Abstract
Basal cells are multipotent stem cells responsible for the repair and regeneration of all the epithelial cell types present in the proximal lung. In mice, the elusive origins of basal cells and their contribution to lung development were recently revealed by high-resolution, lineage tracing studies. It however remains unclear if human basal cells originate and participate in lung development in a similar fashion, particularly with mounting evidence for significant species-specific differences in this process. To address this outstanding question, in the last several years differentiation protocols incorporating human pluripotent stem cells (hPSC) have been developed to produce human basal cells in vitro with varying efficiencies. To facilitate this endeavour, we introduced tdTomato into the human TP63 gene, whose expression specifically labels basal cells, in the background of a previously described hPSC line harbouring an NKX2-1GFP reporter allele. The functionality and specificity of the NKX2-1GFP;TP63tdTomato hPSC line was validated by directed differentiation into lung progenitors as well as more specialised lung epithelial subtypes using an organoid platform. This dual fluorescent reporter hPSC line will be useful for tracking, isolating and expanding basal cells from heterogenous differentiation cultures for further study.
Similar content being viewed by others
Introduction
Respiratory diseases are leading causes of death and disability in the world. About 65 million people suffer from chronic obstructive pulmonary disease (COPD) and 3 million die from it each year, making it the third leading cause of death worldwide1. In order to develop preventative and/or curative strategies against lung diseases, understanding lung development, regeneration and repair are key to dissecting disease mechanisms.
The lungs are made up of an extensive branching network of epithelial tubes. Air enters the nasal passages, flows down the trachea into increasingly smaller branches of bronchioles until it reaches the alveoli where gas exchange takes place. In addition to the epithelial cells that line the respiratory tract, the lungs are also home to various other cell types such as smooth muscle cells, fibroblasts, immune cells and neurons, all of which play important roles in lung homeostasis and function. With the constant interaction between the epithelial cells and the external environment, cell loss due to natural turnover, damage or disease is inevitable. The adult lung, although relatively quiescent compared to organs with high cellular turnover such as the intestine, possesses a remarkable ability to regenerate and repair itself following acute injury2,3. Rodent injury models, lineage tracing and transcriptome analyses have been useful in the identification and study of the cell types involved in this process4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19.
Basal cells are a population of multipotent stem cells with the ability to self-renew and regenerate all the epithelial subtypes present in the proximal region of the lung20. Named because of their location next to the basal lamina, basal cells are present throughout the trachea and terminal bronchioles in humans. Basal cells can be identified through expression of Transformation-related protein 63 (TP63), Cytokeratin 5 (KRT5), Nerve Growth Factor Receptor (NGFR) and Integrin subunit Αlpha 6 (ITGA6)21. Expression of TP63 appears to be crucial to basal cell identity as mice without functional Trp63 lack basal cells and die at birth22,23,24. Lung development begins at embryonic day (E)9 in the mouse and around 4 weeks post-conception (pcw) in humans. Expression of Nkx2-1 is crucial to this process as Nkx2.1 mutant mice fail to develop lungs and humans with NKX2-1 mutations develop congenital lung diseases25,26,27. These Nkx2-1 + cells in the anterior foregut form the lung buds that then undergo branching morphogenesis. Proximal–distal patterning occurs whereby acquisition of Sox2 or Sox9 expression directs differentiation of these early lung progenitors toward proximal or distal lineages respectively. Basal cells were thought to emerge later during lung development28 until a recent study by Yang et al. demonstrated using multiple Cre recombinase mouse driver lines, that Trp63 + basal cells appear shortly after the initiation of lung development at E9.5. These early basal cells, although capable of contributing to both proximal and distal epithelial cell lineages, become more lineage-restricted by E10.529.
Tremendous progress has been made in understanding lung development with the aid of murine models. However, with increasing evidence of biologically significant differences between murine and human lungs30,31, it is important that this knowledge gap be filled in order to understand human-specific disease mechanisms. Nonetheless, even with the lack and/or sparse amount of human data, several groups have developed protocols largely based on mimicking in vivo mouse lung development to direct differentiation of human pluripotent stem cells (hPSCs), whether human embryonic stem cells (hESCs) or human induced pluripotent stem cells (hiPSCs), into lung epithelial cells32,33,34,35,36,37,38,39. These protocols generate lung progenitors with varying degrees of efficiency that can be further matured into a variety of lung epithelial cell subtypes. Initial studies were aimed at increasing the yield of NKX2-1 + lung progenitors. Interest is however mounting in directing the differentiation of hPSCs into specific lung lineages36,37,39. Despite these efforts, the origins and development of human lung basal cells remain unknown. Given the important role these cells play in lung homeostasis and repair, elucidating the molecular mechanisms of their development can potentially inform the development of protocols to direct hPSC differentiation into basal cells, which will be invaluable in applications such as disease modelling, regenerative medicine as well as for the understanding of normal human lung development. To this end, we have generated an NKX2-1GFP;TP63tdTomato dual fluorescence reporter line that will facilitate the investigation of human lung basal cell biology.
Results
Generation of the NKX2-1 GFP ;TP63 tdTomato dual fluorescence reporter line
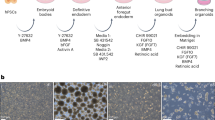
As TP63 is important to basal cell identity and development, we introduced the tdTomato fluorescent reporter into the human TP63 gene locus. The TP63 gene is transcribed from two promoters, generating two isoforms with an N-terminal P53-homologous transactivation domain (TAp63) or without (ΔNp63). These isoforms undergo alternative splicing, yielding 10 different isoforms with 5 different C-termini designated α, β, γ, δ, and ε40,41,42,43. The α isoform is the longest isoform, incorporating exons 11 through 14 that encode the Sterile Alpha Motif (SAM) and a Post-Inhibitory Domain (PID). As ΔNp63α is the most highly expressed isoform in airway epithelial cells44,45, we chose to generate a ΔNp63α reporter allele in which tdTomato is inserted at the 3′ end of exon 14 in the previously described BU3 NKX2-1GFP hiPSC line (Fig. 1A)46 that allows the specific isolation of lung basal cells (Fig. 1B)47.
Schematic representation of the targeting strategy used to insert tdTomato into the endogenous TP63 locus in the NKX2-1GFP hiPSC reporter line46. (a) Schematic of targeted NKX2-1 allele in BU3 NKX2-1GFP hiPSC line from Hawkins et al.46. (b) Single guide RNAs were designed targeting the 3′ end of exon 14. Donor template consists of 800 bp homology arms flanking sequences encoding a P2A peptide (2A) and tdTomato. Expression of the α isoform of TP63 (both TAp63 and ΔNp63) is expected to lead to expression of tdTomato.
Using the CHOPCHOP web tool (https://chopchop.cbu.uib.no), two single guide RNAs (sgRNAs) targeting the end of exon 14 were identified (Supplementary Fig. 1A). The T7EI assay was performed to assess cleavage efficiencies of the two sgRNAs. Cas9-mediated cleavage is predicted to result in a product of approximately 880 bp. Successful cleavage was observed with sgRNA 2 (Supplementary Fig. 1B). This sgRNA was then transfected into BU3 NKX2-1GFP hiPSCs along with the donor template using Lipofectamine STEM. The donor template was constructed with 800 bp 5′ and 3′ homology arms flanking the P2A-tdTomato sequence. A total of 169 colonies were screened and only one potential homozygous clone was obtained (Supplementary Fig. 1C). This clone was then expanded, subcloned, genotyped and sequenced to confirm bi-allelic insertion of P2A-tdTomato (Supplementary Fig. 1D,E).
Characterization of the NKX2-1 GFP ; TP63 tdTomato hiPSC line
NKX2-1GFP;TP63tdTomato hiPSC colonies maintained typical ESC colony morphology (Supplementary Fig. 2A) and expressed the pluripotency marker OCT4 at levels similar to the BU3 parental line (Supplementary Fig. 2B,C). The modified hiPSC line displayed no gross chromosomal abnormalities (Supplementary Fig. 2D) and was also capable of differentiating into cells derived from all 3 germ layers (Supplementary Fig. 3), indicating that the insertion of tdTomato did not impact pluripotency.
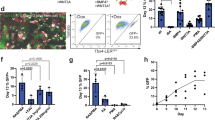
To assess tdTomato reporter functionality, the parental and the NKX2-1GFP;TP63tdTomato hiPSC lines were differentiated into early lung progenitors using a modified version of the protocols published by McCauley et al.38 (Fig. 2A). At day 15, NKX2-1GFP+ cells were observed in both lines, with a minority of cells tdTomato-positive (tdTomato+) (0.88 ± 1.01, n = 11) (Fig. 2B). Both lines differentiated into NKX2-1GFP+ lung progenitors at similar efficiencies (Fig. 2C). The insertion of P2A-tdTomato into the TP63 locus therefore does not appear to impact lung differentiation efficiency.
Assessment of tdTomato reporter functionality. (a) Schematic of lung differentiation protocol based on the work of McCauley et al. (2017)38. hPSC: human pluripotent stem cell; DE: definitive endoderm; AFE: anterior foregut endoderm; LP: lung progenitor. (b) Flow cytometry analysis of GFP + tdTomato + cells at Day 15 of the differentiation protocol. Representative dot plot shown. (c) Quantification of NKX2-1GFP+ cells at Day 15 as a measure of lung differentiation efficiency. Data are represented as means ± SD, n = 11 (NKX2-1GFP), n = 15 (NKX2-1GFP; TP63tdTomato). ns, not statistically significant. t test. Graph was made and statistical analysis was done using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) (www.graphpad.com).
To assess the specificity of the TP63tdTomato reporter, the population of tdTomato+ cells that emerged on day 15 was collected via FACS sorting and mRNA harvested for analysis of TP63 and tdTomato expression. Both TP63 and tdTomato expression were found to be enriched in the tdTomato+ fraction (Fig. 3A). Immunostaining of tdTomato + cells with an anti-TP63 antibody showed co-localization of tdTomato and TP63 (Fig. 3B), demonstrating the faithfulness of the TP63tdTomato reporter allele. Western blot was also performed to confirm enrichment of TP63 in the tdTomato + fraction (TP63TOM+). HEK293T cells overexpressing tdTomato were used as a negative control and primary human bronchial epithelial cells (priHBEC) were used as a positive control for TP63 (Fig. 3C). TP63 protein was detected only in the TP63TOM + and priHBEC samples. Uncleaved TP63-P2A-tdTomato protein (approximate expected size 110 kDa) was not detected in the TP63TOM+ samples, indicating efficient cleavage of the P2A sequence, consistent with previous reports on P2A being the most efficient self-cleaving peptide sequence48,49.
Assessment of tdTomato reporter specificity. (a) QPCR analysis of TP63 (total), ∆Np63, TP63α and tdTomato expression in tdTomato- and tdTomato + fractions. n = 3. **p < 0.01, ***p < 0.001, ****p < 0.0001. t test. Graphs were made and statistical analyses were done using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) (www.graphpad.com). (b) Isolated tdTomato + cells plated onto Matrigel-coated plates and immunostained for TP63. Scale bar indicates 100 µm. (c) Western blot analysis of TP63 and Tubulin. 30 µg of whole cell lysates prepared from HEK293T cells overexpressing tdTomato (HEK293T), TP63TOM + cells, and primary human bronchial epithelial cells (priHBEC). Blots were probed with anti-TP63 antibody with Tubulin used as a loading control. Bands corresponding to TP63 and Tubulin are indicated by black arrows. The asterisk indicates where the uncleaved TP63-P2A-tdTomato protein of about 110 kDa is expected to be, if present. The full uncropped blots are presented in Supplementary Fig. 5.
Differentiation of the NKX2-1 GFP ;TP63 tdTomato hiPSC line into lung organoids
McCauley et al. and Jacob et al. have shown that modulation of WNT signalling in early lung progenitors can influence the specification and development of lung cell types38,39. Specifically, activation of WNT signalling induces distal lung fates while its absence promotes proximal lung fates. Indeed, BU3 NKX2-1GFP hiPSCs can be differentiated into lung organoids expressing mainly proximal and distal lung markers in low-WNT and high-WNT conditions, respectively (Supplementary Fig. 4). We therefore hypothesized that low-WNT conditions should generate more NKX2-1GFP+;TP63tdTomato+ cells compared to high WNT conditions. NKX2-1GFP+ cells were thus isolated from differentiated NKX2-1GFP;TP63tdTomato hiPSCs via FACS sorting, embedded in Matrigel drops and then grown as organoids for 2 weeks in proximalizing38 or distalizing39 culture medium (Fig. 4A). Organoids that formed in the proximalizing conditions were observed to contain more GFP+ tdTomato+ double positive (DP) cells compared to the distalizing conditions (Fig. 4B). Flow cytometry was performed on dissociated organoids to quantify the cells in the double negative, tdTomato+, GFP+ and DP fractions (Fig. 4C). Proximalizing conditions yielded higher numbers of DP as well as tdTomato+ cells compared to the distalizing conditions (Fig. 4D). Consistent with previous reports38,39, the CHIR99021-containing conditions promoted a more distal lung fate. QPCR analysis of organoids did not show significantly higher levels of TP63 expression in the proximalized organoids (Fig. 5A). Interestingly, expression of KRT5 was also found to be higher in the distalized conditions. The distal marker NKX2-1 was expectedly enriched in distalized organoids, while the basal cell marker NGFR was found to be higher in the proximalized organoids. Miller et al. recently reported that the expression of EGFR, IL-33, S100A2 and F3 is enriched in early lung basal cells, and that EGFR and F3 can be used to purify these early lung basal cells from heterogeneous lung differentiation cultures50. Expression of these markers was also assessed via QPCR and expression of EGFR was significantly higher in the proximalized organoids (Fig. 5B).
Differentiation of NKX2-1GFP;TP63tdTomato hiPSCs into lung organoids. (a) Protocol for differentiating NKX2-1GFP;TP63tdTomato hiPSCs into proximalized and distalized lung organoids based on McCauley et al.38 and Jacob et al.39. hPSC human pluripotent stem cell, DE definitive endoderm, AFE anterior foregut endoderm, LP lung progenitors, ORG lung organoid. (b) Representative images of proximalized and distalized lung organoids derived from the NKX2-1GFP;TP63tdTomato hiPSC line. White arrows indicate lung organoids containing double positive cells. (c) Flow cytometry analysis of lung organoids cultured in proximalizing and distalizing conditions. Representative dot plots shown. (d) Quantification of the 4 populations in the lung organoids grown in the differentiation culture conditions. DN double negative (GFP- tdTOM-), DP double positive (GFP + tdTOM +), tdTomato + tdTomato-positive only (GFP- tdTOM +), GFP GFP-positive only (GFP + tdTomato-). Data are represented as means ± SD, n = 5. *p < 0.05. t test. Graphs were made and statistical analyses were done using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) (www.graphpad.com).
Gene expression analysis of NKX2-1GFP;TP63tdTomato hiPSC-derived lung organoids. (a) QPCR analysis of lung marker NKX2-1 and basal cell markers TP63 (total), ΔNp63, TP63α, KRT5, and NGFR. Expression levels were normalized to housekeeping gene, ACTB. n = 3. *p < 0.05. t test. (b) QPCR analysis of additional markers identified by Miller et al. (2020)50 to be associated with basal cells. Expression levels were normalized to ACTB. n = 3. **p < 0.01. All graphs were made and statistical analyses were done using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) (www.graphpad.com).
Characterization of NKX2-1GFP;TP63tdTomato double positive (DP) cells
To confirm that the DP cells are specified to the basal cell lineage, the expression of other lung basal cell markers was assessed via QPCR. TP63 was detected in both the DP and tdTomato+ fractions, confirming the specificity and functionality of the fluorescence reporter line (Fig. 6A). NKX2-1 expression was present in both DP and GFP+ fractions and at low levels in the tdTomato+ fraction. KRT5 was found to be expressed in the DP fraction but at higher levels in the tdTomato+ fraction. Expression of NGFR was not found to be increased in the DP cells compared to the double negative (DN) fraction. NGFR expression, however, was high in the tdTomato+ fraction. Expression levels of EGFR, IL-33, S100A2 and F3 were also assessed (Fig. 6B). Both DP and tdTomato+ cells expressed high levels of EGFR, S100A2 and F3 with DP cells expressing higher levels of EGFR and S100A2. IL-33 was expressed at low levels in both DP and tdTomato+ cells.
Analysis of basal cell marker expression in 4 cell fractions isolated from Day 30 proximalized lung organoids. (a) QPCR analysis of basal cell markers in DN, DP, tdTomato + and GFP + fractions isolated from Day 30 proximalized lung organoids. Expression levels were normalized to ACTB. DN double negative (GFP- tdTOM-), DP double positive (GFP + tdTOM +), tdTomato + tdTomato-positive only (GFP- tdTOM +), GFP GFP-positive only (GFP + tdTomato-), hiPSC undifferentiated BU3 NKX2-1GFP hiPSCs. n = 4. **p < 0.01, ***p < 0.001, ****p < 0.0001. ANOVA followed by Tukey’s posthoc test. (b) QPCR analysis of other basal cell markers EGFR, F3, S100A2 and IL-33 in the DN, DP, tdTomato + and GFP + fractions. Expression levels were normalized to ACTB. n = 4. **p < 0.01, ***p < 0.001, ****p < 0.0001. ANOVA followed by Tukey’s post hoc test. All graphs were made and statistical analyses were done using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) (www.graphpad.com).
DP cells isolated from proximalized organoids (Passage number, P0) were passaged until P3 in proximalized culture conditions (Fig. 7A). Isolated DP (P0) cells were observed to be proliferative (Fig. 7B). However, we observed a gradual decrease in the proportion of DP cells with increasing passage number (Fig. 7C). DP cells at P3 were still capable of forming organoids in culture (Fig. 7D). While DP cells at P3 were still strongly TP63-positive and were capable of forming organoids in Matrigel, NKX2-1 expression was observed to be weak (Fig. 7D,E).
DP cells are proliferative and are capable of self-renewal. (a) Schematic of experimental setup. DP cells were serially passaged up to 3 passages. ORG: lung organoid; DP: double positive; MG: Matrigel. (b) Growth kinetics of DP cells at passage number 1 (P1). n = 3. (c) DP cells quantified at passage numbers P0, P1, P2 and P3 using flow cytometry. n = 3. (b,c) were made and statistical analyses were done using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) (www.graphpad.com). (d) Representative images of organoids derived from DP cells at P3. Scale bars indicate 500 µm. (e) Representative flow cytometry dot plot of dissociated organoids derived from DP P3 cells.
We next assessed whether DP cells are capable of multilineage differentiation like endogenous airway basal cells. DP cells were plated onto transwells, grown to confluence and then cultured at an air–liquid interface to induce terminal differentiation (Fig. 8A). Airway basal cells were also reported to respond to interleukin-6 (IL-6) and IL-13, factors purported to be able to increase differentiation of basal cells toward multiciliated and secretory cells, respectively51,52,53,54,55. Terminal differentiation was therefore performed in the presence of 10 ng/ml IL-6 or 10 ng/ml IL-13. DP cells were capable of differentiating into acetylated tubulin (AcTUB +) multiciliated cells and MUC5AC + secretory cells, and the addition of IL-6 or IL-13 altered the proportion of these cells in the ALI cultures (Fig. 8B). Ciliary movement could be observed in the ALI cultures (Supplementary video 1). Consistent with previous reports on the effects of IL-6 or IL-13 on differentiating airway epithelial cells, QPCR analysis for the expression of multiciliated cell markers (FOXJ1, DNAH5 and SNTN) was increased in culture conditions containing IL-6 and IL-13 compared to the ALI only conditions (Fig. 8C)51. Although FOXJ1 expression was not significantly different between IL-6- and IL-13-treated samples, levels of DNAH5 and SNTN were observed to be lower in the IL-13 conditions compared to the IL-6 conditions, suggesting that in the presence of IL-6, basal cells preferentially differentiate into multiciliated cells over secretory cells. Moreover, DP cells differentiated in the presence of IL-13 express higher levels of SPDEF and MUC5AC and lower levels of MUC5B compared to the ALI only and IL-6-containing conditions. This is also in agreement with previous reports on the ability of IL-13 to skew the differentiation of basal cells toward the secretory cell lineage by increasing MUC5AC expression via STAT6/SPDEF52,53,54,56.
DP cells possess multilineage differentiation potential. (a) Schematic of differentiation protocol. Isolated DP cells were seeded onto transwells and then cultured at an air–liquid interface (ALI) upon reaching confluence in the absence or presence of 10 ng/ml IL-6 or 10 ng/ml IL-13. (b) Representative images of differentiation cultures immunostained for acetylated α-tubulin (AcTUB) and MUC5AC. Scale bars indicate 5 µm. n = 3. (c) QPCR analysis of multiciliated cell markers (FOXJ1, DNAH5, SNTN), secretory cell markers (SPDEF, MUC5AC, MUC5B). n = 3. Graphs were made and statistical analyses were done using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) (www.graphpad.com).
Taken together, our data show that the TP63tdTomato faithfully reports endogenous TP63 expression and can therefore be used to track and monitor basal cell development.
Discussion
The airway epithelium is incredibly complex, consisting of many different specialized cell types. Lineage-tracing studies and the discovery of cell type-specific markers shed some light on the functions of these cell types in homeostasis and repair of the lung.
Here, we report the generation of an NKX2-1GFP;TP63tdTomato hiPSC reporter line using CRISPR/Cas9 gene editing. Two other groups recently reported the generation of similar NKX2-1GFP;TP63tdTomato hiPSC reporter lines57,58. Like Hawkins et al., we performed gene editing in the BU3 hiPSC line that harbours a bi-allelic integration of GFP into the endogenous NKX2-1 locus46. In the second study, Drick et al. introduced the tdTomato reporter into the TP63 locus in a previously characterized MHHi002-A-2 NKX2-1GFP reporter hiPSC line57,59. However, the gene targeting strategies used by the aforementioned groups differ from ours in several ways. First, Drick et al. employed TALENs and inserted tdTomato into exon 10, while Hawkins et al. used CRISPR/Cas9 and inserted tdTomato into exon 4. Both groups selected exons present in all isoforms and splice variants of TP6341,57,58. We chose to introduce tdTomato at the 3′ end of exon 14 to monitor the expression of the ΔNp63α isoform, which is the most abundantly expressed isoform in airway epithelial cells. QPCR analyses showed that the ΔNp63α isoform is indeed the dominant isoform expressed in the hPSC-derived airway epithelial cultures and its expression pattern was similar to that of tdTomato. Insertion of the tdTomato reporter did not interfere with the maintenance of pluripotency and was also shown to be functional and specific to ΔNp63α expression. Second, the tdTomato reporter was integrated into only one allele in the reporter lines generated by both Drick et al. and Hawkins et al., whereas our gene targeting approach resulted in homozygous integration of the tdTomato reporter in the TP63 locus. Third, one additional, though likely subtle difference among the three gene-targeting strategies, is that Drick et al. and Hawkins et al. relied on the use of antibiotic selection cassettes while our targeting strategy did not. In the case of Hawkins et al. , the puromycin cassette was subsequently removed via Cre-loxP excision. Finally, modifications to the TP63 locus in this study result in no loss of wild-type TP63 expression, which is particularly important given the key role TP63 plays in a multitude of cellular processes and that heterozygous mutations in TP63 have been reported to result in developmental disorders and diseases in humans60,61,62,63,64,65,66,67,68,69,70,71.
The NKX2-1GFP; TP63tdTomato dual fluorescent reporter hiPSC line was differentiated into proximalized and distalized lung organoids using published protocols to assess tdTomato reporter specificity and functionality. Proximalized lung organoids derived from the NKX2-1GFP;TP63tdTomato hiPSC reporter line were observed to contain significantly higher numbers of NKX2-1GFP+;TP63tdTomato+ DP cells compared to the distalized lung organoids, quantified by flow cytometry. This is in line with previous reports by McCauley et al. and Jacob et al.38,39. However, QPCR analyses of these proximalized and distalized organoids did not indicate statistically significant higher levels of TP63 (total), ΔNp63 and TP63α in the proximalized compared to the distalized organoids. KRT5 expression was observed to be higher in the distalized organoids although this is not statistically significant. This observation could be attributed to the NKX2-1GFP-;TP63tdTomato+ (TOM +) cells often present at the bottom of the wells underneath the organoids in the distalizing culture conditions. It is unclear if these cells have been derived from NKX2-1GFP+;TP63tdTomato- (GFP +) or DP populations or simply non-lung cell types that were not eliminated during FACS sorting on day 15. It is possible that the TOM + population represents mature basal cells, but this needs to be confirmed via lineage tracing experiments. This underscores the importance of quantifying the number of DP present in the culture conditions via flow cytometry instead of QPCR.
We show that DP cells express basal cell markers (KRT5, PDPN and ITGA6) as well as markers recently identified by Miller et al. such as EGFR, F3, and S100A250. Co-expression of KRT5 and NGFR are thought to identify mature basal cells in both mice and humans20,72 and have been used as read-outs for basal cell maturity73,74. Interestingly, the DP population did not appear to express significantly higher levels of NGFR compared to the DN population. The low expression of NGFR in DP cells was also reported by Hawkins et al. who further showed that culturing DP cells in PneumaCULT Ex medium supplemented with A-83–01, Dorsomorphin and Y-27632 (BC medium), increased NGFR expression and induced basal cell maturity58. The TOM + cell population was observed to also express these markers at similar or higher levels (in particular, NGFR and KRT5) compared to the DP population. However, KRT5 and NGFR are also expressed in basal cells of other epithelial tissues/organs such as the skin, cornea, esophagus, mammary gland and prostate75,76,77,78,79. It is therefore possible that these cells are non-lung cell types. The presence of the NKX2-1GFP fluorescent reporter is particularly important in facilitating the specific isolation of airway basal cells.
Finally, we validated our dual fluorescent reporter hiPSC line by further characterizing the DP cell population. DP cells are proliferative but the proportion of DP cells was observed to decrease with increasing passage number. This could be due to the proximalizing culture conditions being sub-optimal for long term culture of airway basal cells. Similarly, primary airway basal cells have a short lifespan in culture, which can be extended through the use of various culture methods such as the small molecule inhibitors or feeder cells80,81. Indeed, Hawkins et al. showed that the hiPSC-derived basal cells can be maintained and expanded up to 10 passages when cultured in PneumaCULT-Ex medium supplemented with A-83–01, Dorsomorphin and Y-2763258. NKX2-1 expression was also observed to be decreased in these DP cells and their derived organoids. The downregulation of NKX2-1 expression could be due to the DP cells becoming more lineage-restricted with increasing time in culture. During lung development in the mouse, expression of NKX2-1 becomes increasingly confined to distal airway cells as branching morphogenesis occurs82,83. In addition, knockout mouse models indicate that Nkx2-1 is necessary for distal lung development but appears to be dispensable for proximal lung specification and morphogenesis25,84. Like primary airway basal cells, DP cells can also be terminally differentiated into multiciliated and secretory cells in ALI cultures. DP cells are also responsive to IL-6 and IL-13, which bias differentiation of basal cells toward multiciliated and secretory cells, respectively.
We anticipate that this dual fluorescent reporter hiPSC line will be very useful for the identification and isolation of lung basal cells for the study of basal cell development and differentiation. In addition, this line will also be a valuable tool in the development of culture conditions that enable the expansion and long-term maintenance of basal cells for a variety of applications85.
Materials and methods
Human pluripotent stem cell culture
BU3 NKX2-1GFP hiPSCs were a kind gift from Professor Darrell Kotton, Boston University University School of Medicine, and were routinely cultured on Vitronectin-coated tissue culture dishes in Essential 8 (E8) medium (Life Technologies). All cells were maintained in an incubator with 5% CO2, at 37 °C.
Construction of CRISPR sgRNA vectors and donor template
CRISPR sgRNAs targeting exon 14 of the human TP63 gene were designed using CHOPCHOP (https://chopchop.cbu.uib.no/). Two sgRNAs were identified (TP63 sgRNA 1 and sgRNA2), annealed and cloned into the pHF1-Cas9 plasmid. The donor template was constructed with the P2A-tdTomato cassette flanked by 800 bp homology arms amplified from genomic DNA extracted from the BU3 NKX2-1GFP hiPSC line. Site-directed mutagenesis was performed to remove the PAM site on the donor template to prevent cleavage by Cas9. Primer sequences are found in Supplementary Table 1.
Transfection
BU3 NKX2-1GFP hiPSCs were plated onto vitronectin-coated tissue culture plates at 30% confluence one day before transfection. 2 µg of sgRNA and 7 µg donor template were diluted in OptiMEM, mixed at a 1:4 ratio with Lipofectamine STEM (Life Technologies) and then applied to the cells. E8 culture medium containing 5.25 µg/ml Blasticidin was supplied to the cells 24 h after transfection. Selection continued for two days, after which cells were fed fresh E8 medium daily until colonies were large enough for picking and genotyping.
T7EI assay
BU3 NKX2-1GFP hiPSCs transfected with 2 µg of empty vector or sgRNA using Lipofectamine STEM were harvested 7 days post-transfection. Genomic DNA was extracted using the QiAmp DNA mini kit (Qiagen). Gene-specific primers were used to amplify genomic regions flanking the sgRNA target sites via PCR. PCR products were purified using the QIAQuick PCR purification kit, denatured and allowed to reanneal in NEB buffer 2. The PCR products were then treated with or without T7 endonucleause I (NEB) at 37 °C for 1 h before they were resolved on a 2% agarose gel and visualized using an UVIpro Gel documentation system (UVItec).
Genotyping
Genomic DNA was extracted from picked colonies in a 20 µl reaction volume containing 1X detergent (0.05% IGEPAL CA630, 0.05% Tween-20), proteinase K and 1X TE buffer, incubated at 55 °C for 1 h followed by a 5 min incubation at 95 °C. 0.5 to 1 µl of the reaction was then used in a 10 µl PCR reaction containing 1X Primestar Max Mastermix (Takara) and 1 µl 10 µM forward and reverse primers. The thermal cycling conditions used were as follows: 10 s at 98 °C, followed by 35 cycles of 10 s at 98 °C, 5 s at 55 °C and 1 min at 72 °C.
The primers used for genotyping were designed to target outside the 5′ and 3′ homology arms. A positive clone (with insertion of P2A-tdTomato) would yield a PCR product of 3.1 kb while a negative clone (wild-type) would yield a PCR product of 1.5 kb. Positive clones identified were sent for Sanger sequencing to confirm presence of P2A-tdTomato without any unwanted mutations. Primer sequences can be found in Supplementary Table 1.
Clonal expansion
Positive BU3 NKX2-1GFP hiPSC clones with P2A-tdTomato correctly integrated into the locus were dissociated into single cells using Accutase and seeded at very low densities onto vitronectin-coated plates in E8 medium supplemented CloneR (Stem Cell Technologies) per manufacturer’s instructions. E8 medium was refreshed daily until colonies emerged and were large enough for picking and genotyping. Homozygous clones were expanded in culture and frozen stocks were made using Cryostor (Stem Cell Technologies).
Trilineage differentiation
hiPSCs were washed with DPBS once and then incubated with Accutase for 5 min at 37 °C. A P1000 pipette was then used to resuspend the cells and break up large cell clumps. Accutase was diluted out using DMEM F12 Advanced. The cell suspension was centrifuged at 1200 rpm for 5 min to collect cells into a pellet. The pellet was then resuspended in MEF medium (15% FBS, 1% Glutamax in DMEM F12 Advanced) and transferred into low adhesion tissue culture dishes to form embryoid bodies. The medium was changed every other day for 7 days after which the embryoid bodies were collected and allowed to attach onto 0.1% gelatin-coated tissue culture dishes. Cellular outgrowths were fed every other day for a further 7 days before they were fixed with 4% PFA for analysis of differentiation markers via immunofluorescence.
Karyotype analysis
NKX2-1GFP;TP63tdTomato hiPSC subclone 31.1 (Passage number P35 + 8, 8 passages post-subcloning) was grown to 70–80% confluency on a vitronectin-coated dish in E8 medium before cells were sent to the KK Women’s and Children’s Hospital (Singapore) Cytogenetics department for karyotype analysis.
Flow cytometry
Cells were washed once with DPBS and then incubated with TrypLE express for 5 min at 37 °C. TrypLE express was then diluted out with DMEM F12 Advanced. Cells were collected into a pellet by centrifugation at 1200 rpm for 5 min at room temperature. Cell pellet was resuspended and fixed in 4% PFA and left to incubate for 20 min at room temperature with agitation every 5 min to prevent clumping. PFA was then removed and DPBS was added to wash the cells of residual PFA. The cell pellet was then collected via centrifugation at 1200 rpm for 5 min and then resuspended in 200 µl FACS buffer. 8 µl of isotype control antibodies or OCT4 antibodies were added and left to incubate in the dark for 1 h at room temperature. Excess and unbound antibodies were then removed and washed off by rinsing the cells with FACS buffer three times. Cells were then resuspended in 300 µl FACS buffer and passed through a 40 µm cell strainer to remove any cell clumps prior to analysis on the BD FACS Calibur. For the OCT4 staining experiment, the flow cytometry gates were set using Day 15 differentiated cells as a negative control. FlowJo was used to analyse all flow cytometry data. Antibodies used for FACS analyses can be found in Supplementary Table.
Immunofluorescence
Cells were washed with DPBS and then fixed in 4% PFA for 20 min at room temperature. The PFA was removed and cells were washed with DPBS before they were blocked and permeabilized with blocking buffer (10% donkey serum, 0.1% Triton X-100 in DPBS) for 1 h at room temperature. Cells were then incubated with primary antibodies diluted in staining buffer (1% donkey serum, 0.1% Triton X-100 in DPBS) overnight at 4 °C. Excess and unbound primary antibodies were removed and the cells were washed 3 times with DPBS before incubated with fluorescence-conjugated secondary antibodies diluted in staining buffer for 1 h in the dark at room temperature. Cells were washed 3 times with DPBS to remove excess and unbound antibodies. NucBlue Fixed Cell ReadyProbes Reagent (DAPI, ThermoFisher Scientific) diluted in DPBS at 1 drop/ml was then added to each well to enable visualization of nuclei. Cells were then imaged using the Leica FV-3000 and ImageJ was used for image processing and analysis. The list of primary and secondary antibodies used can be found in the Supplementary Table 2.
To image organoids, media was removed from the wells and organoids embedded in Matrigel were carefully washed with DPBS once. The organoids were then fixed with 4% PFA for 20 min at room temperature. The PFA was then removed and the organoids were washed with DPBS once. 1 ml DPBS was then added to each well. 1 drop of DAPI solution was added to each well. Organoids were imaged using the Leica FV-3000 and images were analysed and processed using ImageJ.
RNA extraction, cDNA synthesis and quantitative real-time PCR (QPCR)
Total RNA was extracted from cells using the Qiagen RNeasy kit per manufacturer’s instructions. 500 ng of purified RNA was converted into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). QPCR was performed using the QuantStudio 7 Flex Real-Time PCR System. Samples were run in duplicates and normalized to β actin expression. Primer sequences can be found in Supplementary Table 3. Adult lung mRNA used as a positive control for QPCR experiments was obtained from Biochain (Total RNA – Human Adult Normal Tissue: Lung lower left lobe; Cat #R1234152-50; Lot #B6050780).
Protein extraction and western blot
Whole cell lysates were prepared by lysing cells in Pierce RIPA buffer (ThermoFisher Scientific) supplemented with complete protease inhibitor cocktail (Calbiochem). 30 µg protein was resolved on a 7.5% SDS PAGE gel and then transferred onto PVDF membranes via semi-dry transfer method (Bio-rad). Membranes were blocked in 5% skim milk in Tris-buffered saline (TBST: 0.05 M Tris, 0.138 M NaCl, 0.0027 M KCl, pH 8.0) with 0.1% Tween-20 (Sigma-Aldrich) for an hour at room temperature. Membranes were then incubated with primary antibodies at 4 °C overnight. Membranes were then washed 3 times in TBST and then incubated with HRP-linked secondary antibodies for 1 h at RT. Membranes were washed 3 times with TBST before proteins were visualized using the SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific) and Amersham Hyperfilm ECL (GE Healthcare). Primary and secondary antibodies can be found in Supplementary Table 2. Primary human bronchial epithelial cells used as a positive control for TP63 was obtained from Promocell (C-12640).
Differentiation of hiPSCs into lung progenitors
hiPSCs were washed with DPBS and then incubated with Accutase for 5 min at 37 °C. A P1000 pipette was used to resuspend and break up clumps into single cells. Acctuase was diluted out using DMEM F12 Advanced and the cells were collected into a pellet by centrifuging at 1200 rpm for 5 min. The cell pellet was resuspended in E8 medium supplemented with 10 µM Y-27632. 400,000 cells were seeded into each 12-well and left in the incubator to attach overnight. Fresh E8 media was added to the cells the next day (Day -1, D-1). At D0, hiPSCs were differentiated into definitive endoderm (DE) cells using a protocol previously published by Vallier et al.86. To differentiate the DE cells into anterior foregut and subsequently lung progenitors, the protocol published by McCauley et al. was used38. Briefly, on D0, hiPSCs were treated 100 ng/ml Activin A, 80 ng/ml FGF2, 10 µM LY294002, 3 µM CHIR99021, 10 ng/ml BMP4 in CDM-PVA medium. On D1, hiPSCs were treated with 100 ng/ml Activin A, 80 ng/ml FGF2, 10 µM LY294002 and 10 ng/ml BMP4 in CDM-PVA medium. On D2, hiPSCs were treated with 100 ng/ml Activin A, 80 ng/ml FGF2, 1X B27, 1X NEAA in RPMI medium. To drive DE cells towards anterior foregut formation, cells were treated with 10 µM SB431542 and 2 µM Dorsomorphin (DSM) in basal medium (1X B27, 1X N2, 1X Glutamax, 1 mM HEPES in DMEM F12 Advanced) for 3 days. Finally, to differentiate AFE cells into lung progenitors, cells were treated with 3 µM CHIR, 10 ng/ml BMP4, 10 ng/ml FGF7, 10 ng/ml FGF10 and 50 nM RA in basal medium for 9 days.
Lung organoid culture
Day 15-sorted GFP + cells were resuspended in basal medium containing 250 ng/ml FGF2, 100 ng/ml FGF10, 10 ng/ml FGF7, 50 nM Dexamethasone (Sigma-Aldrich), 0.1 mM 8-Bromoadenosine 30,50-cyclic monophosphate sodium salt (cAMP, Sigma-Aldrich) and 0.1 mM 3-Isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich), with or without the addition of 3 µM CHIR99021. Undiluted growth factor-reduced Matrigel (Corning) was added to the cell suspension at a 1:1 ratio after which 40 µl of this cell suspension was added to the middle of each 24-well plate to create a Matrigel drop. The plates were then returned to the incubator to allow the Matrigel drops to solidify for at least 30 min before medium is overlaid over the drops. Y-27632 was included in the medium for the first 24 h to improve cell survival. Medium was refreshed every other day for 2 weeks (Day 30) prior to analysis by flow cytometry.
Fluorescence-activated cell sorting (FACS)
For sorting at Day 15, differentiated hiPSCs were washed once with DPBS and then incubated with TryPLE express for 15 min at 37 °C. A P1000 pipette was used to resuspend the cells to help break down large clumps into single cells. TrypLE express was diluted out with DMEM Advanced F12 and removed by centrifugation at 1200 rpm for 5 min. The cell pellet was resuspended in FACS buffer supplemented with 2% pen/strep and 10 µM Y-27632, passed through a 40 µm cell strainer to remove cell clumps and then sent to the Singapore Immunology Network (SIgN) Flow cytometry facility for sorting. H9 cells that are GFP- and tdTomato- were used as a negative control and used to set the gating parameters.
For sorting at Day 30, lung organoids embedded in Matrigel were washed with DPBS once. Organoids were then incubated with TrypLE express for 30 min at 37 °C. A P1000 pipette was used to resuspend and break the organoids down into single cells. DMEM F12 advanced was used to dilute out TrypLE express and then cells were collected into a pellet by centrifugation at 1200 rpm for 5 min. The cells were resuspended in FACS buffer supplemented with 2% pen/strep and 10 µM Y-27632, passed through a 40 µm cell strainer and then sent to the Singapore Immunology Network (SIgN) Flow cytometry facility for sorting.
Terminal differentiation of DP cells
DP cells were seeded onto Matrigel-coated Transwell inserts. When cells reached 100% confluence, media was removed from both chambers and PneumaCULT ALI medium (Stem Cell Technologies) with or without 10 ng/ml IL-6 or 10 ng/ml IL-13 was added only to the bottom chamber. Medium was refreshed every other day for 2 weeks before cells were harvested for mRNA or fixed in 4% PFA for immunostaining.
Statistical analysis
All graphs were made and statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA). All error bars indicate mean ± standard deviation (SD). All experiments were performed with at least 3 biological replicates. The two-tailed Student’s T test was used to test for statistical significance where only a single parameter was compared between two groups. Where comparisons across > 2 groups had to be made, a one-way Analysis of Variance (ANOVA) was done followed by Tukey’s multiple comparisons analysis. A p value of < 0.05 was considered statistically significant.
References
Society, E. R. Forum of International Respiratory Societies. The Global Impact of Respiratory Disease – Second Edition. Sheffield,. (2017).
Bowden, D. H. Cell turnover in the lung. Am. Rev. Respir. Dis. 128, 2 (1983).
Kauffman, S. L. Cell proliferation in the mammalian lung. Int. Rev. Exp. Pathol. 22, 131–191 (1980).
Adamson, I. Y. R. & Bowden, D. H. The type 2 cell as progenitor of alveolar epithelial regeneration: A cytodynamic study in mice after exposure to oxygen. Lab. Investig. 30, 35–42 (1974).
Adamson, I. Y. R. & Bowden, D. H. Origin of ciliated alveolar epithelial cells in bleomycin induced lung injury. Am. J. Pathol. 87, 569–580 (1977).
Adamson, I. Y. R. & Bowden, D. H. Bleomycin-induced injury and metaplasia of alveolar type 2 cells. Relationship of cellular responses to drug presence in the lung. Am. J. Pathol. 96, 531–544 (1979).
Bowden, D. H., Davies, E. & Wyatt, J. P. Cytodynamics of pulmonary alveolar cells in the mouse. Arch. Pathol. 86, 667–670 (1968).
Cabral-Anderson, L. J., Evans, M. J. & Freeman, G. Effects of NO2 on the lungs of aging rats I. Morphology. Exp. Mol. Pathol. 27, 353–365 (1977).
Evans, M. J., Cabral, L. C., Stephens, R. J. & Freeman, G. Acute kinetic response and renewal of the alveolar epithelium following injury by nitrogen dioxide. Chest 65, 62S-64S (1974).
Evans, M. J., Cabral, L. J., Stephens, R. J. & Freeman, G. Renewal of alveolar epithelium in the rat following exposure to NO2. Clin. Res. 21, 278 (1973).
Evans, M. J., Cabral, L. J., Stephens, R. J. & Freeman, G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp. Mol. Pathol. 22, 142–150 (1975).
Evans, M. J., Cabral Anderson, L. J. & Freeman, G. Role of the clara cell in renewal of the bronchiolar epithelium. Lab. Investig. 38, 648–655 (1978).
Rawlins, E. L. & Hogan, B. L. M. Epithelial stem cells of the lung: Privileged few or opportunities for many?. Development 133, 2455–2465 (2006).
Rawlins, E. L., Ostrowski, L. E., Randell, S. H. & Hogan, B. L. M. Lung development and repair: Contribution of the ciliated lineage. Proc. Natl. Acad. Sci. U. S. A. 104, 410–417 (2007).
Rawlins, E. L. & Hogan, B. L. M. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am. J. Physiol. Lung Cell Mol. Physiol. 295, 2 (2008).
Rawlins, E. L. et al. Epithelial stem/progenitor cells in lung postnatal growth, maintenance, and repair. Cold Spring Harb. Symp. Quant. Biol. 73, 291–295 (2008).
Borthwick, D. W., Shahbazian, M., Krantz, Q. T., Dorin, J. R. & Randell, S. H. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 24, 662–670 (2001).
Lynch, T. J. et al. Submucosal gland myoepithelial cells are reserve stem cells that can regenerate mouse tracheal epithelium. Cell Stem Cell 22, 653-667.e5 (2018).
Tata, A. et al. Myoepithelial cells of submucosal glands can function as reserve stem cells to regenerate airways after injury. Cell Stem Cell 22, 668-683.e6 (2018).
Rock, J. R., Randell, S. H. & Hogan, B. L. M. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. DMM Dis. Model. Mech. 3, 545–556 (2010).
Rock, J. R. et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. U. S. A. 106, 12771–12775 (2009).
Yang, A. et al. P63 is essential for regenerative proliferation in limb craniofacial and epithelial development. Nature 398, 714–718 (1999).
Daniely, Y. et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am. J. Physiol. Cell Physiol. 287, 2 (2004).
Romano, R. A. et al. ΔNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development 139, 772–782 (2012).
Minoo, P., Su, G., Drum, H., Bringas, P. & Kimura, S. Defects in tracheoesophageal and lung morphogenesis in Nkx21(-/-) mouse embryos. Dev. Biol. 209, 60–71 (1999).
Tata, P. R. et al. Developmental history provides a roadmap for the emergence of tumor plasticity. Dev. Cell 44, 679-693.e5 (2018).
Devriendt, K., Vanhole, C., Matthijs, G. & De Zegher, F. Deletion of thyroid transcription factor-1 gene in an infant with neonatal thyroid dysfunction and respiratory failure [5]. N. Engl. J. Med. 338, 1317–1318 (1998).
Plopper, C. G. et al. Tracheobronchial epithelium in vivo: composition, differentiation and response to hormones. In D. G. Thomassen and P. Nettesheim, editors. Biology, Toxicology and Carcinogenesis in the Respiratory Epithelium. Hemisphere, New York. 380, (1990).
Yang, Y. et al. Spatial-temporal lineage restrictions of embryonic p63+ progenitors establish distinct stem cell pools in adult airways. Dev. Cell 44, 752-761.e4 (2018).
Kho, A. T. et al. Transcriptomic analysis of human lung development. Am. J. Respir. Crit. Care Med. 181, 54–63 (2010).
Nikolić, M. Z. et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife 6, 2 (2017).
Green, M. D. et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 29, 267–273 (2011).
Mou, H. et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10, 385–397 (2012).
Wong, A. P. et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 30, 876–882 (2012).
Huang, S. X. L. et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84–91 (2013).
Firth, A. L. et al. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. PNAS https://doi.org/10.1073/pnas.1403470111 (2014).
Konishi, S. et al. Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Rep. 6, 18–25 (2016).
McCauley, K. B. et al. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 20, 2 (2017).
A, J. et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21, 472–488 (2017).
Mangiulli, M. et al. Identification and functional characterization of two new transcriptional variants of the human p63 gene. Nucleic Acids Res. 37, 6092–6104 (2009).
Levrero, M. et al. The p53/p63/p73 family of transcription factors: Overlapping and distinct functions. J. Cell Sci. 113, 1661–1670 (2000).
Van Bokhoven, H. & McKeon, F. Mutations in the p53 homolog p63: Allele-specific developmental syndromes in humans. Trends Mol. Med. 8, 133–139 (2002).
Yang, A. et al. P63, a P53 homolog At 3Q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 (1998).
Warner, S. M. B. et al. Transcription factor p63 regulates key genes and wound repair in human airway epithelial Basal cells. Am. J. Respir. Cell Mol. Biol. 49, 978–988 (2013).
Arason, A. J. et al. DeltaNp63 has a role in maintaining epithelial integrity in airway epithelium. PLoS ONE 9, 2 (2014).
Hawkins, F. et al. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. J. Clin. Invest. 127, 2277–2294 (2017).
Sethi, I. et al. A global analysis of the complex landscape of isoforms and regulatory networks of p63 in human cells and tissues. BMC Genom. 16, 2 (2015).
Kim, M.-K. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines zebrafish and mice. PLoS ONE 6, e18556 (2011).
Wang, Y., Wang, F., Wang, R., Zhao, P. & Xia, Q. 2A self-cleaving peptide-based multi-gene expression system in the silkworm Bombyx mori. Sci. Rep. 5, 2 (2015).
Miller, A. J. et al. In vitro and in vivo development of the human airway at single-cell resolution. Dev. Cell 53, 117-128.e6 (2020).
Tadokoro, T. et al. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc. Natl. Acad. Sci. U. S. A. 111, 2 (2014).
Seibold, M. A. Interleukin-13 stimulation reveals the cellular and functional plasticity of the airway epithelium. Ann. Am. Thorac. Soc. 15, S98–S106 (2018).
Woodruff, P. G. et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 180, 388–395 (2009).
Kondo, M., Tamaoki, J., Takeyama, K., Nakata, J. & Nagai, A. Interleukin-13 induces goblet cell differentiation in primary cell culture from guinea pig tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 27, 536–541 (2002).
Laoukili, J. et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J. Clin. Invest. 108, 1817–1824 (2001).
Yu, H., Li, Q., Kolosov, V. P., Perelman, J. M. & Zhou, X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun. Adhes. 17, 83–92 (2011).
Drick, N. et al. Generation of a NKX2.1–p63 double transgenic knock-in reporter cell line from human induced pluripotent stem cells (MHHi006-A-4). Stem Cell Res. 42, 2 (2020).
Hawkins, F. et al. Derivation of airway basal stem cells from human pluripotent stem cells. Cell Stem Cell https://doi.org/10.1101/2020.02.21.959395 (2020).
Olmer, R. et al. Generation of a NKX2.1 knock-in reporter cell line from human induced pluripotent stem cells (MHHi006-A-2). Stem Cell Res. 39, 2 (2019).
Galoczova, M., Coates, P. & Vojtesek, B. STAT3, stem cells, cancer stem cells and p63. Cell. Mol. Biol. Lett. 23, 2 (2018).
Yoh, K. & Prywes, R. Pathway regulation of p63, a director of epithelial cell fate. Front. Endocrinol. 6, 2 (2015).
Keyes, W. M. & Mills, A. A. A new link between senescence and aging. Cell Cycle 5, 260–265 (2006).
Gonfloni, S., Caputo, V. & Iannizzotto, V. P63 in health and cancer. Int. J. Dev. Biol. 59, 87–93 (2015).
Kouwenhoven, E. N. et al. Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO Rep. 16, 863–878 (2015).
Moll, U. M. & Slade, N. p63 and p73: Roles in development and tumor formation. Mol. Cancer Res. 2, 371–386 (2004).
Boughner, J. C. et al. P63 expression plays a role in developmental rate, embryo size, and local morphogenesis. Dev. Dyn. 247, 779–787 (2018).
Vanbokhoven, H., Melino, G., Candi, E. & Declercq, W. P63, a story of mice and men. J. Invest. Dermatol. 131, 1196–1207 (2011).
Westfall, M. D. & Pietenpol, J. A. p63: Molecular complexity in development and cancer. Carcinogenesis 25, 857–864 (2004).
Qu, J. et al. Mutant p63 affects epidermal cell identity through rewiring the enhancer landscape. Cell Rep. 25, 3490-3503.e4 (2018).
Pattison, J. M. et al. Retinoic acid and BMP4 cooperate with p63 to alter chromatin dynamics during surface epithelial commitment. Nat. Genet. 50, 1658–1665 (2018).
Soares, E. & Zhou, H. Master regulatory role of p63 in epidermal development and disease. Cell. Mol. Life Sci. 75, 1179–1190 (2018).
Bilodeau, M., Shojaie, S., Ackerley, C., Post, M. & Rossant, J. Identification of a proximal progenitor population from murine fetal lungs with clonogenic and multilineage differentiation potential. Stem Cell Rep. 3, 634–649 (2014).
de Carvalho, A. L. R. T. et al. Glycogen synthase kinase 3 induces multilineage maturation of human pluripotent stem cell-derived lung progenitors in 3D culture. Dev. 146, 2 (2019).
Hawkins, F. J. et al. Derivation of airway basal stem cells from human pluripotent stem cells. BioRxiv https://doi.org/10.1101/2020.02.21.959395 (2020).
Reis-Filho, J. S. et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology 49, 10–21 (2006).
Daiko, H. et al. Molecular profiles of the mouse postnatal development of the esophageal epithelium showing delayed growth start. Int. J. Mol. Med. 18, 1057–1066 (2006).
Di Girolamo, N. et al. Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J. Cell. Mol. Med. 12, 2799–2811 (2008).
Jiang, M. et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 550, 529–533 (2017).
Toivanen, R. & Shen, M. M. Prostate organogenesis: Tissue induction, hormonal regulation and cell type specification. Dev. 144, 1382–1398 (2017).
Mou, H., Vinarsky, V., Tata, P. R., Brazauskas, K. & Soon, H. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 19, 217–231 (2016).
Liu, X. et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 180, 599–607 (2012).
Stahlman, M. T., Gray, M. E. & Whitsett, J. A. Expression of thyroid transcription factor-1 (TTF-1) in fetal and neonatal human lung. J. Histochem. Cytochem. 44, 673–678 (1996).
Yatabe, Y., Mitsudomi, T. & Takahashi, T. TTF-1 expression in pulmonary adenocarcinomas. Am. J. Surg. Pathol. 26, 767–773 (2002).
Yuan, B. et al. Inhibition of distal lung morphogenesis in Nkx2.1(-/-) embryos. Dev. Dyn. 217, 180–190 (2000).
Trott, J. et al. Long-term culture of self-renewing pancreatic progenitors derived from human pluripotent stem cells. Stem Cell Reports 8, 1675–1688 (2017).
Vallier, L. et al. Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cells 27, 2655–2666 (2009).
Acknowledgements
We are grateful to Professor Darrell Kotton, the National Center for Advancing Translational Sciences (NCATS; grant number: U01TR001810) and the Center for Regenerative Medicine for the BU3 NKX2-1GFP hiPSC line. We would also like to acknowledge the support of Seri Mustafah from the Agency for Science, Technology and Research Singapore (A*STAR) Flow Cytometry Core Facility for technical assistance with FACS sorting experiments. K.J.G, L.H. and S.R. are supported by A*STAR. E.K.T. and N.R.D. are supported by start-up funds provided by the Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore.
Author information
Authors and Affiliations
Contributions
K.J.G. and N.R.D. conceived the study. K.J.G., E.K.T., H.L and S.R generated the data in the manuscript. K.J.G. and N.R.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goh, K.J., Tan, E.K., Lu, H. et al. An NKX2-1GFP and TP63tdTomato dual fluorescent reporter for the investigation of human lung basal cell biology. Sci Rep 11, 4712 (2021). https://doi.org/10.1038/s41598-021-83825-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83825-6
This article is cited by
-
Reconstructing the pulmonary niche with stem cells: a lung story
Stem Cell Research & Therapy (2022)
-
A Maverick Review of Common Stem/Progenitor Markers in Lung Development
Stem Cell Reviews and Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.