Abstract
Wheat is a major food crop worldwide. The plant architecture is a complex trait mostly influenced by plant height, tiller number, and leaf morphology. Plant height plays a crucial role in lodging and thus affects yield and grain quality. In this study, a wheat population was genotyped by using Illumina iSelect 90K single nucleotide polymorphism (SNP) assay and finally 22,905 high-quality SNPs were used to perform a genome-wide association study (GWAS) for plant architectural traits employing four multi-locus GWAS (ML-GWAS) and three single-locus GWAS (SL-GWAS) models. As a result, 174 and 97 significant SNPs controlling plant architectural traits were detected by ML-GWAS and SL-GWAS methods, respectively. Among these SNP makers, 43 SNPs were consistently detected, including seven across multiple environments and 36 across multiple methods. Interestingly, five SNPs (Kukri_c34553_89, RAC875_c8121_1490, wsnp_Ex_rep_c66315_64480362, Ku_c5191_340, and tplb0049a09_1302) consistently detected across multiple environments and methods, played a role in modulating both plant height and flag leaf length. Furthermore, candidate SNPs (BS00068592_51, Kukri_c4750_452 and BS00022127_51) constantly repeated in different years and methods associated with flag leaf width and number of tillers. We also detected several SNPs (Jagger_c6772_80, RAC875_c8121_1490, BS00089954_51, Excalibur_01167_1207, and Ku_c5191_340) having common associations with more than one trait across multiple environments. By further appraising these GWAS methods, the pLARmEB and FarmCPU models outperformed in SNP detection compared to the other ML-GWAS and SL-GWAS methods, respectively. Totally, 152 candidate genes were found to be likely involved in plant growth and development. These finding will be helpful for better understanding of the genetic mechanism of architectural traits in wheat.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum L.) is a staple crop worldwide, providing 20% of total food needs of the world population1,2. Plant architecture is a complex trait mainly depends on the three dimensional structure of the plant stature including branching pattern, morphology of leaves and flower organs3. Plant height directly indicates the ability of plant to compete for light, influencing plant growth and development4. It is of prime importance, strongly influencing plant defense against environmental stress, potential grain yield, and plant adaptability for better cultivation and harvesting5,6. Leaf morphology can regulate many important aspects related to plant growth and development7. In cereals, flag leaves have prominent role in photosynthesis and contribute about 43% of the total carbohydrates required for gain filling8. To date, several loci have been identified associated with flag leaf related traits in cereals9,10. Productive tillers in wheat are of great importance which may directly affect spike number and thus influence the final yield. The plant stature and the number of tillers influence many factors, including the process of photosynthesis, the flowering and grain set in plant11. It is thus understandable that the genetic elucidation of tillers at various plant growth stages is an important component in wheat breeding research programs11.
Considerable work has been done to dissect the genetic background of plant height in wheat. To date, 25 height reducing genes have been identified across different chromosomes in wheat12,13. One of the great achievements of the Green Revolution, was mainly based on modifying plant architecture by selecting the cultivars with reduced height that can carry more yield with enhanced resistance against lodging14. Achieving optimal plant height is of prime importance for the stability, productivity and yield potential of the cultivars15,16. Improvement in wheat yield during the Green Revolution was achieved through the introduction of Reduced height (Rht) dwarfing genes. Among them, the Rht-B1 and Rht-D1 loci ensured short stature by limiting the response to the growth-promoting hormone gibberellin (GA). In addition, a newly discovered gene for reduced height Rht24 belongs to GA-sensitive type and was predicated to be commercial importance in worldwide wheat breeding17. Till now, more than 50 loci have been detected for plant height16,17. For instance, Wei et al.18, detected several stable SNPs on chromosomes 2A, 2B, 2D, 3B, 4B, 5A, 5D, 7B, and 7D associated with plant height. Griffiths et al.16, identified several height related genes on all chromosomes except for 3D, 4A, and 5D. Further studies are required to discover, to fine map and to clone more new semi-dwarf genes thus expanding the portfolio of Rht genes for the breeding of the favorable plant architecture.
Currently research has been accomplished through GWAS in wheat9, maize19, rice20, and cotton21. Comparatively hexaploid wheat has larger genome (≈ 17.9 Gb) than rice (≈ 400 Mb) and maize (≈ 2.32 Gb)9. Over the past decade, the absence of fully sequenced reference genome has limited the gene discovery of wheat. Recent advances in functional genomics have provided breeders with a new impetus to achieve their goals22. However, substantially more work is required because of the experimental bottleneck emerging from the absence of inconsistency among studies, and the utilization of low-density marker platforms in gene mapping studies. Despite the current research on plant height in wheat, the effects of important loci and several other candidate loci responsible for fine-tuning of plant height in hexaploid wheat is still an intrinsic part in wheat breeding17. The availability of high quality reference genome allows for previously impossible follow-up analysis. The application of SNPs as molecular markers provides better understanding of variation in an organism or individual part and further provide high-throughput maps for detecting candidate loci and genes for target traits. Molecular markers are mostly used in segregation analysis, forensic examination, genetic mapping and diagnosis, and numerous biological applications23,24,25. In the present study we were interested to gain a comprehensive picture about the candidate loci responsible for modulating plant height and related traits in wheat through a series of different GWAS models.
Thus the present study was designed to conduct GWAS in a set of 319 wheat accessions employing high-density wheat iSelect 90K SNP array. The objectives of this study included: (1) the investigation of marker-trait associations (MTAs) for plant architectural traits (2) appraising the correlation among these traits and further highlighting SNPs common to more than one trait (3) detecting candidate genes responsible for corresponding morphological traits. Overall, this study will provide insights by integrating three single-locus and four newly developed multi-locus GWAS methods, and will be helpful to establish a regulatory network in the genetic improvement of wheat architectural traits.
Results
Statistical analysis of phenotypic traits
In the present study, we evaluated wheat germplasm collections for plant architectural traits, including plant height (PH), flag leaf length (FLL), flag leaf width (FLW) and the number of tillers per plant. The phenotypic characteristics for plant height across four environments and flag leaf length, flag leaf width and number of tillers per plant across two environments as shown in Supplementary Table S1 and Supplementary Fig. S1. All the traits exhibited the normal distribution pattern each year, indicating the quantitative nature of these traits (Fig. 1). Descriptive statistics revealed large phenotypic variations for all the traits as given in Supplementary Table S1. PH ranged from 48.40 to 124.82 cm with coefficient of variations (CVs) ranged from 11.09 to 16.11%. The FLL varied from 16.06 to 31.57 cm, FLW varied from 1.22 to 2.75 cm and for tillers, the number of tillers per plant ranged from 8.10 to 14.67. Analysis of variance indicated highly significant differences (P < 0.001) for all the studied traits (Supplementary Table S2).
Broad sense heritability was also estimated for PH, FLL, FLW and number of tillers with values ranged from 0.79 (FLL) to 0.91 (PH), suggesting the stability of these traits. The correlation analysis revealed significant correlation between different environments for each of the four traits, indicating the consistency of these traits across various environments (Fig. 2). Furthermore, PH was significantly and positively correlated with FLL in almost all the environments. These results are further confirmed by GWAS results, which revealed several SNPs have common association to both PH and FLL. However, both PH and FLL significantly but negatively correlated with FLW in most of the environments indicating competition of these traits to assimilate at the plant growth stage. Finally, the relatively weak correlation of tillers with other traits suggested the independency of this trait.
Population structure analysis
Population structure is important due to the large number of diverse genotypes used in the study may produce false associations between the phenotypic values and unlinked markers. Therefore, a comprehensive analysis of population structure is prerequisite for evaluating successful association mapping. The number of subpopulations were calculated by the rate of change in the log probability of data between successive K-values. ΔK was calculated for increasing the number of K-value determined by STRUCTURE analysis according to the procedure of Evanno26. At K = 2, a break in the slope was observed followed by flattening of the curve (Supplementary Fig. S1a). Hence, the most likely number of subpopulations was two (K = 2) (Supplementary Fig. S1b). Moreover, this result was confirmed by PCA based on standardized covariance of genetic distances of SNP markers (Supplementary Fig. S1c). Linkage disequilibrium (LD) analysis indicating the mapping resolution and robustness was done using TASSEL v.5.0. software. LD for whole genome presented in (Supplementary Fig. S2a). The r2 value for the A, B, and D sub-genomes decreased gradually with increasing the genetic distance (Supplementary Fig. S2b). The LD analysis for the A, B, and D sub-genomes indicated the highest marker density on B (58%) followed by A (34.6%) and then D (7.4%). Among the chromosomes, 2B has the highest marker density, while 4D has the lowest. More details about the description of LD and population structure analysis have been reported in our previous study27.
GWAS using four multi-locus models
To obtain more reliable results, the SNPs that were simultaneously detected in at least two years or by at least two methods were considered as most stable SNPs. After removing the repeated SNPs, a total of 113 and 62 significant SNPs were identified by ML-GWAS and SL-GWAS methods, respectively (Fig. 3a).
Significant SNPs detected through different GWAS methods in multiple environments. The traits include PH (Plant height); FLL (Flag leaf length); FLW (Flag leaf width) and TILL (Number of tillers per plant). (a) Significant SNPs detected via ML-GWAS and SL-GWAS methods, (b) Significant SNPs detected through four ML-GWAS methods i.e. FASTmrMLM, FASTmrEMMA, mrMLM, and pLARmEB, (c) Significant SNPs detected across multiple years and different ML-GWAS methods, (d) Significant SNPs detected through three SL-GWAS methods i.e. FarmCPU, MLM, and MLMM.
ML-GWAS models i.e. FASTmrMLM, FASTmrEMMA, mrMLM, and pLARmEB screened 47, 32, 46, and 49, significant SNPs, respectively for PH (117 SNPs) across four environments and for FLL (24), FLW (15) and Tillers (18) across two environments (Fig. 4, Tables 1, 2, 3). Among these SNPs, 30, 8, 5, and 4 were detected by FASTmrMLM for PH, FLL, FLW, and Tillers, respectively (Fig. 3b, Table 1). By using FASTmrEMMA the number of significant SNPs identified for the above-mentioned traits were 24, 4, 1, and 3, respectively. Also 27, 6, 9, and 4 significant SNPs were detected by mrMLM approach for the said traits, respectively. Finally, the pLARmEB model identified 36, 6, and 7 significant association signals with PH, FLL, and Tillers, respectively (Fig. 3b, Table 1).
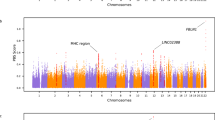
Manhattan and quantile–quantile (Q-Q) plots for plant architectural traits across different environments; (a,b) Manhattan and Q-Q plots for Plant height (2015–2018); (c) Manhattan and Q-Q plots for flag leaf length (2017–2018); (d) Manhattan and Q-Q plots for flag leaf width (2017–2018); (e) Manhattan and Q-Q plots for number of tillers (2017–2018). Note: Number of detected SNPs by four multi-locus GWAS methods. For brevity, we presented here the Manhattan and Q–Q plots of mrMLM model. The rest of the Manhattan and Q–Q plots are given in Supplementary Fig. S3.
To validate the findings, we further compared the results across multiple environments and found six and one SNPs were co-identified in at least two of the environments for PH and FLL, respectively (Fig. 3c, Table 2). These environment-stable SNPs were located on different chromosomes. For PH there was one SNP on chromosome 2B, one on 3A, one located on 3B, one on 6A and two SNPs located on chromosome 6B. The LOD scores ranged from 3.05 to 7.92. One stable SNP across two environments located on chromosome 5A associated with FLL with LOD value ranging from 3.88 to 6.55 (Table 2). Comparing the results across different methods, we found 36 common SNPs were co-detected simultaneously by at least two approaches (Fig. 3c, Table 1). Among these, four significant SNPs (RAC875_c8121_1490, Ku_27771_508, Tdurum_contig42962_2138, BS00022127_51) were detected by all four methods (Table 1).
We further checked the co-detected common SNPs simultaneously in multiple environments and different methods and screened five most stable SNPs (Kukri_c34553_89, RAC875_c8121_1490, wsnp_Ex_rep_c66315_64480362, Ku_c5191_340, and tplb0049a09_1302). Among these, one SNP was associated with FLL and the rest of four were identified for PH across multiple environments and methods (Fig. 3c, Table 3). Finally, we extended our screening criteria for significant QTLs and detected several major QTLs with phenotypic variation explained ranged from 5.5 to 13.8% associated with all the studied traits (Supplementary Table S3). Comparatively, the four ML-GWAS models (FASTmrMLM, FASTmrEMMA, mrMLM, and pLARmEB) to uncover genomic regions associated with plant height and related traits, the pLARmEB model detected the most SNPs (49), most of which associated with PH (36 SNPs), while, FASTmrEMMA identified the least SNPs (32; Fig. 4b).
GWAS using three single-locus models
Three single-locus GWAS (SL-GWAS) methods i.e. FarmCPU, MLM, and MLMM were used to further analyzed the results of the same plant architectural traits. A total of 97 significant SNPs were detected by the three SL-GWAS methods for the above mentioned traits across multiple environments (Fig. 3d, Supplementary Table S4). Among these SNPs, chromosome 5A harbored most of the SNPs (28) followed by 3B (14) and 3A (11). In the three SL-GWAS methods, FarmCPU detected 56 significant SNPs, MLM detected 19, and MLMM detected 22 significant SNPs associated with different traits in multiple environments (Fig. 3d, Supplementary Table S4). We further checked the common SNPs detected by all three SL-GWAS methods across multiple environments and methods and found three most stable SNPs (RAC875_c8121_1490, BS00049008_51, and tplb0049a09_1302) were repeated consistently by all methods in most of the environments (Supplementary Table S4). We further extended the screening criteria for significant SNPs and detected a total of 19 SNPs co-detected by using ML-GWAS and SL-GWAS methods together (Supplementary Table S5). This practice adds an extra screening to the GWAS approaches and thus makes us more confident about the results. By comparing the results of all three SL-GWAS methods, the FarmCPU model identified the most SNPs (56), while MLM detected the least SNPs (19). Manhattan and Q-Q plots of the above three single-locus GWAS models for plant architectural traits are presented in Supplementary Fig. S4.
Traits having common associations
SNPs associated with more than one trait are very useful for marker-assisted selection. A total of five SNPs (Jagger_c6772_80, RAC875_c8121_1490, BS00089954_51, Excalibur_01167_1207, and Ku_c5191_340) were detected associated with more than one trait across multiple environments (Supplementary Table S6). Among these, one SNP (Excalibur_01167_1207) on chromosome 5A associated with PH and FLW. The rest of four pleiotropic SNPs were associated with PH and FLL across multiple environments. The presence of pleiotropic effects of these SNPs controlling plant height and flag leaf length were confirmed by the correlation analysis (Fig. 2). These pleiotropic SNPs (Jagger_c6772_80, RAC875_c8121_1490, BS00089954_51, and Ku_c5191_340) were located on chromosome 1A, 3A, 3B, and 6B, respectively. Moreover, these pleiotropic associations suggest that the aforementioned SNPs have multifaceted role in plant architectural traits and highlight the significance of flag leaf length and width to plant height.
Candidate genes identification
To further understand the genetic basis of plant architectural traits, we predicted a total of 152 candidate genes that were surrounding the peak SNPs. Interestingly, several major candidate genes that were directly associated with the consensus SNPs had exact same annotations (Fig. 5, Supplementary Table S7). For instance, several putative candidate genes for PH and FLL, annotated as Laccase which is used for lignin polymerization to help in a variety of functions in plant development28. Similarly, the putative genes responsible for PH and FLL annotated as Cysteine proteinase inhibitor, has a function in plant growth and defense29. We also found a significant hit for auxin related gene which regulates cell and organ growth in rice30, and plays a prominent role in shoot apical meristem growth31. Three putative candidate genes, TraesCS6A01G142000, TraesCS5A01G533200, and TraesCS5A01G533300 were revealed homology to the transcription factor basic helix-loop-helix 74 (bHLH74) which was reported to be involved in cell elongation and plant development32,33,34. Another gene (TraesCS6A01G174700) corresponds to Cytochrome P450, which is a part of ent-kaurenoic acid oxidase, an enzyme of the gibberellin acid (GA) metabolism35. Additionally, six putative candidate genes surrounding significant SNPs associated with number of tillers have annotations as F-box family protein, involved in plant vegetative and reproductive growth36. Further examples are given in Fig. 5 and Supplementary Table S7. Despite these results, further research is required to validate the possibility of these candidate genes with the architectural traits, these results will provide useful information for designing functional markers and for future work.
Discussion
In this study, we employed four multi-locus GWAS models and three single-locus models to identify SNPs that significantly associated with plant height, flag leaf length, flag leaf width, and number of tillers across environments in hexaploid wheat. Plant height is a key factor in crop breading as it plays a crucial role in reshaping plant architecture and affects lodging and grain traits16,37. The real success of green revolution was the use of semi dwarf wheat cultivars. We identified some significant SNPs across multiple years by different ML-GWAS approaches (Tables 1, 2, 3, Fig. 4a–e). A total of 24 SNPs was consistently detected by most of the ML-GWAS methods for plant height (Table 1 and Fig. 3c). Most of them located on chromosomes 5A and 7A, consistent with some SNPs reported in previous studies38,39,40. Chromosome 5A was revealed to harbor the highest number of significant SNPs for plant architectural traits as showed in Table 1 in this study and has been confirmed of having the most useful and reproducible regions in wheat genome41,42,43,44. Sukumaran et al.45 reported the most numbers of significant SNPs for yield traits on chromosomes 5A and 6A in a spring wheat population. Similarly, the SNPs detected on chromosomes 5A and 6A are most likely the MTAs reported previously42,46.
Five SNPs were detected across both multiple environments and methods for plant height and flag leaf length, of which two were located on chromosome 6B (Table 3). These results revealed the significance of chromosome 6B on plant height, consistent with the findings of some previous studies47,48,49. Among the five stable SNPs, one (Kukri_c34553_89) was located on chromosome 2B with LOD ranging from 3.2–6.4, which was also detected as one of the environment-stable SNPs and was revealed the positive effects on harvest index50. Two consensus SNPs (RAC875_c8121_1490 on chromosome 3A and Ku_c5191_340 on chromosome 6B), were also reported for plant height across different wheat populations51. The stable SNP (tplb0049a09_1302) located on chromosome 5A, was also reported by Ain et al.38, they used 90K array to identify several genomic regions associated with yield related traits in historical wheat genotypes of Pakistan. Another SNP (BobWhite_c5694_1201) located on chromosome 4B is likely same to the QTL identified in a spring wheat population by Zou et al.52.
In addition to the stable SNPs, if the other SNPs in this study were considered, a wider co-localization was found between the SNPs in this study and in previous studies. For example, the SNP, D_contig10675_778 (located in chromosome 2D at 12.3 cM) was found to be co-localized with the QPht/Sl.cau-2D.1 (BobWhite_rep_c63957_1472 located in chromosome 2D at 12.24 cM) reported in a previous study53, both at the physical location 20.8 MB with a dwarf gene (Rht8-19.6 Mb). The cultivars that harbors the reduced height gene Rht8, short stature tended to get more spike number in unit area9. In addition, the Rht8 has the ability to improve the early vigor of semi-dwarf wheat53. Thus, the SNP D_contig10675_778 identified in this study is of interest for further genetic studies and molecular breeding.
The SNPs BS00023152_51 and tplb0049a09_1302 (located on chromosome 5A) detected in two environments in this study falls in the region of the SNP (AX_110446653, 671.2 Mb) reported by a previous study1. It should be mentioned, except the Rht8, the SNPs in this study were not well co-mapped with some semi-dwarf genes such as the Rht1, 2, 14, 16 and 18. Similarly, in a previous study, where a total of 14 SNPs for plant height was mapped on chromosomes 1A, 1B, 2A, 3A, 3B, 4D, 5A, 5B, 6B and 7A, however, only the AX_108916749 on chromosome 4D is at the same position as Rht-D154. The reasons might be (i) that a relatively small population from a limited region was used in studies, (ii) that the 90K array in which the lower density of makers is not reliable for detected the SNPs with small effects and for the comparison of the SNPs across different studies and (iii) the inconsistency of SNPs with previous studies possibly indicated the identification of potentially new genes.
Flag leaves are the primary source of carbohydrate production to sustain proper crop growth and development, thus the importance of flag leaf morphology on increasing grain yield has widely been studied7,8. In present study, we detected several consensus SNPs associated with flag leaf length and width. For flag leaf length (FLL), three consensus SNPs were detected on chromosomes 4B, 5A, and 6D (Table 1). Bilgrami et al. 11 reported a total of 47 significant SNPs associated with number of tillers in breed wheat. The SNP (BS00021881_51) associated with FLL simultaneously detected via two ML-GWAS approaches i.e. FASTmrMLM and pLARmEB was reported earlier in QTL mapping55. Number of tillers have been considered the primary trait for increasing cereal yield no matter in favorable or unfavorable environments11. In the present study, we highlighted several prominent SNPs controlling number of tillers. Two stable SNPs (BS00022127_51 and wsnp_BE499835B_Ta_2_5) associated with the number of tillers per plant corresponded to the previously reported SNPs in wheat56,57. Among the stably detected SNPs for number of tillers, BS00022127_51 located on chromosome 7B, consistently detected by all four ML-GWAS methods (Table 1). Bilgrami et al. 11 reported a total of 47 significant SNPs associated with number of tillers in breed wheat. These SNPs might be the best target for improving the ability of light harvesting and the tiller number of plants. The comprehensive understanding of leaf morphology will provide new insights to the genetic mechanism of crop growth and development.
By further reviewing the significant SNPs results, 174 and 97 SNPs were detected by ML-GWAS and SL-GWAS models, respectively, these results signify the importance of ML-GWAS over SL-GWAS approaches. In earlier studies, mostly SL-GWAS methods were adopted, but only few SNPs for each trait have been identified due to its procedural limitations58. According to our results of SL-GWAS models, MLM model detected the least SNPs (19), which reveal the setting of very high threshold, due to which many small-effect loci are missed59. To make up for the limitations of these methods, some multi-locus approaches such as FASTmrMLM60, FASTmr EMMA61, mrMLM59, and pLARmEB62 have been used in this study. These models can improve the accuracy of SNPs with high detection power and less stringent criteria, and no Bonferroni multiple test correction is needed59,61. In our results, the number of significant SNPs by ML-GWAS were comparatively higher than SL-GWAS models, which suggest the significance of ML-GWAS models. Jaiswal et al.63 verified that ML-GWAS has more detection power than SL-GWAS by revealing ten MTAs through SL-GWAS while, 22 MTAs through multi locus mixed model (MLMM) and 58 MTAs through multi-trait mixed model (MTMM). Furthermore, we detected a total of 19 SNPs co-detected by using ML-GWAS and SL-GWAS methods together (Supplementary Table S5), which reveal the credibility of these SNPs as highlighted by several approaches. Zhu et al.64 suggested the combination of both SL-GWAS and ML-GWAS methods, which contributes efficiently to the detection of significant loci associated with pre-harvest sprouting tolerance in wheat. According to Li et al.65, the power of QTN detection in association analysis can be improved by combining single locus and multi-locus GWASs. Through integrating the results of ML-GWAS and SL-GAWS methods led to the verification of the significance of ML-GWAS models. However, some recent findings revealed the reliability of association studies can be improved by combining single-locus and multi-locus GWAS approaches65,66,67,68.
Taken together, four multi-locus and three single-locus GWAS models were used for parsing the genetic background of plant architectural traits (PH, FLL, FLW, and TILL) in hexaploid wheat. A total of 271 significant SNP was detected across multiple environments and in different methods. Comparatively, 174 and 97 significant association signals were detected by ML-GWAS and SL-GWAS models, respectively which signifies the importance of ML-GWAS over SL-GWAS approaches. By further appraising these GWAS methods, the pLARmEB and FarmCPU models outperformed in SNP detection compared to the other ML-GWAS and SL-GWAS methods, respectively. Taken together, the results of ML-GWAS, revealed five most stable SNPs i.e. Kukri_c34553_89, RAC875_c8121_1490, wsnp_Ex_rep_c66315_64480362, Ku_c5191_340, and tplb0049a09_1302 which were consistently detected across multiple environments and methods.
Our study will provide new insights to the genetic basis of plant architectural traits and can serve as a basis for further functional investigation. The loci and significant SNP markers identified in this study can be used for pyramiding favorable alleles in developing varieties with desirable plant architecture and potentiality in the genetic improvement of grain yield. Among them, the stable SNPs identified across years in this study are of great importance. Secondly, based on the correlation between traits and the direction of SNP effects, we can design the combinations or find the accessions with a high percentage of favorable alleles. For example, the plant height and flag leaf length generally positive correlated with each other, but both have less correlation with flag leaf width and tiller numbers. These information, together with the SNPs identified, will be beneficial for breeding design. However, to achieve these goal, the larger populations and higher density genetic maps are required. It is necessary (i) to narrow down the SNP confidence interval thus that the markers tightly linked to the genes of interest should be much reliable for marker-assisted selection and for fine mapping and subsequent cloning of the candidate genes, (ii) to estimate the effects of number of alleles for desirable phenotypic values for each traits as reported by some previous studies1,69 and to convert the SNPs of interest into kompetitive allele-specific PCR (KASP) markers, and to further verify in bi-parental populations and (iii) to jointly investigate the SNPs for the other agronomic important traits including grain yield and grain qualities across multiple genetic mapping populations thus to gain a comprehensive picture for breeding design.
Materials and methods
Plant materials and phenotyping
A total of 319 wheat germplasm accessions from the collection at the Hubei Academy of Agricultural Science in Hubei Province, China, which represent a wheat gene pool adapted to central China and the Yangzi River regions. The plant materials were grown in randomized complete blocks with three replicates at the experimental farm of Huazhong Agricultural University, Wuhan, China for four consecutive winter seasons (2015–2018). Twenty individuals from each variety (line) were grown in two rows with a distance of 15 cm between plants in each row and 20 cm between rows. Field management essentially followed normal local wheat cropping practices. The lines were harvested individually at maturity to prevent seed contamination among lines. Four phenotypic traits were evaluated, including plant height across four environments (2015–2018) and the rest of three traits i.e. flag leaf length, flag leaf width, and the number of tillers per plant across two environments (2017–2018). The measurements of these traits were performed by selecting five random individual plants in the middle of the row for each accession. Plant height was measured after physiological maturity by measuring the distance between the stem base and the top of the spike excluding awns. Flag leaf length was measured as the distance from the base to the tip of the leaf. Flag leaf width as the width of the widest section of the leaf. Number of tillers were recorded by counting the total number of fertile tillers per plant.
Genotyping
A total of 319 wheat accessions were genotyped using the Illumina iSelect 90K SNP array 70 in the genotyping Laboratory of North Dakota State University in Fargo as described in our previously published study27. A quality preprocessing of genotyping data was done for sample call rate, SNP call rate, minor allele frequency (MAF) and Hardy–Weinberg equilibrium (HWE). This preprocessing was implemented in PLINK software (https://zzz.bwh.harvard.edu/plink/)71.
Statistical analysis
Descriptive analysis, ANOVA, correlation analysis and heritability estimates were conducted in the R statistical package72. The broad sense heritability for the traits was estimated by the formula H2 = VG/(VG + VE) where VG and VE represent estimates of genetic and environmental variance, respectively73. Variance components for the studied traits were analyzed according to our previous study27, using general linear model to detect the effect of genotypes, environment, replication and genotype × environment interaction. All sources of variation were considered as random effects.
Population structure and kinship analysis
The SNP markers and estimated methods for population structure and linkage disequilibrium (LD) were the same as in Muhammad et al.27. Population structure using a Bayesian cluster analysis was estimated by STRUCTURE 2.3.4 software74, and the obtained results were visualized with the STRUCTURE HARVESTER software75. A putative number of subpopulations ranging from K = 1 to 7 was assessed using 100,000 burn-in iterations followed by 500,000 recorded Markov-Chain iterations. To estimate the sampling variance (robustness) of inferred population structure, 10 independent runs were carried out for each K. K was estimated using an ad-hoc statistic ∆K based on the rate of change in log probability of data between successive values26. Principle component analysis (PCA) was calculated by R software for evaluating the population structure and compared to the result of STRUCTURE9. LD among markers was calculated using observed vs. expected allele frequencies of the markers in TASSEL v.5.038.
Genome-wide association studies
In this study, we used mrMLM software for four ML-GWAS (FASTmrMLM, FASTmrEMMA, mrMLM, and pLARmEB) and three SL-GWAS (FarmCPU, MLM, and MLMM) implemented by Genomic Association and Prediction Integrated Tool (GAPIT) in R76. Previously, SL-GWAS methods were mostly applied such as GLM and MLM. However, single-locus approaches have some limitations such as GLM leads to high false-positive rates (FPRs), while MLM utilizes Bonferroni corrections for loci detection to reduce the FPRs77. Though, this procedure is so stringent that results in missing significant SNPs59. Therefore, multi-locus GWAS approaches are the best alternatives. The stringent Bonferroni multiple test correction in the SL-GWAS analysis is substituted by a flexible selection criterion in multi-locus GWAS analysis, that reduces the possibility of missing out significant loci59,61. The four ML-GWAS methods were performed with default parameters, and the screening criteria for significance were set with LOD scores 3 or > 359,61,62. However, for SL-GWAS models, the threshold for P-value was calculated based on the number of the markers (P = 1/n, n = total SNP used) according to the method of78. Significant markers were visualized with a Manhattan plot using Haploview 4.2 software79. Important p-value distributions (expected vs. observed p-values on a – log10 scale) were shown with a quantile–quantile plot.
Candidate gene analysis
Candidate gene sites were aligned and downloaded from the ViroBLAST database (https://urgi.versailles.inra.fr/blast/docs/aboutviroblast.html). The R Package Pathway Association Study Tool (PAST) version 1.0.1 was used to identify genes around the peak SNPs with a window size of 200 kb. To find candidate genes or putative related proteins of SNP flanking-regions, BLASTx search was conducted for significant marker-trait associations (MTAs) against recently released genome sequence IWGSC RefSeq v1.080.
References
Li, F. et al. Genetic architecture of grain yield in bread wheat based on genome-wide association studies. BMC Plant Biol. 19, 168 (2019).
Muhammad, A. et al. Survey of wheat straw stem characteristics for enhanced resistance to lodging. Cellulose 27, 2469–2484. https://doi.org/10.1007/s10570-020-02972-7 (2020).
Reinhardt, D. & Kuhlemeier, C. Plant architecture. EMBO Rep. 3, 846–851 (2002).
Wang, X., Singh, D., Marla, S., Morris, G. & Poland, J. Field-based high-throughput phenotyping of plant height in sorghum using different sensing technologies. Plant Methods 14, 53 (2018).
Li, X., Wang, X., Peng, Y. & Li, T. In 2016 IEEE International Conference on Functional-Structural Plant Growth Modeling, Simulation, Visualization and Applications (FSPMA). 117–124 (IEEE).
Borrelli, G. M., De Vita, P., Mastrangelo, A. M. & Cattivelli, L. 327–354 (Elsevier, 2009).
Liu, L., Sun, G., Ren, X., Li, C. & Sun, D. Identification of QTL underlying physiological and morphological traits of flag leaf in barley. BMC Genet. 16, 29 (2015).
Ma, J. et al. Flag leaf size and posture of bread wheat: Genetic dissection, QTL validation and their relationships with yield-related traits. Theor. Appl. Genet. 133, 297–315 (2020).
Sun, C. et al. Genome-wide association study for 13 agronomic traits reveals distribution of superior alleles in bread wheat from the Yellow and Huai Valley of China. Plant Biotechnol. J. 15, 953–969 (2017).
Wang, P., Zhou, G., Cui, K., Li, Z. & Yu, S. Clustered QTL for source leaf size and yield traits in rice (Oryza sativa L.). Mole. Breed. 29, 99–113 (2012).
Bilgrami, S. S. et al. Detection of genomic regions associated with tiller number in Iranian bread wheat under different water regimes using genome-wide association study. Sci. Rep. 10, 1–17 (2020).
McIntosh, R. et al. Catalogue of gene symbols for wheat: 2015–2016 supplement. Komugi Wheat Genet. Resour. Database. (2016).
Mo, Y. et al. Identification and characterization of Rht25, a locus on chromosome arm 6AS affecting wheat plant height, heading time, and spike development. Theor. Appl. Genet. 131, 2021–2035 (2018).
Guo, Z. et al. Genome-wide association analyses of plant growth traits during the stem elongation phase in wheat. Plant Biotechnol. J. 16, 2042–2052 (2018).
Bognár, Z., Láng, L. & Bedő, Z. Effect of environment on the plant height of wheat germplasm. Cereal Res. Commun. 35, 281–284 (2007).
Griffiths, S. et al. Meta-QTL analysis of the genetic control of crop height in elite European winter wheat germplasm. Mol. Breed. 29, 159–171 (2012).
Würschum, T., Langer, S. M. & Longin, C. F. H. Genetic control of plant height in European winter wheat cultivars. Theor. Appl. Genet. 128, 865–874 (2015).
Wei, T. M., Chang, X. P., Min, D. H. & Jing, R. L. Analysis of genetic diversity and tapping elite alleles for plant height in drought-tolerant wheat varieties. Acta Agron. Sin. 36, 895–904 (2010).
Samayoa, L., Cao, A., Santiago, R., Malvar, R. & Butrón, A. Genome-wide association analysis for fumonisin content in maize kernels. BMC Plant Biol. 19, 166 (2019).
Chen, J. et al. Genome-wide association analyses reveal the genetic basis of combining ability in rice. Plant Biotechnol. J. 17, 2211–2222 (2019).
Dilnur, T. et al. Association analysis of salt tolerance in Asiatic cotton (Gossypium arboretum) with SNP markers. Int. J. Mol. Sci. 20, 2168 (2019).
Pingault, L. et al. Deep transcriptome sequencing provides new insights into the structural and functional organization of the wheat genome. Genome Biol. 16, 29 (2015).
Lam, H.-M. et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 42, 1053 (2010).
Singh, H. et al. Highly variable SSR markers suitable for rice genotyping using agarose gels. Mol. Breed. 25, 359–364 (2010).
Sonah, H. et al. Genome-wide distribution and organization of microsatellites in plants: An insight into marker development in Brachypodium. PLoS ONE 6, e21298 (2011).
Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 14, 2611–2620 (2005).
Muhammad, A. et al. Appraising the genetic architecture of kernel traits in hexaploid wheat using GWAS. Int. J. Mol. Sci. 21, 5649 (2020).
Berthet, S. et al. In Advances in Botanical Research Vol. 61, 145–172 (Elsevier, 2012).
Li, R. et al. Overexpression of a cysteine proteinase inhibitor gene from Jatropha curcas confers enhanced tolerance to salinity stress. Electron. J. Biotechnol. 18, 368–375 (2015).
Wang, B., Sang, Y., Song, J., Gao, X.-Q. & Zhang, X. Expression of a rice OsARGOS gene in Arabidopsis promotes cell division and expansion and increases organ size. J. Genet. Genomics 36, 31–40 (2009).
Zhang, D. & Yuan, Z. Molecular control of grass inflorescence development. Annu. Rev. Plant Biol. 65, 553–578 (2014).
Zhang, L.-Y. et al. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21, 3767–3780 (2009).
Ikeda, M., Fujiwara, S., Mitsuda, N. & Ohme-Takagi, M. A triantagonistic basic helix-loop-helix system regulates cell elongation in Arabidopsis. Plant Cell 24, 4483–4497 (2012).
Liu, Y., Li, X., Li, K., Liu, H. & Lin, C. Multiple bHLH proteins form heterodimers to mediate CRY2-dependent regulation of flowering-time in Arabidopsis. PLoS Genet. 9, e1003861 (2013).
Zanke, C. D. et al. Whole genome association mapping of plant height in winter wheat (Triticum aestivum L.). PLoS ONE 9, e113287 (2014).
Peng, J. et al. Arabidopsis F-box gene FOA1 involved in ABA signaling. Sci. China Life Sci. 55, 497–506 (2012).
Hedden, P. The genes of the Green Revolution. Trends Genet. 19, 5–9 (2003).
Ain, Q.-U. et al. Genome-wide association for grain yield under rainfed conditions in historical wheat cultivars from Pakistan. Front. Plant Sci. 6, 743 (2015).
Sheoran, S. et al. Uncovering genomic regions associated with 36 agro-morphological traits in Indian spring wheat using GWAS. Front. Plant Sci. 10, 527 (2019).
Gao, F. et al. Genome-wide linkage mapping of QTL for yield components, plant height and yield-related physiological traits in the Chinese wheat cross Zhou 8425B/Chinese Spring. Front. Plant Sci. 6, 1099 (2015).
Cuthbert, J. L., Somers, D. J., Brûlé-Babel, A. L., Brown, P. D. & Crow, G. H. Molecular mapping of quantitative trait loci for yield and yield components in spring wheat (Triticum aestivum L.). Theor. Appl. Genet. 117, 595–608 (2008).
Huang, X., Kempf, H., Ganal, M. & Röder, M. Advanced backcross QTL analysis in progenies derived from a cross between a German elite winter wheat variety and a synthetic wheat (Triticum aestivum L.). Theor. Appl. Genet. 109, 933–943 (2004).
Marza, F., Bai, G.-H., Carver, B. & Zhou, W.-C. Quantitative trait loci for yield and related traits in the wheat population Ning7840× Clark. Theor. Appl. Genet. 112, 688–698 (2006).
Quarrie, S. et al. A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring× SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor. Appl. Genet. 110, 865–880 (2005).
Sukumaran, S., Dreisigacker, S., Lopes, M., Chavez, P. & Reynolds, M. P. Genome-wide association study for grain yield and related traits in an elite spring wheat population grown in temperate irrigated environments. Theor. Appl. Genet. 128, 353–363 (2015).
Börner, A. et al. Mapping of quantitative trait loci determining agronomic important characters in hexaploid wheat (Triticum aestivum L.). Theor. Appl. Genet. 105, 921–936 (2002).
Wu, Q. et al. QTL mapping of flag leaf traits in common wheat using an integrated high-density SSR and SNP genetic linkage map. Euphytica 208, 337–351 (2016).
Yang, D. et al. Genetic dissection of flag leaf morphology in wheat (Triticum aestivum L.) under diverse water regimes. BMC Genet. 17, 94 (2016).
Fan, X. et al. QTLs for flag leaf size and their influence on yield-related traits in wheat (Triticum aestivum L.). Mol. Breed. 35, 24 (2015).
Chen, J. et al. Genome-wide association study of six quality traits reveals the association of the TaRPP13L1 gene with flour colour in Chinese bread wheat. Plant Biotechnol. J. 17, 2106–2122 (2019).
Gao, L., Zhao, G., Huang, D. & Jia, J. Candidate loci involved in domestication and improvement detected by a published 90K wheat SNP array. Sci. Rep. 7, 44530 (2017).
Zou, J. et al. QTLs associated with agronomic traits in the Attila× CDC Go spring wheat population evaluated under conventional management. PLoS ONE 12, e0171528 (2017).
Chai, L. et al. Dissection of two quantitative trait loci with pleiotropic effects on plant height and spike length linked in coupling phase on the short arm of chromosome 2D of common wheat (Triticum aestivum L.). Theor. Appl. Genet. 131, 2621–2637 (2018).
Peng, J. et al. ‘Green revolution’genes encode mutant gibberellin response modulators. Nature 400, 256–261 (1999).
He, X. et al. QTL characterization of Fusarium head blight resistance in CIMMYT bread wheat line Soru# 1. PLoS ONE 11, e0158052 (2016).
Downie, R. C. et al. Assessing European wheat sensitivities to Parastagonospora nodorum necrotrophic effectors and fine-mapping the Snn3-B1 locus conferring sensitivity to the effector SnTox3. Front. Plant Sci. 9, 881 (2018).
Naraghi, S. M. et al. Deciphering the genetics of major end-use quality traits in wheat. G3 Genes Genom. Genet. 9, 1405–1427 (2019).
Pace, J., Yu, X. & Lübberstedt, T. Genomic prediction of seedling root length in maize (Zea mays L.). Plant J. 83, 903–912 (2015).
Wang, S.-B. et al. Improving power and accuracy of genome-wide association studies via a multi-locus mixed linear model methodology. Sci. Rep. 6, 19444 (2016).
Zhang, Y.-M. & Tamba, C. L. A fast mrMLM algorithm for multi-locus genome-wide association studies. bioRxiv 341784 (2018).
Wen, Y.-J. et al. Methodological implementation of mixed linear models in multi-locus genome-wide association studies. Brief. Bioinform. 19, 700–712 (2017).
Zhang, J. et al. pLARmEB: Integration of least angle regression with empirical Bayes for multilocus genome-wide association studies. Heredity 118, 517–524 (2017).
Jaiswal, V. et al. Genome wide single locus single trait, multi-locus and multi-trait association mapping for some important agronomic traits in common wheat (T. aestivum L.). PLoS ONE 11, e0159343 (2016).
Zhu, Y. et al. Genome-wide association study of pre-harvest sprouting tolerance using a 90K SNP array in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 132, 2947–2963 (2019).
Li, C., Fu, Y., Sun, R., Wang, Y. & Wang, Q. Single-locus and multi-locus genome-wide association studies in the genetic dissection of fiber quality traits in upland cotton (Gossypium hirsutum L.). Front. Plant Sci. 9, 1083 (2018).
Chang, F. et al. Genome-wide association studies for dynamic plant height and number of nodes on the main stem in summer sowing soybeans. Front. Plant Sci. 9, 1184 (2018).
Xu, Y. et al. Genome-wide association mapping of starch pasting properties in maize using single-locus and multi-locus models. Front. Plant Sci. 9, 1311 (2018).
He, L. et al. Genome-wide association studies for pasmo resistance in flax (Linum usitatissimum L.). Front. Plant Sci. 9, 1982 (2018).
Hu, Z. et al. Genetic loci simultaneously controlling lignin monomers and biomass digestibility of rice straw. Sci. Rep. 8, 1–11 (2018).
Wang, S. et al. Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol. J. 12, 787–796 (2014).
Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Team, R. C. R: A Language and Environment for Statistical Computing (2013).
Arora, S. et al. Genome-wide association study of grain architecture in wild wheat Aegilops tauschii. Front. Plant Sci. 8, 886 (2017).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Earl, D. A. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361 (2012).
Lipka, A. E. et al. GAPIT: Genome association and prediction integrated tool. Bioinformatics 28, 2397–2399 (2012).
Ma, L. et al. Genetic dissection of maize embryonic callus regenerative capacity using multi-locus genome-wide association studies. Front. Plant Sci. 9, 561 (2018).
Li, H. et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 45, 43 (2013).
Barrett, J. C., Fry, B., Maller, J. & Daly, M. J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2004).
Consortium, I. W. G. S. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361, eaar7191 (2018).
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (31771775 and 31171524), Program of Introducing Talents of Discipline to Guangxi University (A3310051010 and EE101701).
Author information
Authors and Affiliations
Contributions
J.W. and L.W. designed the project. A.M. and W.H. performed experimental works. J.Y., J. L., S.U.K. and M.H.U.K. performed computational analysis. A.M. and G.X. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muhammad, A., Li, J., Hu, W. et al. Uncovering genomic regions controlling plant architectural traits in hexaploid wheat using different GWAS models. Sci Rep 11, 6767 (2021). https://doi.org/10.1038/s41598-021-86127-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86127-z
This article is cited by
-
Identification of genetic loci for flag leaf traits in wheat (Triticum aestivum L.)
Euphytica (2024)
-
E-GWAS: an ensemble-like GWAS strategy that provides effective control over false positive rates without decreasing true positives
Genetics Selection Evolution (2023)
-
Genome-wide association study of leaf-related traits in tea plant in Guizhou based on genotyping-by-sequencing
BMC Plant Biology (2023)
-
Utilization of wheat 55K SNP array for QTL mapping of plant height and flag leaf in a RIL population
Cereal Research Communications (2023)
-
Unlocking the genetic control of spring wheat kernel traits under normal and heavy metals stress conditions
Plant and Soil (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.