing domain was assessed using category fluency, trail making A, and WAIS-III digit symbol-coding. The sustained attention domain was assessed using d-Prime of clear version and d-Prime of blurred version. The working memory domain was assessed using verbal working memory and nonverbal working memory.
Functional outcomes
We measured functional outcomes using the QLS1 and the GAF Scale of the DSM-IV2. QLS is a tool to provide the rating of functional outcomes in schizophrenia, including social activity, social initiatives, social withdrawal, sense of purpose, motivation, curiosity, anhedonia, aimless inactivity, capacity for empathy, emotional interaction8. GAF is a tool to provide a measure for assessing global psychological, social, and occupational functioning in schizophrenia2.
Statistical analysis
The Student’s t test was conducted to measure the difference in the means of two continuous variables37. We performed the chi-square test for categorical data. The criterion for significance was set at P < 0.05 for all tests. Data are presented as the mean ± standard deviation.
With the assumption that a 95% confidence level and a proportion of 0.5, a simplified formula38 was used to calculate sample sizes as follows: n = N / (1 + N (e)2), where n is the sample size, N is the population size, and e is the level of precision. In this study, we assumed that N = 230,000 and e = 0.06.
Bagging ensemble predictive models
We employed a key ensemble machine learning technique called bagging predictors25 and utilized the Waikato Environment for Knowledge Analysis (WEKA) software (which is available from https://www.cs.waikato.ac.nz/ml/weka/)26 to carry out the bagging ensemble predictive framework. In addition, other machine learning software tools can be employed, for example, Pattern Recognition for Neuroimaging Toolbox (PRoNTo; http://www.mlnl.cs.ucl.ac.uk/pronto/) and NeuroMiner (https://github.com/neurominer-git). All the experiments were conducted on a computer with Intel (R) Core (TM) i5-4210U, 4 GB RAM, and Windows 721. It should be noted that we utilized the repeated tenfold cross-validation method to examine the generalization of bagging predictors21, 32, 39.
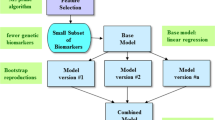
Figure 1 shows the illustrative diagram of the bagging ensemble predictive framework with feature selection. The technique of the bagging ensemble predictive algorithm is used to combine the predictive performance of multiple versions of a base predictor to achieve an aggregated predictor with higher accuracy. The multiple versions of the base predictor are formed by the bootstrap method, where the bootstrap method is one of the most popular data resampling methods used in statistical analysis. The technique of the bagging ensemble predictive algorithm tends to reduce variance and avoid overfitting. The base predictor we employed was MFNNs or SVM. Here, we used the default parameters of WEKA, such as 100 for the batch size, 100 for the percentage of the bag size, and 10 for the number of iterations21, 40.
The schematic illustration of the bagging ensemble predictive algorithm with feature selection. First, the M5 Prime feature selection algorithm is performed to select a subset of features, which serves as the input to the bagging ensemble predictive algorithm. The idea of the bagging ensemble predictive algorithm is to generate the multiple versions of a base predictor by bootstrap replications. The final prediction is then produced by averaging the predictive performance of the multiple versions. The base predictor was chosen as multi-layer feedforward neural networks (MFNNs) or support vector machine (SVM) in this study. GAF = Global Assessment of Functioning; QLS = Quality of Life Scale.
Machine learning algorithms for benchmarking
For the benchmarking task in the present study, we utilized 4 state-of-the-art machine learning algorithms including MFNNs, SVM, linear regression, and random forests. We carried out the analyses for these 4 machine learning algorithms using the WEKA software26 and a computer with Intel (R) Core (TM) i5-4210U, 4 GB RAM, and Windows 721. Other machine learning software tools such as PRoNTo (http://www.mlnl.cs.ucl.ac.uk/pronto/) and NeuroMiner (https://github.com/neurominer-git) could be also used. It should be noted that we utilized the repeated tenfold cross-validation method to examine the generalization of these 4 machine learning algorithms21, 32, 39.
An MFNN framework consists of one input layer, one or multiple hidden layers, and one output layer, where each layer contains neuron structures and connections among neuron structures contain no directed cycles21, 41. In general, the back-propagation algorithm42 is widely leveraged to train the MFNN framework, where the back-propagation algorithm updates the weights of neuron structures in the layers of the MFNN framework21, 43. In this study, we used the architecture containing 1 hidden layer. For example, we used the following WEKA’s parameters for training the MFNN model with 1 hidden layer: the momentum = 0.01, the learning rate = 0.01, and the batch size = 10021, 40.
The SVM algorithm44 is a popular technique for pattern recognition and classification21. The SVM algorithm, which is based on statistical learning theory, finds a linear relationship between input variables and the dependent variable (that is, the predicted output)44, 45. The best model for the predicted output is obtained by minimizing both the coefficients of the cost function and the predictive errors, where the cost function consists of the regression coefficients and an error term44, 45. In this study, we used the polynomial kernel with the exponent value of 1.024.
The random forests model combines a collection of decision trees, where a decision tree is defined as an inverted tree with three types of nodes such as a root node, internal nodes, and leaf nodes21, 46. The random forests model is conceptualized to obtain a better prediction by aggregating the predictive results from a collection of decision trees21, 46. Here, we used the default parameters of WEKA for the random forests model; for example, 100 for the batch size and 100 for the number of iterations21.
The linear regression model, the standard method for prediction problems in clinical applications, was used as a basis for comparison21, 26. Linear regression is suitable for assessing the relationship between a scalar response (that is, a dependent variable) and explanatory variables (that is, independent variables) by fitting a linear equation to the data 21, 26.
M5 Prime feature selection algorithm
In the present study, we utilized an Akaike information criterion (AIC)-based approach called the M5 Prime algorithm26 for the feature selection task. The M5 Prime algorithm constructs a decision tree with multivariate linear models at the terminal nodes and iteratively removes the feature with the smallest standardized coefficient until no further improvement in the estimated error defined by the AIC47, 48. Moreover, we utilized the tenfold cross-validation method to examine the generalization of the feature selection task21, 32, 39.
To predict the QLS and GAF, we used the M5 Prime algorithm to select features from 2 different feature datasets (Fig. 1). The first feature dataset includes 3 clinical symptom scales. The second feature dataset includes 11 cognitive function scores.
Evaluation of the predictive performance
In this study, we utilized one of the most popular criteria, the RMSE, to assess the performance of predictive models32, 45, 49. The RMSE calculates the difference between the measured values and the estimated values by a predictive model. The better the prediction model, the lower the RMSE32, 49. Moreover, we utilized the repeated tenfold cross-validation method to examine the generalization of predictive models21, 32, 39. First, the whole dataset was randomly split into ten separate segments. Second, the predictive model was trained using nine-tenths of the data and was tested using the remaining tenth of data to evaluate the predictive performance. Next, the previous step was repeated nine more times by leaving out distinct nine-tenths of the data as training data and a distinct tenth of data as testing data. Finally, the average estimation was reported over all runs by processing the aforementioned tenfold cross-validation for 10 times with distinct batches of data. We estimated the performance of all predictive models using the repeated tenfold cross-validation method.
References
Heinrichs, D. W., Hanlon, T. E. & Carpenter, W. T. Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr. Bull. 10, 388–398 (1984).
Startup, M., Jackson, M. C. & Bendix, S. The concurrent validity of the Global Assessment of Functioning (GAF). Br. J. Clin. Psychol. 41, 417–422 (2002).
Bechi, M. et al. Exploring functioning in schizophrenia: predictors of functional capacity and real-world behaviour. Psychiatry Res. 251, 118–124 (2017).
Galderisi, S. et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 13, 275–287 (2014).
Lin, C.-H. et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr. Res. 146, 231–237 (2013).
Santesteban-Echarri, O. et al. Predictors of functional recovery in first-episode psychosis: a systematic review and meta-analysis of longitudinal studies. Clin. Psychol. Rev. 58, 59–75 (2017).
Vesterager, L. et al. Cognitive and clinical predictors of functional capacity in patients with first episode schizophrenia. Schizophr. Res. 141, 251–256 (2012).
Narvaez, J.-M. et al. Subjective and objective quality of life in schizophrenia. Schizophr. Res. 98, 201–208 (2008).
Sim, K. et al. Physical comorbidity, insight, quality of life and global functioning in first episode schizophrenia: a 24-month, longitudinal outcome study. Schizophr. Res. 88, 82–89 (2006).
Ventura, J., Hellemann, G. S., Thames, A. D., Koellner, V. & Nuechterlein, K. H. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr. Res. 113, 189–199 (2009).
Milev, P., Ho, B.-C., Arndt, S. & Andreasen, N. C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 162, 495–506 (2005).
Campellone, T. R., Sanchez, A. H. & Kring, A. M. Defeatist performance beliefs, negative symptoms, and functional outcome in schizophrenia: a meta-analytic review. Schizophr. Bull. 42, 1343–1352 (2016).
Horan, W. P. et al. Social cognition in schizophrenia, part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr. Bull. 38, 865–872 (2012).
Davies, G. & Greenwood, K. A meta-analytic review of the relationship between neurocognition, metacognition and functional outcome in schizophrenia. J. Mental Health, 1–11 (2018).
Katsanis, S. H., Javitt, G. & Hudson, K. Public health. A case study of personalized medicine. Science 320, 53–54. https://doi.org/10.1126/science.1156604 (2008).
Snyderman, R. Personalized health care: from theory to practice. Biotechnol. J. 7, 973–979. https://doi.org/10.1002/biot.201100297 (2012).
Lane, H. Y., Tsai, G. E. & Lin, E. Assessing gene-gene interactions in pharmacogenomics. Mol. Diagn. Ther. 16, 15–27. https://doi.org/10.2165/11597270-000000000-00000 (2012).
Lin, E. & Chen, P. S. Pharmacogenomics with antidepressants in the STAR*D study. Pharmacogenomics 9, 935–946. https://doi.org/10.2217/14622416.9.7.935 (2008).
Lin, E. & Lane, H. Y. Genome-wide association studies in pharmacogenomics of antidepressants. Pharmacogenomics 16, 555–566. https://doi.org/10.2217/pgs.15.5 (2015).
Lin, E. & Lane, H.-Y. Machine learning and systems genomics approaches for multi-omics data. Biomark. Res. 5, 2 (2017).
Lin, E., Lin, C.-H., Hung, C.-C. & Lane, H.-Y. An ensemble approach to predict schizophrenia using protein data in the N-methyl-D-aspartate receptor (NMDAR) and tryptophan catabolic pathways. Front. Bioeng. Biotechnol. 8, 569 (2020).
Lin, E. et al. Combination of G72 genetic variation and G72 protein level to detect Schizophrenia: machine learning approaches. Front. Psych. 9, 566 (2018).
Lin, E. et al. A deep learning approach for predicting antidepressant response in major depression using clinical and genetic biomarkers. Front. Psych. 9, 290 (2018).
Lin, E. et al. Prediction of antidepressant treatment response and remission using an ensemble machine learning framework. Pharmaceuticals 13, 305 (2020).
Breiman, L. Bagging predictors. Mach. Learn. 24, 123–140 (1996).
Witten, I. H. F., E. (Morgan Kaufmann Publishers, 2005).
Koutsouleris, N. et al. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. Lancet Psychiatry 3, 935–946 (2016).
Koutsouleris, N. et al. Prediction models of functional outcomes for individuals in the clinical high-risk state for psychosis or with recent-onset depression: a multimodal, multisite machine learning analysis. JAMA Psychiat. 75, 1156–1172 (2018).
Rabinowitz, J. et al. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr. Res. 137, 147–150 (2012).
Tomotake, M. Quality of life and its predictors in people with schizophrenia. J. Med. Invest. 58, 167–174 (2011).
Craig, T., Fennig, S., Tanenberg-Karant, M. & Bromet, E. J. Six-month clinical status as a predictor of 24-month clinical outcome in first-admission patients with schizophrenia. Ann. Clin. Psychiatry 11, 197–203 (1999).
Huang, L. C., Hsu, S. Y. & Lin, E. A comparison of classification methods for predicting Chronic Fatigue Syndrome based on genetic data. J. Transl. Med. 7, 81. https://doi.org/10.1186/1479-5876-7-81 (2009).
Shahamat, H. & Pouyan, A. A. Feature selection using genetic algorithm for classification of schizophrenia using fMRI data. J. AI Data Min. 3, 30–37 (2015).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Andreasen, N. C. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry 155, 49–52 (1989).
Hamilton, M. in Assessment of depression 143–152 (Springer, 1986).
Lin, E., Kuo, P.-H., Liu, Y.-L., Yang, A. & Tsai, S.-J. Association and interaction effects of interleukin-12 related genes and physical activity on cognitive aging in old adults in the Taiwanese population. Front. Neurol. 10, 1065 (2019).
Yamane, T. Statistics: an introductory analysis. (1967).
Lin, E. & Hsu, S. Y. A Bayesian approach to gene–gene and gene–environment interactions in chronic fatigue syndrome. Pharmacogenomics 10, 35–42. https://doi.org/10.2217/14622416.10.1.35 (2009).
Lin, E., Mukherjee, S. & Kannan, S. A deep adversarial variational autoencoder model for dimensionality reduction in single-cell RNA sequencing analysis. BMC Bioinformatics 21, 1–11 (2020).
Bishop, C. M. Neural Networks for Pattern Recognition. (Clarendon Press, 1995).
Rumelhart, D. E. H., G.E.; William, R.J. in The Micro-Structure of Cognition Vol. 1 (MIT Press, 1996).
Kung, S. Y. H., J.N. Neural networks for intelligent multimedia processing. Proc. IEEE 86, 1244–1272 (1998).
Vapnik, V. The Nature of Statistical Learning Theory. (Springer, 2013).
Lin, E. & Hwang, Y. A support vector machine approach to assess drug efficacy of interferon-alpha and ribavirin combination therapy. Mol Diagn Ther 12, 219–223 (2008).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Bozdogan, H. Model selection and Akaike’s information criterion (AIC): The general theory and its analytical extensions. Psychometrika 52, 345–370 (1987).
Quinlan, J. R. in 5th Australian joint conference on artificial intelligence. 343–348 (World Scientific).
Linden, A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J. Eval. Clin. Pract. 12, 132–139. https://doi.org/10.1111/j.1365-2753.2005.00598.x (2006).
Funding
This work was supported by National Health Research Institutes, Taiwan (NHRI- EX109-10731NI), Ministry of Science and Technology in Taiwan (MOST 109-2622-B-039-001-CC2; 109-2314-B-039-001; 109-2314-B-039-039-MY3), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW109-TDU-B-212-114004), China Medical University Hospital (DMR-110-236).
Author information
Authors and Affiliations
Contributions
E.L., C-.H.L., and H-.Y.L. designed the study. C-.H.L. and H-.Y.L. conducted the study. E.L. analyzed the data. EL drafted the manuscript. E.L., C-.H.L., and H-.Y.L. revised the manuscript. All authors provided the final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, E., Lin, CH. & Lane, HY. Applying a bagging ensemble machine learning approach to predict functional outcome of schizophrenia with clinical symptoms and cognitive functions. Sci Rep 11, 6922 (2021). https://doi.org/10.1038/s41598-021-86382-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86382-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.