Abstract
Immortelle (Helichrysum italicum (Roth) G. Don; Asteraceae) is a perennial plant species native to the Mediterranean region, known for many properties with wide application mainly in perfume and cosmetic industry. A total of 18 wild H. italicum populations systematically sampled along the eastern Adriatic environmental gradient were studied using AFLP markers to determine genetic diversity and structure and to identify loci potentially responsible for adaptive divergence. Results showed higher levels of intrapopulation diversity than interpopulation diversity. Genetic differentiation among populations was significant but low, indicating extensive gene flow between populations. Bayesian analysis of population structure revealed the existence of two genetic clusters. Combining the results of FST - outlier analysis (Mcheza and BayeScan) and genome-environment association analysis (Samβada, LFMM) four AFLP loci strongly associated with the bioclimatic variables Bio03 Isothermality, Bio08 Mean temperature of the wettest quarter, Bio15 Precipitation seasonality, and Bio17 Precipitation of driest quarter were found to be the main variables driving potential adaptive genetic variation in H. italicum along the eastern Adriatic environmental gradient. Redundancy analysis revealed that the partitioning of genetic variation was mainly associated with the adaptation to temperature oscillations. The results of the research may contribute to a clearer understanding of the importance of local adaptations for the genetic differentiation of Mediterranean plants and allow the planning of appropriate conservation strategies. However, considering that the identified outlier loci may be linked to genes under selection rather than being the target of natural selection, future studies must aim at their additional analysis.
Similar content being viewed by others
Introduction
The Mediterranean basin area is one of the largest biodiversity hotspots in the world1. It occupies only 2% of the world's land area and is the habitat for great number of species2. Heterogeneous environments and numerous past events are responsible for significant plant diversity and a large number of endemic species in this area2. The Mediterranean basin is the meeting point of three continents; a place where humans began to exert their influence on biota and environment very early3. Today, human activity continues to pose a threat to the biodiversity in the Mediterranean, mainly because population density is highest along the coastal region, where numerous refugia have been discovered4. Refugia are known to act as climate-stable zones that are crucial for the long-term survival of species and genetic diversity. Populations that survived in glacial refugia show high rarity index values (DW; frequency down-weighted marker values) and high genetic diversity due to long-term isolation5. Populations that emerged after the Last Glacial Maximum show lower genetic variation6 and lower rarity as a result of consecutive founder events during postglacial colonization7,8. It is very important to preserve these areas of numerus refugia, which are vital for the evolutionary processes of Mediterranean plant species4.
Climate change, overexploitation of natural populations, degradation, and fragmentation of natural habitats are the direct drivers affecting biodiversity9,10, and the impact of each factor on the evolutionary processes of a species must be considered for appropriate conservation planning.
Local adaptation is an important tool of species through which natural populations are phenotypically and genotypically separated to better adapt to new habitat conditions and survive11,12. From a theoretical perspective, the interaction between gene flow and natural selection has been well studied13, but there are still too few empirical studies to clarify the establishment of local adaptation and maintenance at the molecular level in natural populations. For non-model perennial and outcrossing species, genomic and evolutionary research is more difficult to conduct, and the genetic background of adaptive traits remains poorly understood14,15. Plant species that have wide geographic distribution and thereby are exposed to a range of environmental conditions, are often locally adapted, in part because of higher gene flow14,15,16. Discovering the mechanisms of adaptability of plant species is important for predicting their survival due to climate change or to what extent certain plant species will develop or activate adaptive mechanisms to the new conditions17,18.
Genetic signatures of natural selection are revealed by genome scanning19,20,21. Genotyping of random loci throughout the genome allows the detection of outlier loci characterized by a higher degree of differentiation between populations than would be expected for neutral loci20,22,23,24,25. Identification of genetic regions under selection is commonly performed using two methods: outlier loci detection and genome-environment association analyzes. Both approaches assume that only a small number of loci are under selection26. Detection of outlier loci is a population-level analysis based on estimates of population genetic differentiation (FST), whereas genome-environment analysis examines correlations between population allele frequencies and environmental variables27,28,29. Using these methods, local adaptation along environmental gradients was studied in several plant species: Eruca sativa Mill.30; Eucalyptus camaldulensis Dehnh.31; Abies alba Mill.32; Liriodendron chinense (Hemsl.) Sarg.21; Geropogon hybridus (L.) Sch.Bip.33; Diplotaxis harra (Forssk.) Boiss.34, Populus tremula L.35, and Festuca pallescens (St.-Yves) Parodi36.
To study local adaptation along the eastern Adriatic environmental gradient characterized by increasing temperatures and precipitation, we chose H. italicum, a typical Mediterranean representative of thermophilic species. The species is naturally distributed along the eastern Adriatic coast and on the islands along the northwest-southeast environmental gradient. It grows on calcareous and well-drained soils: from sea level to 2200 m a.s.l.37. It is an outcrossing, thermophilic perennial and entomophilous plant species. As an anemochorous species, it is easily dispersed by wind, which contributes significantly to the spread of the species. It belongs to the genus Helichrysum Mill. and the family Asteraceae. The genus Helichrysum has more than 500 species worldwide38, with the species H. italicum distributed in the Mediterranean region. Herrando-Moraira et al.39 revised the classification of the entire H. italicum complex and proposed a new division of the H. italicum subspecies: (1) H. italicum subsp. italicum (grows in Italy, Croatia, on the eastern Mediterranean coast of France and Corsica, in Bosnia and Herzegovina, Greece (Aegean islands and Cyprus), (2) H. italicum ssp. microphyllum (endemic to Crete), (3) H. italicum ssp. siculum (endemic to Sicily), and (4) H. italicum ssp. tyrrhenicum (disjunct distribution area between islands Corsica, Sardinia, Mallorca, and Dragonera islet). Numerous studies on the medicinal properties and biological activity of H. italicum were conducted, and are summarized in Guinoiseau et al.40, Maksimovic et al.41, and Ninčević et al.42. There have been several studies on the genetic diversity of Helichrysum spp.37,43,44,45,46 but none of them focused on the eastern Adriatic natural populations. Owing to many beneficial properties it is used in many commercial products, which increased the demand for H. italicum. This has led to overexploitation of natural populations, especially on the eastern Adriatic coast, where the collection of H. italicum contributes significantly to the local economy (e.g. in Croatia immortelle is the most collected of all other wild medicinal species). In general, the use of medicinal and aromatic plants (MAP) has a long tradition on the eastern Adriatic coast. Commercial gathering is an important source of main or additional income in many regions47,48. Wild collection is still the main way to supply the market with medicinal and aromatic plants, as production is still insufficient. The negative impacts of wild collection are manifested in the loss of biodiversity, inconsistent plant material and lower prices. Increasing demand and resulting overexploitation, destructive harvesting techniques along with habitat loss and degradation49 put great pressure on many medicinal plants, leading to a decrease in genetic diversity and a reduction in the species potential to respond to environmental changes47,50. Therefore, it is of great importance to assess genetic biodiversity for efficient conservation of plant genetic resources and their use in plant breeding programs48.
In this research, an AFLP genome scan was performed on a total of 18 natural H. italicum populations systematically sampled along the eastern Adriatic environmental gradient to elucidate genetic diversity within and among populations, identify candidate loci subjected to selection, and investigate their relationship with environmental conditions. Possible effects of past climatic variability and overexploitation on the genetic diversity and structure of natural H. italicum populations are considered, as well as the importance of local adaptations to climatic conditions as important promoters of genetic differentiation.
Results
Within‑population diversity
Diversity within and between 18 populations of H. italicum was analyzed using AFLP markers. Four combinations of primers resulted in 693 polymorphic AFLP markers. The overall error rate for all combinations of selective PCR primers was 4.87%.
The Shannon information index ranged from 0.330 (P16 Živogošće) to 0.383 (P04 Rab) with an average value of 0.355 (Table 1; Fig. 1a). The average frequency of rare alleles (DW) was 50.82. The highest frequency of rare alleles (DW) was 76.10 for the population P06 Miškovići, Pag, and the lowest 33.53 for population P17 Slano (Fig. 1b). Expected heterozygosity (HE) was calculated within each of the analyzed populations based on gene frequencies assuming equilibrium according to Hardy–Weinberg (Table 1). The lowest gene diversity was found in the population P16 Živogošće (HE = 0.134), and the highest in the population P04 Rab (HE = 0.153). The total gene diversity (HT) was 0.147, and an average gene diversity within populations (HW) was 0.142.
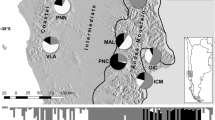
Genetic diversity and relationships among 18 H. italicum populations along eastern Adriatic coast: (a) Shannon’s information index, (b) Frequency down-weighted marker values, (c) Genetic structure derived from Bayesian analysis using STRUCTURE at K = 2, (d) the Fitch-Margoliash tree based on Nei’s genetic distance matrix between populations. Bootstrap values greater than 50% based on 1000 pseudorepeats are marked on the branches. In (a) and (b), the size of the dots is directly proportional to the depicted values. Maps were generated using QGIS 3.10.7 (https://qgis.org/).
Population differentiation and structure
The Wright coefficient of genetic differentiation (FST) at the level of all populations was 0.036. The highest value of FST was calculated between populations P04 Rab and P13 Hvar (FST = 0.069), and the lowest between populations P09 Kistanje and P10 Unešić (FST = 0.002).
The results of molecular variance analysis showed that 93.08% of diversity belongs to diversity within-population, and 6.92% to diversity between populations. The results were obtained after 10,000 permutations with significance P(φ) < 0.001. A matrix of φST values was calculated between all pairs of populations by individual analyzes (Table S1). The φST values ranged from 0.010 between P09 Kistanje and P10 Unešić to 0.127 between populations P05 Zrće, Pag, and P13 Hvar, with a mean of 0.068.
Assuming Hardy-Weinberg equilibrium, a Nei’s genetic distance was calculated between 18 H. italicum populations. The Nei’s distance varied from 0.0003 between populations P09 Kistanje and P10 Unešić to 0.0128 between populations P04 Rab and P13 Hvar, with a mean value of 0.0062. The Fitch-Margoliash tree grouped the populations according to their geographical origin. This grouping showed strong differentiation between the populations of Kvarner bay (P01 Krk, P02 Cres, P03 Lošinj, P04 Rab, P05 Pag (Zrće), P06 Pag (Miškovići), and P07 Obrovac) and the populations of Dalmatia (P08 Benkovac, P09 Kistanje, P10 Unešić, P11 Seget, P12 Brač, P13 Hvar, P14 Sinj, P15 Omiš, P16 Živogošće, P17 Slano, and P18 Cavtat) supported by bootstrap value of 96% (Fig. 1d).
The results of Bayesian model-based clustering using STRUCTURE are shown in Fig. 1c. The log-likelihood of the hypotheses [ln P(X|K)] and the rate of change between successive values of K (ΔK) for different numbers of ancestral populations (K = 1–11) were obtained. The highest value of ΔK was found at K = 2 (ΔK 386.84) followed by K = 3 (ΔK = 101.29). For K values greater than three, the average [ln P(X|K)] values decreased, while the standard deviations between the different runs for each K increased considerably and, therefore, the ΔK values were notably lower (from 6.50 to 0.08) (Fig. S1).
The proportions of membership of each individual in each of the gene pools were calculated for K = 2 based on the run with the highest [ln P(X|K)], and populations were assigned to a particular cluster (A and B). The results were congruent with those obtained using the distance-based method. At K = 2 populations mainly from North Adriatic and Kvarner Bay (P01 Krk, P02 Cres, P03 Lošinj, P04 Rab, P05 (Zrće) Pag, P06 (Miškovići) Pag, and P07 Obrovac) were assigned to the cluster A, while populations from central and south of investigated area (Dalmatia) (P08 Benkovac, P11 Seget, P13 Hvar and P15 Omiš) were assigned to the cluster B.
As maximum ΔK at K = 2 could be an artifact resulting from significantly low likelihoods for K = 151, we used the BAPS program to verify data obtained from STRUCTURE. The results showed agreement with those from STRUCTURE for K = 2. The best partitions obtained log marginal likelihoods of −89,691.83 at P = 1 (without using geographic coordinates as informative priors) and −90,102.87 at P = 1 (with spatially informative priors). Both analyzes, with or without spatially informative priors classified the populations identically into two clusters as revealed by the STRUCTURE analysis (Fig. S2).
The correlation between the matrix of FST / (1−FST) values and the matrix of natural logarithms of geographic distances (in km) between the analyzed populations was r = 0.510 and was highly significant (PMantel < 0.0001). The coefficient of determination was R2 = 0.260, revealing that 26% of the genetic differentiation between the analyzed populations can be explained by their spatial distance (Fig. S3).
Bioclimatic variation
Nineteen bioclimatic variables accounted for in this study were highly correlated. A strong positive correlation (r > 0.70) was found in 26 cases, and in five cases a strong negative correlation (r < −0.70), out of 171 pairs examined, respectively (Table S2. Principal component analysis (PCA), based on the correlation matrix, showed that the first four principal components had eigenvalues greater than 1 and together explained 93.82% of the variance (Table 2). The first principal component explained 39.36% of the total variance. A strong positive correlation with the first principal component (PC1) was found for five environmental variables (Bio01 Average annual temperature, Bio06 Minimum temperature of the coldest month, Bio09 Average temperature of the driest quarter, Bio10 Average temperature of the warmest quarter, Bio11 Average temperature of the coldest quarter). The second principal component explained 24.48% of the total variance and was positively correlated with four environmental variables (Bio12 Annual precipitation, Bio13 Precipitation in the wettest month, Bio16 Precipitation in the wettest quarter, and Bio19 Precipitation in the coldest quarter). The first principal component separated the southern Adriatic populations (P16 Živogošće, P15 Omiš, P13 Hvar and P12 Brač) from sampled sites characterized by higher temperatures and lower precipitation, from the northern Adriatic populations (P01 Krk, P02 Cres, P03 Lošinj, P04 Rab, P05 Zrće, Pag and P06 Miškovići, Pag) and central Adriatic populations (P14 Sinj, P11 Seget, P10 Unešić, P09 Kistanje, P08 Benkovac, and P07 Obrovac) where lower temperatures and higher precipitation were recorded. The second principal component separated the populations from the Kvarner Bay where higher precipitation and lower temperatures were recorded, from the populations from central Dalmatia where higher temperatures and lower precipitation were recorded (Fig. 2).
Biplot obtained by Principal Component Analysis (PCA) based on 19 bioclimatic variables for 18 H. italicum sampling sites. Red vectors represent temperature-related variables and blue vectors precipitation-related variables. Northern Adriatic H. italicum populations (P01–P06) are colored in red, central populations in green (P07–P10) and southern populations (P11–P18) in blue color.
Adaptive genetic variation
The outlier loci were detected on a set of 446 AFLP markers (markers with a frequency under 3% or above 97% were removed). With a 99% confidence level using the Mcheza program, a total of 21 outlier loci (4.71%) under possible selection were detected, of which ten (2.24%) were under positive selection (directional selection) and 11 (2.47%) under balancing selection (Fig. 3a). BayeScan identified nine (2.02%) loci under positive selection that exceeded the critical value of log10(PO) threshold used to determine the significance of loci that showed atypical values at a false-positive probability level of 0.01 [FDR < 0, 01; PO = 29.03; log10(PO) = 1.463]. No locus was under balancing selection (Fig. 3b). Mcheza and BayeScan together detected a set of seven (1.57%) markers that were potentially under positive selection. By calculating logistic regressions between all possible marker pairs and bioclimatic variable (8,474 models), the Samβada program revealed 184 (2.17%) significant models that included 50 (11.21%) markers associated with one to 13 bioclimatic variables. The Mcheza and BayeScan, identified 12 loci, and the Samβada program 41. Bioclimatic variable associated with more than 20 markers were Bio08 average temperature of the wettest quarter, Bio14 amount of precipitation in the driest month, Bio15 coefficient of precipitation variation, Bio17 amount of precipitation in the driest quarter, and Bio18 amount of precipitation in the warmest quarter (Table 3). Of 50 markers detected by Samβada, five markers were also identified by Mcheza and BayeScan to be under positive selection. Latent factor mixed model (LFMM) identified 50 markers associated with one to 15 bioclimatic variables. Out of the total 446 markers, four markers (0.89%) were identified by all four methods, as shown in the Wenn diagram (Fig. 3c). The bioclimatic variables Bio03 Isothermality, Bio08 Mean temperature of wettest quarter, Bio15 Precipitation seasonality, and Bio17 Precipitation of driest quarter, were correlated with the highest number of loci and may have played a key role in adaptive divergence in populations H. italicum in the eastern Adriatic (Table 3).
Identification of FST outlier loci using (a) Mcheza and (b) BayeScan and (c) the Venn diagram summarizing the number of loci identified as FST outlier loci by Mcheza and BayeScan and significantly associated with environmental variables by Samβada and LFMM. In (a) FST values were plotted against its heterozygosity (HE). The dashed lines represent the 99% confidence intervals. Loci under positive selection are indicated as red dots, those under balancing selection as blue dots, and neutral as grey dots. Loci under positive selection detected also by BayeScan are underlined while those identified by Samβada are shown in italics. In (b) FST values were plotted against the log10 of the posterior odds (PO). The vertical line shows the critical PO used for identifying outlier markers [FDR < 0.01; PO = 29,03; log10(PO) = 1463]. Loci under positive selection detected also by Mcheza are underlined while those identified by Samβada are shown in italics.
Six uncorrelated bioclimatic variables, four temperature-related (i.e., Bio03, Bio04, Bio08, Bio10) and two precipitation-related (i.e., Bio18, Bio19) were selected for linear model redundancy analysis (RDA). The optimal RDA model included three variables: Bio18, Bio04 and Bio03. The model was highly significant (P < 0.0001) and explained 20.17% (adjusted R2 = 0.2017) of the inherent genetic variation, indicating an important role of these environmental variables in shaping the distribution of AFLP genotypes. The first two RDA axes were significant and explained 19.82% and 8.10% of the variation, respectively. Variable Bio18 Precipitation of warmest quarter, significantly associated with RDA axis 1, contributed to the partitioning among northern, central and southern H. italicum populations. Both variables Bio03 Isothermality and Bio04 Temperature seasonality were associated with RDA axis 2 differentiating central H. italicum populations from the rest (Fig. 4).
Triplot obtained by Redundancy analysis (RDA) based on three bioclimatic variables included in the optimal RDA model showing the relative contribution of bioclimatic variables in shaping the genetic structure of 18 H. italicum populations. Red vectors represent temperature-related variables (Bio3 and Bio04) and blue vector precipitation-related variable (Bio18). Northern Adriatic H. italicum populations (P01–P06) are colored in red, central populations in green (P07–P10) and southern populations (P11–P18) in blue color. Small empty boxes represent AFLPs.
Discussion
Molecular analyzes of the genetic diversity and structure of H. italicum populations from the eastern Adriatic coast using AFLP markers revealed high intrapopulation diversity and low differentiation between populations as well as the population structure characterized by a pattern of isolation by distance. The dynamics of gene set in species is strongly influenced by life history traits such as life form, geographic range, reproductive system, seed dispersal mechanism and successional status52,53,54.
Recent studies have been conducted in the study area and have contributed significantly to the knowledge of species that are typical representatives of the Mediterranean climate, e.g., Dalmatian pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch. Bip.)55 and sage (Salvia officinalis L.)56. Our results are comparable with studies on the above species, because the species have similar life traits. They are thermophilic, xerophytic, outcrossing perennials. Although these species generally have a wider range, their distribution pattern overlaps on the eastern Adriatic coast. The overall genetic diversity of H. italicum populations, indicated by the Shannon information index, showed intermediate values (0.355) compared to the populations of S. officinalis (0.387) and T. cinerariifolium (0.223). All three species are of great economic value, either as medicinal plants or, in the case of T. cinerariifolium, as a source of the potent natural insecticide pyrethrin. Overexploitation has been observed in all three species but may have most affected the genetic diversity of T. cinerariifolium, the species that has been extensively collected over long periods. In the case of H. italicum, collection of wild populations has increased more recently, in the last decade, and the impact on overall genetic diversity will become apparent in the future. Collection of immortelle from natural habitats is legally regulated in Croatia by the Nature Protection Act57 and the Ordinance on Collection of Native Wild Species58. Nevertheless, it is necessary to systematically monitor habitats, as harvesting regulations are often not respected. Results of the research showed that differentiation between populations of H. italicum was low, indicating extensive gene flow between populations59, and compared to S. officinalis and T. cinerariifolium it was the lowest. The AMOVA results showed greater intrapopulation genetic variation in H. italicum, which is typical for outcrossing plant species54. The greater genetic variation within populations compared to variation between populations was also observed in another outcrossing plant species from the family Asteraceae Xanthium italicum Moretti, naturally distributed on Corsica60. The similar partitioning of genetic diversity between and within populations was also found in the studies by Grdiša et al.55 and Jug-Dujaković et al.56.
The results of the model-based methods showed clustering of H. italicum populations into two distinct cluster: A, with populations from the north (Kvarner Bay) and B, with populations from the central and southern part of the study area (Dalmatia). These findings are congruent with the Fitch-Margoliash tree. The eastern Adriatic coast and the coastal Dinarides were heavily glaciated at various times during the Pleistocene61,62,63, which is well documented in the study of Quaternary sediments along the north of east Adriatic coast and the islands of Krk, Rab and Pag, as well as northern Dalmatia63. Much evidence suggests that glaciers were the barriers along the eastern Adriatic coast64 and may have been the reason for the separation of these two ancestral groups for a long time. We assume that the populations of H. italicum survived in the mini-refugia (microecologically favorable pockets) during various unfavorable climatic events and reconnected when climatic conditions became favorable again. This theory is confirmed by the existence of four refugia for the eastern Adriatic coast and the coastal Dinarides out of a total of 52 recognized putative refugia in the Mediterranean65. Many authors also confirmed the existence of several Pleistocene microrefugia for this study area55,66,67,68,69,70,71,72,73,74.
The separation of populations of H. italicum was found below the Kvarner Bay, near the Zrmanja river canyon (Obrovac), which is supported in a study by Marjanac and Marjanac64 that there is evidence of glaciation in the Obrovac area. Grdiša et al.55, also identified strong genetic differentiation of the north Adriatic genetic group for T. cinerariifolium with similar phylogeographic split at Kvarner Bay, near the Zrmanja canyon. In another study, Liber et al.75 found a distinct cluster at Kvarner Bay for S. officinalis, which also suggests the existence of refugia in the northern Adriatic. In the study by Rešetnik et al.72, a phylogeographic split is found between genetic clusters of S. officinalis located further south (at the border between northern and central Dalmatia). Glasnović et al.73, in their studies of Edraianthus tenuifolius, also indicated the possible presence of these two separate "refugia within refugia" during the LGM. The results can also be compared with studies of Edraianthus tenuifolius68, Campanula pyramidalis76 and Cardamine maritime77, which showed a similar phylogeographic split, but in the area of the Neretva Valley (Central Dalmatia). The rarity (DW value) in the northwestern H. italicum populations was higher than in the other parts of the study area, indicating long-term survival of the populations in the north5. The Shannon information index of H. italicum was also higher in the northern Adriatic populations. The high rarity of the northern Adriatic populations (Kvarner Bay) was also reported by Grdiša et al.55 for T. cinerariifolium and Liber et al.75 for S. officinalis. The lower DW values of H. italicum populations from the central and southern part of the study area might indicate later dispersal.
In 18 H. italicum populations, a total of 446 AFLP markers were used to identify loci thought to be under natural selection. A total of 21 loci that exhibited atypical values (FST) (4.71%) were detected using the Mcheza program, of which 10 (2.24%) were under positive selection and 11 (2.47%) were under balancing selection, while nine (2.02%) loci under positive selection were identified using the BayeScan program. Based on the results of both programs, Mcheza and BayeScan, seven AFLP loci were under positive selection in H. italicum. Less than 5% of the large number of markers commonly examined by this method are identified as outliers18. Nosil et al.25 reported that 5–10% of loci are outliers when summarizing data from available studies. In the study of the species Mikania micrantha Kunth78, 14 outlier loci (2.9%) were identified using the Dfdist and BayeScan programs, while in the analysis of the species Eruca sativa Mill.30 nine loci were identified, but only three of them were confirmed by another method (1.6%). In mountain populations of Sideritis scardica Giseb.79, a lower number of loci were also identified under selection by BayeScan (3.10%) compared to Mcheza (5.31%). Similar results were obtained by other authors who emphasize the more conservative approach of the Bayesian method, that works more efficiently as it detects a larger number of outlier loci with a lower presence of false positives80,81,82. Therefore, it is recommended to use more methods that incorporate different approaches and use different algorithms to increase the probability that the detected loci are truly adaptive.
When adaptive loci are combined with climate variables, there is also the possibility of determining which climate factors are responsible for adaptive evolution83. The correlation between allele frequency variation and climatic variables were assessed by using the Spatial Analysis Method (Samβada), which does not depend on genetic models and operates at the individual level84. Using all three methods (Mcheza, BayeScan and Samβada), five (1.121%) loci under selection associated with bioclimatic traits were identified in H. italicum populations. Comparison with studies of other species confirms that only a small number of loci are potentially affected by natural selection. Müller et al.33 detected 11 (8.9%) outlier loci correlated to annual precipitation in the species Geropogon hybridus (L.) Sch. Bip. using the Mcheza, BayeScan and the spatial analysis method (SAM). Yang et al.21 used both frequency based (Dfdist and BayeScan) and correlation based (MLM) methods and showed that six outlier loci were strongly associated with at least one climate factor (temperature, precipitation, and radiation being the most important factors) in Liriodendron chinense (Hemsl.) Sarg. Authors Oberprieler et al.34 reported that using three methods (Mcheza, BayeScan, and Samβada) 732 AFLP loci screened revealed only 1.6% (full dataset) and 0.4% (reduced data set) of all loci were found to be under selection in Diplotaxis harra (Forssk.) Boiss. In the study of Sideritis scardica Giseb.79, seven outlier loci were identified by Mcheza, BayeScan, and Samβada and associated with bioclimatic variables with precipitation identified as a significant environmental factor driving adaptive genetic variation. Temperature and precipitation were the most important environmental factors triggering adaptation in Rhododendron oldhamii85, Keteleeria davidiana var. formosana86, Salix species87, and alpine species11,88,89,90,91. Another method, LFMM has been used to test gene-environment associations while estimating the effects of hidden factors that represent background residuals of population structure. The advantage of the LFMM method is that it has a low rate of false positives and negatives92, and it also offers the best compromise between detection capabilities and error rates when dealing with complex hierarchical neutral genetic structure93. The LFMM has recently been used in several studies. For example94 genetic divergence in Pinus bungeana Zucc. ex Endl. was investigated and six environmental variables were identified that were related to the ecological habitat of the species and were correlated with the highest number of environmentally associated loci. In the study of Li et al.95, annual mean temperature, annual precipitation and slope were considered to be the most important environmental factors associated with adaptive genetic divergence in Cunninghamia konishii Hayata. Our results obtained by combining FST—outlier analysis (Mcheza and BayeScan) and genome-environment association analysis (Samβada, LFMM) showed that the most important environmental variables for adaptive genetic divergence in H. italicum populations on the eastern Adriatic coast were: Bio3 Isothermality, Bio08 Mean temperature of wettest quarter, Bio15 Precipitation seasonality, and Bio17 Precipitation of driest quarter. The observed natural populations of H. italicum inhabit sites under Mediterranean climate characterized by hot summers and cool winters with unevenly distributed precipitation prevailing in winter. Mediterranean plants cope with drought by developing different mechanisms that allows them to survive unfavorable or insufficient precipitation distribution96. In our study, the variable Bio15 Precipitation seasonality describes the differences between minimum and maximum precipitation values; therefore, our results suggest that H. italicum populations might be adapted to large fluctuations/amplitudes in the amount of precipitation. In Mediterranean climates, large rainfall amplitudes are common, and Mediterranean species strive to adapt and survive extremes. As Mediterranean region is highly vulnerable to climate change96 future projections for global climate change state that more uneven temporal distributions of precipitation are expected97. Climate models suggest that the hydrological cycle will intensify due to rising temperatures and increasing evaporation, leading to more storms and precipitation in particular areas, while drought will occur in areas far from storms98. According to Giorgi and Lionello99 and Matusik et al.100 the decrease of precipitation and increase of warming is expected in the future, together with increased inter-annual variability of both precipitation and temperature, which will cause greater frequency of extremely arid periods. Plant adaptation to seasonal water stress in Mediterranean climate is an important driver of genetic differentiation96.
The RDA approach used to study the contribution of bioclimatic variables to the genetic structure of natural populations of H. italicum also identified Bio03 Isothermality as important bioclimatic variable, together with Bio04 Temperature seasonality and Bio18 Precipitation of the warmest quarter as the main bioclimatic variables distinguishing northern, southern and central Adriatic populations. The variable Bio18 Precipitation of the warmest quarter contributed to the partitioning of the northern populations of H. italicum, as they are exposed to greater amounts of precipitation during the warmer months, leading to an adjustment of the populations in this direction. The two variables Bio03 Isothermality and Bio04 Temperature seasonality influenced the differentiation of the central H. italicum populations from the rest. Both variables are a measure of temperature heterogeneity, with Bio03 Isothermality quantifying how temperatures vary in relation to annual oscillations, while Bio04 temperature seasonality measures temperature changes throughout the year101. As mentioned above, the Mediterranean region is characterized by strong seasonality, both in temperature and precipitation, and the climate results in strong, opposing pressures on species102. Seasonality is an important component of climate that influences the availability of resources and thus the distribution of species in the environment at both temporal and spatial scales103. Central inland H. italicum populations cope with the wide range of daytime and nighttime temperatures, as well as cold winters and high summer temperatures, and are most likely adapted to temperature fluctuations, making them the most likely candidates for species persistence under ongoing climate change.
The development of numerous genome scanning and spatial statistical methods has facilitated analyzes and our knowledge of adaptability in non-model species, but there are some limitations to these methods. AFLPs are useful marker systems with high reproducibility and ability for discovering polymorphism without previous information of the genome89, but they are also poorly informative because they are dominant and biallelic, involving only reduced and anonymous part of the genome. The key weakness of genome scans is that they often detect false positives due to deviations from Hardy–Weinberg equilibrium and population structure model assumption104. Demographic events such as bottleneck, allele surfing during population expansion, secondary contact, and isolation by distance can mimic selection12,105, making it difficult to differ selection from demography106 and consequently to draw inferences about selection. Sampling strategy helps reduce occurrence of false positives by including abundant number of sampled individuals, more than 10 per site for allele frequency-based methods (e.g., FST)107,108. Another way to reduce number of false positives and have more confidence in the outliers found is to use different approaches simultaneously and set strict thresholds, as was performed in this research. Four (0.89%) loci detected by all four methods (Mcheza, BayeScan, Samβada and, LFMM) are expected since only small number of them are affected by natural selection. These loci are possibly linked to genes under selection due to the the ‘hitchhiking effect’, but their location and function is unknown due to a lack of prior knowledge about the genome structure of the species under study. Therefore, this research is the first step in finding evidence for adaptive divergence of H. italicum and additional analyzes involving the discovery of the location and function of detected loci are needed. Adaptation to precipitation and temperature oscillations in H. italicum populations appears to be the most important trait to be further investigated using genotype–phenotype association studies.
The analyzes performed in this study and those proposed for future investigations could be the basis for future conservation strategies of H. italicum on eastern Adriatic coast. Extended sampling in the Mediterranean region should also be included in future analyses as the results obtained and conclusion derived in this study may not be applicable to the entire distribution range of the species. On the eastern Adriatic coast, in addition to longer-term problems such as degradation, habitat loss and climate change, increased demand for H. italicum has led to overharvesting, which could also lead to a long-term decline in genetic diversity. Evaluation of genetic diversity allows the identification of populations that have lower genetic diversity and are more vulnerable so conservation measures can be focused on them. Conservation of entire habitats through monitoring in the wild (in situ) can help legislators respond in a timely manner by adopting regulatory measures regarding the amounts of plant material allowed to be collected. Ex situ conservation should include seed banks with special attention to covering the most genetic diversity of the species109. Another possible solution is to promote the cultivation of H. italicum based on knowledge gained from molecular analysis and the adaptive potential of the species, which should be incorporated into breeding programs.
Materials and methods
Sampling and plant material
Leaf tissue from twenty-five individuals from 18 wild H. italicum populations was collected in July 2014 and 2015. In addition, the seed samples of each individual were collected and stored in the Collection of Medicinal and Aromatic Plants at the University of Zagreb, Faculty of Agriculture under the Accession numbers MAP02672-MAP02689 (data available at the Croatian plant Genetic Resources Database; https://cpgrd.hapih.hr/). The collection of plant and seed specimens was carried out within the National Programme for Conservation and Sustainable Use of Plant Genetic Resources for Food and Agriculture in the Republic of Croatia and therefore in accordance with relevant institutional, national, and international guidelines and legislation. Voucher specimens were identified by Tonka Ninčević and Marija Jug -Dujaković and are deposited in the ZAGR Virtual Herbarium, Zagreb, Croatia (available at: http://herbarium.agr.hr/; Herbarium IDs: 38977, 38981, 38983, 44230–44237, 59856–59862). Sampling sites are listed in Table 1. They were selected to represent the distribution range of H. italicum along the environmental gradient from northwest to southeast on the eastern Adriatic coast. Populations P01 to P06 were sampled in the northern part of the study area, populations P07–P10 in the central part and populations P11 to P18 in the southern part of the study area. The average distance between sampling sites was 145.83 km, with minimum and maximum distance of 7.93 km and 415.94 km, respectively. The climatic data on precipitation and temperature conditions of each sampling site were taken from the WorldClim database at a spatial resolution of approximately 1 km2110 (available at: www.worldclim.org). Generally, the average annual temperature increases from the northern to the southern parts of the study area, while the amount of precipitation decreases. Precipitation deficit becomes greater and lasts longer from the northern to southern part of the eastern Adriatic coast111.
DNA extraction and AFLP fingerprinting
DNA samples were extracted from 23 to 25 individuals per each of the 18 populations. Total genomic DNA was isolated from 25 mg of silica gel dried leaf tissue using a DNeasy Plant Mini Kit (Qiagen®). DNA concentrations were measured using a P300 NanoPhotometer (Implen®).
The AFLP analysis was done as proposed in Vos et al.112 with minor modifications proposed in Carović-Stanko et al.113. Four combinations of selective primers were chosen for amplification: FAM-EcoRI-ACA + MseI-CAC, NED-EcoRI-AGA + MseI-CAC, VIC-EcoRI-ACG + MseI-CGA and PET-EcoRI-AGC + MseI-CGA. The amplified fragments were separated by capillary electrophoresis in an ABI3130xl Genetic Analyzer (Applied Biosystems®, Foster City, CA, USA).
Data analysis
Within-population diversity
AFLP fragments were scored using the GeneMapper v. 4.0 software (Applied Biosystems) and fragments from 100 to 500 bp were analyzed. Error rates were estimated using the scanAFLP v. 1.2114. Genetic diversity within the populations was estimated by calculatating the percentage of polymorphic bands (%P), the number of private alleles (Npr) and the Shannon's information index (I)115. The Shannon’s information index was calculated as I = − Σ (pi log2 pi), where pi is the phenotypic frequency115. The frequency down-weighted marker values (DW) were calculated according to Schönswetter and Tribsch5 using AFLPdat116 in R117.
Population differentiation and structure
Analysis of molecular variance (AMOVA118) was used to partition the total AFLP diversity between and within H. italicum populations. Analysis was performed using the program Arlequin v. 3.5.2.2119 and the significance of the φST values was calculated based on 10,000 permutations. Pairwise population comparisons examined with AMOVA yielded a matrix of φST values corresponding to the proportion of total variance shared between two populations that can be interpreted as the distance average between any two populations120.
Based on the frequency of amplified fragments of each AFLP marker in each analyzed population, allelic frequencies were calculated using the Bayesian method with a non-uniform prior distribution of allele frequencies according to Zhivotovsky121, implemented in AFLP-Surv v. 1.0122. We assumed that populations were in Hardy–Weinberg equilibrium (FIS = 0) due to the outcrossing nature of H. italicum. The calculated allele frequencies were used to analyze genetic diversity within and between populations as described in Lynch and Milligan123. Total gene diversity (HT), average gene diversity within populations (HW), average gene diversity between populations that exceeds that observed within populations (HB), and Wright’s FST were used to describe the genetic structure of populations.
Standard Nei genetic distance (DNEI72) was calculated between all populations pairs of using AFLP-Surv v. 1.0122. An unrooted Fitch–Margoliash tree based on pairwise Nei’s standard genetic distances between populations was created using the FITCH program v. 3.6b (PHYLIP124). The bootstrap method was used to create 1,000 distance matrices using the AFLP-Surv, and the bootstrap values were calculated using the FITCH and CONSENSE programs within PHYLIP software package.
The genetic structure of H. italicum populations was assessed using two Bayesian model-based clustering methods as implemented in STRUCTURE v. 2.3.4125 and BAPS v. 6126,127. In STRUCTURE, the number of clusters (K) was set from 1 to 11 and 30 runs per K were performed on the Isabella computer cluster at the University of Zagreb, University Computing Centre (SRCE). An admixture model with correlated allele frequencies was applied with a burn-in period of 200,000 steps and 1,000,000 MCMC replicates after burn-in. The detection of the most likely number of clusters was performed as described by Evanno et al.128, and implemented in STRUCTURE HARVESTER v. 0.6.94129. Results from independent runs were clustered and averaged using Clumpak130 to obtain the Q-value matrix.
Another Bayesian model-based analysis was performed using BAPS126,127 to verify data obtained from STRUCTURE. Mixture analysis was performed both without the geographic coordinates as an informative prior (‘Clustering of individuals’) and with this prior (‘Spatial clustering of individuals’131. BAPS was run with a maximal number of clusters (K) set to 20 with each run replicated 10 times. The best K value was assessed using the log marginal likelihood values of the best partitions and the distribution of posterior probabilities for different K values. To detect admixture between clusters the results of the mixture analysis were used as input to the population admixture analysis132 with default settings.
Isolation by distance133 was performed by the Mantel's test based on 10,000 permutations using the program NTSys-pc v.2.10 s134 between the matrix of FST / (1−FST) values and the matrix of natural logarithms of geographic distances (in km) between the analyzed populations.
Bioclimatic variation and adaptation
Values of 19 environmental variables (11 temperature-related and eight precipitation-related) representing the annual trends, seasonal variations, and extremes in temperature and precipitation, for each 18 H. italicum sampling site are shown in Table S3. A principal component analysis (PCA) was performed on 19 environmental variables and a biplot with two principal components (PC) showing sampled populations and environmental variables (as vectors) was constructed. To identify candidate loci under selection we applied: (i) a frequentist approach as described by Beaumont and Nichols135 and implemented in Mcheza136, (ii) a Bayesian approach as implemented in BayeScan v. 2.0124, and (iii) a spatial analysis method27 implemented in Samβada v. v0.5.184, (iv) a latent factor mixed model (LFMM)92 implemented in R package lfmm137, and (v) a linear model redundancy analysis (RDA)138 implemented in R package vegan v. 2.5–7139. Analyzes were restricted to loci with dominant allele frequencies between 5 and 95% across the whole dataset to avoid a bias in global FST estimates140.
The methodology used in Mcheza is based on the assumption that loci under directional selection have significantly higher FST values than the majority of neutral loci in a sample. In contrast, loci under balancing selection are expected to exhibit significantly lower FST values. The neutral distribution of FST values was determined by 1,000,000 iterations using the 'Neutral mean FST' and 'Force mean FST options. Outlier loci were selected with a confidence interval (CI) of 99% and a false discovery rate (FDR) of 0.1135,136. BayeScan evaluates individual loci within a hierarchical Bayesian model that decomposes genetic variation into population- and locus-specific effects. For each locus, two models are defined that include or exclude the effect of natural selection. The posterior probabilities of these two models are then estimated using a reversible-jump MCMC approach. We used 20 pilot runs with 5,000 iterations to fit the proposal distribution to acceptance rates between 0.25 and 0.45. Analyzes were performed with a burn-in of 50,000 iterations, a sample size of 10,000, and a thinning interval of 50, resulting in a total number of 550,000 iterations. The logarithm of Posterior Odds [log10(PO)] greater than 1.5 was considered as a 'very strong' evidence for selection24,141. The false discovery rate142 (FDR) was set to 0.01 to fit the corresponding log10(PO) significance threshold.
In Samβada, the spatial analysis method was used to calculate multiple univariate logistic regressions to test the probability of the presence of an allelic variant given the values of environmental variables of the sampling sites. Significance was assessed with both the log-likelihood G ratio and Wald test using Bonferroni correction (P < 0.01). A model was considered significant only if both tests rejected the corresponding null hypothesis84.
Gene-environment associations have also been identified using latent factor mixed models (LFMM92,143) implemented in the R package lfmm137. In LFMMs, associations between genetic variation and environmental variables are tested, while estimating the effects of hidden factors representing background residual levels of population structure. Thus, in LFMMs, environmental variables are introduced as fixed effects, while population structure is modeled by latent factors. The number of latent factors (K) was set to two based on the results of STRUCTURE and BAPS analyses. Regularized least squares were calculated using a ridge penalty (lfmm_ridge) and estimated the genomic inflation factor (GIF) was estimated based on median z-scores (lfmm_test). To account for multiple testing, p values were converted to q values using the R package qvalue144. Significant associations were selected based on a false discovery rate (FDR) of 5% (q < 0.05).
Linear model redundancy analysis (RDA), implemented in the R package vegan v. 2.5–7139, was used to analyse the effects of environmental variables on AFLP variation among populations. The highly correlated environmental variables (|r|> 0.70) were excluded before analysis. Hellinger-transformed allele frequencies145 were calculated in vegan based on the AFLP allele frequencies estimated using AFLP-Surv. The optimal model was determined using the ordiR2step function in vegan using a forward selection procedure with 10,000 permutations. To test the significance of the RDA model and constrained-axis, the vegan function anova.cca was run with 10,000 permutations.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Médail, F. & Myers, N. Mediterranean Basin (in Mittermeier, C G Lamoreux, J Fonseca, G A B). Sierra 144–147 (2004).
Médail, F. & Quézel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Missouri Bot. Gard. 84, 112–127 (1997).
Blondel, J. & Medail, F. Biodiversity and conservation. in The Physical Geography of the Mediterranean (ed. Woodward J. C.) 615–650 (Oxford University Press, 2009).
Médail, F. & Diadema, K. Biodiversité végétale méditerranéenne et anthropisation : approches macro et micro-régionales. Ann. Georgr. 651, 618–640 (2006).
Schönswetter, P. & Tribsch, A. Vicariance and dispersal in the alpine perennial Bupleurum stellatum L. (Apiaceae). Taxon 54, 725–732 (2005).
Wielstra, B. et al. Tracing glacial refugia of Triturus newts based on mitochondrial DNA phylogeography and species distribution modeling. Front. Zool. 10, (2013).
Hewitt, G. M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 58, 247–276 (1996).
Hewitt, G. M. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 68, 87–112 (1999).
Butchart, S. H. M. et al. Global biodiversity: indicators of recent declines. Science (80-. ). 328, 1164–1168 (2010).
Rands, M. R. W. et al. Biodiversity conservation: challenges beyond 2010. Science 1298–1303 (2010). doi:https://doi.org/10.1126/science.1189138
Manel, S., Poncet, B. N., Legendre, P., Gugerli, F. & Holderegger, R. Common factors drive adaptive genetic variation at different spatial scales in Arabis alpina. Mol. Ecol. 19, 3824–3835 (2010).
Schoville, S. D. et al. Adaptive genetic variation on the landscape: methods and cases. Annu. Rev. Ecol. Evol. Syst. 43(43), 23–43 (2012).
Yeaman, S. & Whitlock, M. C. The genetic architecture of adaptation under migration-selection balance. Evolution (N. Y). 65, 1897–1911 (2011).
Savolainen, O., Pyhäjärvi, T. & Knürr, T. Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. System. 595–619 (2007). doi:https://doi.org/10.1146/annurev.ecolsys.38.091206.095646
Neale, D. B. & Kremer, A. Forest tree genomics: growing resources and applications. Nat. Rev. Genet. 111–122 (2011). doi:https://doi.org/10.1038/nrg2931
Aitken, S. N. & Whitlock, M. C. Assisted gene flow to facilitate local adaptation to climate change. Annu. Rev. Ecol. Evol. System. 367–388 (2013). doi:https://doi.org/10.1146/annurev-ecolsys-110512-135747
Nielsen, R. Molecular signatures of natural selection. Annu. Rev. Genet. 39, 197–218 (2005).
Hoffmann, A. A. & Willi, Y. Detecting genetic responses to environmental change. Nat. Rev. Genet. 9, 421–432 (2008).
Bonin, A., Ehrich, D. & Manel, S. Statistical analysis of amplified fragment length polymorphism data: a toolbox for molecular ecologists and evolutionists. Mol. Ecol. 16, 3737–3758 (2007).
Fischer, M. C., Foll, M., Excoffier, L. & Heckel, G. Enhanced AFLP genome scans detect local adaptation in high-altitude populations of a small rodent (Microtus arvalis). Mol. Ecol. 20, 1450–1462 (2011).
Yang, A. H., Wei, N., Fritsch, P. W. & Yao, X. H. AFLP genome scanning reveals divergent selection in natural populations of Liriodendron Chinense (Magnoliaceae) along a latitudinal transect. Front. Plant Sci. 7, 698 (2016).
Lewontin, R. C. & Krakauer, J. Distribution of gene frequency as a test of the theory of the selective neutrality of polymorphisms. Genetics 74, 175–195 (1973).
Storz, J. F. Using genome scans of DNA polymorphism to infer adaptive population divergence. Mol. Ecol. 14, 671–688 (2005).
Foll, M. & Gaggiotti, O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: a Bayesian perspective. Genetics 180, 977–993 (2008).
Nosil, P., Funk, D. J. & Ortiz-Barrientos, D. Divergent selection and heterogeneous genomic divergence. Mol. Ecol. 18, 375–402 (2009).
Strasburg, J. L. et al. What can patterns of differentiation across plant genomes tell us about adaptation and speciation?. Philos. Trans. R. Soc. B-Biological Sci. 367, 364–373 (2012).
Joost, S. et al. A spatial analysis method (SAM) to detect candidate loci for selection: towards a landscape genomics approach to adaptation. Mol. Ecol. 16, 3955–3969 (2007).
Schmidt, P. S. et al. Ecological genetics in the North Atlantic: environmental gradients and adaptation at specific loci. in Ecology 91–107 (2008). doi:https://doi.org/10.1890/07-1162.1
Holderegger, R., Buehler, D., Gugerli, F. & Manel, S. Landscape genetics of plants. Trends in Plant Science 675–683 (2010). doi:https://doi.org/10.1016/j.tplants.2010.09.002
Westberg, E., Ohali, S., Shevelevich, A., Fine, P. & Barazani, O. Environmental effects on molecular and phenotypic variation in populations of Eruca sativa across a steep climatic gradient. Ecol Evol 3, 2471–2484 (2013).
Dillon, S. et al. Characterisation of adaptive genetic diversity in environmentally contrasted populations of Eucalyptus camaldulensis Dehnh. (river red gum). PLoS One 9, e103515 (2014).
Brousseau, L. et al. Local adaptation in European firs assessed through extensive sampling across altitudinal gradients in southern Europe. PLoS One 11, e0158216 (2016).
Muller, C. M. et al. Geropogon hybridus (L.) Sch.Bip. (Asteraceae) exhibits micro-geographic genetic divergence at ecological range limits along a steep precipitation gradient. Plant Syst. Evol. 303, 91–104 (2017).
Oberprieler, C., Zimmer, C. & Bog, M. Are there morphological and life-history traits under climate-dependent differential selection in S Tunesian Diplotaxis harra (Forssk.) Boiss. (Brassicaceae) populations? Ecol Evol 8, 1047–1062 (2018).
Wang, J. et al. A major locus controls local adaptation and adaptive life history variation in a perennial plant. Genome Biol. 19, (2018).
López, A. S., López, D. R., Caballé, G., Siffredi, G. L. & Marchelli, P. Local adaptation along a sharp rainfall gradient occurs in a native Patagonian grass, Festuca pallescens, regardless of extensive gene flow. Environ. Exp. Bot. 171, 103933 (2020).
Galbany-Casals, M., Blanco-Moreno, J. M., Garcia-Jacas, N., Breitwieser, I. & Smissen, R. D. Genetic variation in Mediterranean Helichrysum italicum (Asteraceae; Gnaphalieae): Do disjunct populations of subsp. microphyllum have a common origin? Plant Biol. 13, 678–687 (2011).
The Plant List. The Plant List. Version 1.1. Internet (2013). Available at: http://www.theplantlist.org/. Accessed 7 Jan 2021
Herrando-Moraira, S., Blanco-Moreno, J. M., Sáez, L. & Galbany-Casals, M. Re-evaluation of the Helichrysum italicum complex (Compositae: Gnaphalieae): a new species from majorca (Balearic Islands). Collect. Bot. 35, (2016).
Guinoiseau, E. et al. Biological properties and resistance reversal effect of Helichrysum italicum ( Roth ) G . Don. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education (ed. Méndez Vilas, A.) 1073–1080 (Formatex Research Center, 2013).
Maksimovic, S., Tadic, V., Skala, D. & Zizovic, I. Separation of phytochemicals from Helichrysum italicum: an analysis of different isolation techniques and biological activity of prepared extracts. Phytochemistry 138, 9–28 (2017).
Ninčević, T., Grdiša, M., Šatović, Z. & Jug-Dujaković, M. Helichrysum italicum (Roth) G. Don: Taxonomy, biological activity, biochemical and genetic diversity. Ind. Crops Prod. 138, 111487 (2019).
Angioni, A. et al. Chemical composition, plant genetic differences, and antifungal activity of the essential oil of Helichrysum italicum G. Don ssp. microphyllum (Willd) Nym. J. Agric. Food Chem. 51, 1030–1034 (2003).
Scialabba, A., Agrimonti, C., Abbate, G. M. & Marmiroli, N. Assessment of genetic variation in Sicilian Helichrysum (Asteraceae) and implication to germplasm conservation. Plant Biosyst. 142, 287–297 (2008).
Morone-Fortunato, I. et al. Essential oils, genetic relationships and in vitro establishment of Helichrysum italicum (Roth) G. Don ssp. italicum from wild Mediterranean germplasm. Ind. Crops Prod. 32, 639–649 (2010).
Melito, S. et al. Genetic and metabolite diversity of Sardinian populations of Helichrysum italicum. PLoS One 8, e79043 (2013).
Lange, D. Europe’s medicinal and aromatic plants: their use, trade and conservation. Europe’s Medicinal and Aromatic Plants: their Use, Trade and Conservation (TRAFFIC International, 1998).
Šatović, Z. et al. Conservation of medicinal and aromatic plants in Croatia. NATO Sci. Peace Secur. Ser. C Environ. Secur. (2012). doi:https://doi.org/10.1007/978-94-007-2953-7_24
Yuan, Q. J. et al. Impacts of recent cultivation on genetic diversity pattern of a medicinal plant, Scutellaria baicalensis (Lamiaceae). BMC Genet. 11, (2010).
Yamagishi, M., Nishioka, M. & Kondo, T. Phenetic diversity in the Fritillaria camschatcensis population grown on the Sapporo campus of Hokkaido University. Landsc. Ecol. Eng. 6, (2010).
Vigouroux, Y. et al. Population structure and genetic diversity of New World maize races assessed by DNA microsatellites. Am. J. Bot. 95, 1240–1253 (2008).
Loveless, M. D. & Hamrick, J. L. Ecological determinants of genetic structure in plant populations. Annu. Rev. Ecol. Syst. 15(15), 65–95 (1984).
Hamrick, J. L. & Godt, M. J. W. Conservation genetics of endemic plant species. In Conservation Genetics: Case Histories from Nature (eds. Avise, J. C. & Hamrick, J. L.) 281–304 (Chapman and Hall, 1996). doi:https://doi.org/10.1007/978-1-4757-2504-9_9
Nybom, H. & Bartish, I. V. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect. Plant Ecol. Evol. Syst. 3, 93–114 (2000).
Grdiša, M. et al. Genetic Diversity and Structure of Dalmatian Pyrethrum (Tanacetum cinerariifolium Trevir./Sch./Bip., Asteraceae) within the Balkan Refugium. PLoS One 9, e105265 (2014).
Jug-Dujaković, M., Ninčević, T., Liber, Z., Grdiša, M. & Šatović, Z. Salvia officinalis survived in situ Pleistocene glaciation in ‘refugia within refugia’ as inferred from AFLP markers. Plant Syst. Evol. 306, 38 (2020).
Official Gazette. Croatian Nature Protection Act. 80/13, (2013).
Official Gazette. The Croatian ordinance on Collection of Native Wild Species. 144/17, (2017).
Wright, S. The genetical structure of populations. Ann. Eugen. 15, 323–354 (1951).
Tang, J. S. & Ma, M. Genetic diversity and genetic differentiation of invasive weed Xanthium italicum in China. C. R. Biol. 343, 63–72 (2020).
Marjanac, L. & Marjanac, T. Glacial history of the Croatian Adriatic and Coastal Dinarides. In Quartenary Glaciations—Extent and Chronology, Part I: Europe: Developments in Quaternary Science (eds. Ethelrs, J. & Gibbard, P. L.) 19–26 (Elsevier, 2004). doi:https://doi.org/10.1016/S1571-0866(04)80053-8
Marjanac, L. Pleistocene glacial and periglacial sediments of Kvarner, Northern Dalmatia and Southern Velebit Mt.—evidence of Dinaric glaciation. (Faculty of Science, University of Zagreb, 2012).
Hughes, P. D., Woodward, J. C., van Calsteren, P. C. & Thomas, L. E. The glacial history of the Dinaric Alps, Montenegro. Quat. Sci. Rev. 30, 3393–3412 (2011).
Marjanac, L. & Marjanac, T. Sedimentological evidence of extensive Dinaric glaciation. In Book of Abstracts 3rd Scientific INQUA Meeting Quaternary Geology in Croatia with International Participation (eds. Marjanac, L. & Mauch Lenardić, J.) 37–38 (Hrvatska akademija znanosti i umjetnosti, Geološki zavod Slovenije, 2013).
Medail, F. & Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 36, 1333–1345 (2009).
Bardy, K. E., Albach, D. C., Schneeweiss, G. M., Fischer, M. A. & Schönswetter, P. Disentangling phylogeography, polyploid evolution and taxonomy of a woodland herb (Veronica chamaedrys group, Plantaginaceae s.l.) in southeastern Europe. Mol. Phylogenet. Evol. 57, 771–786 (2010).
Kucera, J., Marhold, K. & Lihova, J. Cardamine maritima group (Brassicaceae) in the amphi-Adriatic area: a hotspot of species diversity revealed by DNA sequences and morphological variation. Taxon 59, 148–164 (2010).
Surina, B., Schonswetter, P. & Schneeweiss, G. M. Quaternary range dynamics of ecologically divergent species (Edraianthus serpyllifolius and E. tenuifolius, Campanulaceae) within the Balkan refugium. J. Biogeogr. 38, 1381–1393 (2011).
Slovák, M., Kučera, J., Turis, P. & Zozomová-Lihová, J. Multiple glacial refugia and postglacial colonization routes inferred for a woodland geophyte, Cyclamen purpurascens: patterns concordant with the Pleistocene history of broadleaved and coniferous tree species. Biol. J. Linn. Soc. 105, 741–760 (2012).
Temunović, M. et al. Environmental heterogeneity explains the genetic structure of continental and mediterranean populations of Fraxinus angustifolia Vahl. PLoS One 7, e42764 (2012).
Kutnjak, D. et al. Escaping to the summits: phylogeography and predicted range dynamics of Cerastium dinaricum, an endangered high mountain plant endemic to the western Balkan Peninsula. Mol. Phylogenet. Evol. 78, 365–374 (2014).
Rešetnik, I. et al. Genetic diversity and demographic history of wild and cultivated/naturalised plant populations: evidence from Dalmatian Sage (Salvia officinalis L., Lamiaceae). PLoS One 11, e0159545 (2016).
Glasnovic, P. et al. Understanding biogeographical patterns in the western Balkan Peninsula using environmental niche modelling and geostatistics in polymorphic Edraianthus tenuifolius. AoB Plants 10, (2018).
Radosavljević, I. et al. Morphological, genetic and epigenetic aspects of homoploid hybridization between Salvia officinalis L. and Salvia fruticosa Mill. Sci. Rep. 9, 1–13 (2019).
Liber, Z. et al. Genetic diversity of dalmatian sage (Salvia officinalis L.) as assessed by RAPD markers. Agric. Conspec. Sci. 79, 77–84 (2014).
Lakušić, D. et al. Molecular phylogeny of the Campanula pyramidalis species complex (Campanulaceae) inferred from chloroplast and nuclear non-coding sequences and its taxonomic implications. Taxon 62, 505–524 (2013).
Kucera, J., Tremetsberger, K., Vojta, J. & Marhold, K. Molecular study of the Cardamine maritima group (Brassicaceae) from the Balkan and Apennine Peninsulas based on amplified fragment length polymorphism. Plant Syst. Evol. 275, 193–207 (2008).
Wang, T., Chen, G., Zan, Q., Wang, C. & Su, Y. juan. AFLP genome scan to detect genetic structure and candidate loci under selection for local adaptation of the invasive weed Mikania micrantha. PLoS One 7, e41310 (2012).
Grdiša, M. et al. Divergent selection and genetic structure of Sideritis scardica populations from southern Balkan Peninsula as revealed by AFLP fingerprinting. Sci. Rep. 9, 12767 (2019).
Pérez-Figueroa, A., García-Pereira, M. J., Saura, M., Rolán-Alvarez, E. & Caballero, A. Comparing three different methods to detect selective loci using dominant markers. J. Evol. Biol. 23, 2267–2276 (2010).
Narum, S. R. & Hess, J. E. Comparison of FST outlier tests for SNP loci under selection. Mol. Ecol. Resour. 11, 184–194 (2011).
Vilas, A., Pérez-Figueroa, A. & Caballero, A. A simulation study on the performance of differentiation-based methods to detect selected loci using linked neutral markers. J. Evol. Biol. 25, 1364–1376 (2012).
Wang, J., Street, N. R., Scofield, D. G. & Ingvarsson, P. K. Natural selection and recombination rate variation shape nucleotide polymorphism across the genomes of three related populus species. Genetics 202, 1185–1200 (2016).
Stucki S. & Joost. Samβada: User manual, Version v0.5.1. (2015).
Huang, C. L. et al. Influences of environmental and spatial factors on genetic and epigenetic variations in Rhododendron oldhamii (Ericaceae). Tree Genet. Genomes 11, 823 (2015).
Fang, J. Y. et al. Divergent Selection and Local Adaptation in Disjunct Populations of an Endangered Conifer, Keteleeria davidiana var. formosana (Pinaceae). PLoS One 8, e70162 (2013).
Huang, C. L. et al. Genetic relationships and ecological divergence in Salix species and populations in Taiwan. Tree Genet. Genomes 11, (2015).
Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. (Springer-Verlag, 2003). doi:https://doi.org/10.1659/0276-4741(2001)021[0202:aplfpe]2.0.co;2
Poncet, B. N. et al. Tracking genes of ecological relevance using a genome scan in two independent regional population samples of Arabis alpina. Mol. Ecol. 19, 2896–2907 (2010).
Bothwell, H. et al. Identifying genetic signatures of selection in a non-model species, alpine gentian (Gentiana nivalis L.), using a landscape genetic approach. Conserv. Genet. 14, 467–481 (2013).
Di Pierro, E. A. et al. Climate-related adaptive genetic variation and population structure in natural stands of Norway spruce in the South-Eastern Alps. Tree Genet. Genomes 12, (2016).
Frichot, E., Schoville, S. D., Bouchard, G. & François, O. Testing for associations between loci and environmental gradients using latent factor mixed models. Mol. Biol. Evol. 30, 1687–1699 (2013).
De Villemereuil, P., Frichot, É., Bazin, É., François, O. & Gaggiotti, O. E. Genome scan methods against more complex models: when and how much should we trust them?. Mol. Ecol. 23, 2006–2019 (2014).
Zhang, X. X. et al. Landscape genetics reveals that adaptive genetic divergence in Pinus bungeana (Pinaceae) is driven by environmental variables relating to ecological habitats. BMC Evol. Biol. 19, 1–13 (2019).
Li, Y. S., Shih, K. M., Chang, C. T., Chung, J. D. & Hwang, S. Y. Testing the effect of mountain ranges as a physical barrier to current gene flow and environmentally dependent adaptive divergence in Cunninghamia konishii (Cupressaceae). Front. Genet. 10, 742 (2019).
Nardini, A., Lo Gullo, M. A., Trifilò, P. & Salleo, S. The challenge of the Mediterranean climate to plant hydraulics: responses and adaptations. Environ. Exp. Bot. 103, 68–79 (2014).
Allan, R. P. & Soden, B. J. Atmospheric warming and the amplification of precipitation extremes. Science (80-. ). 321, 1481–1484 (2008).
NASA’s Jet Propulsion Laboratory. Climate Change and Global Warming. Internet (2021). Available at: https://climate.nasa.gov/.
Giorgi, F. & Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Change 63, 90–104 (2008).
Matusick, G., Ruthrof, K. X., Brouwers, N. C., Dell, B. & Hardy, G. S. J. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. Eur. J. For. Res. 132, 497–510 (2013).
O’Donnell, M. S. & Ignizio, D. A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States. (2012).
Médail, F. Mediterranean. in Encyclopedia of Ecology 2296–2308 (Academic Press, 2008). doi:https://doi.org/10.1016/B978-008045405-4.00348-7
Dayton, G. H. Seasonality. in Encyclopedia of Ecology 3168–3171 (Elsevier Inc., 2008). doi:https://doi.org/10.1016/B978-008045405-4.00545-0
Excoffier, L., Hofer, T. & Foll, M. Detecting loci under selection in a hierarchically structured population. Heredity (Edinb). 103, 285–298 (2009).
Hahn, M. W. Toward a selection theory of molecular evolution. Evolution (N. Y). 62, 255–265 (2008).
Li, H. P. A new test for detecting recent positive selection that is free from the confounding impacts of demography. Mol. Biol. Evol. 28, 365–375 (2011).
Lotterhos, K. E. & Whitlock, M. C. The relative power of genome scans to detect local adaptation depends on sampling design and statistical method. Mol. Ecol. 24, 1031–1046 (2015).
Hoban, S. et al. Finding the genomic basis of local adaptation: pitfalls, practical solutions, and future directions. Am. Nat. 188, 379–397 (2016).
Laguna, E. et al. The role of small reserves in plant conservation in a region of high diversity in eastern Spain. Biol. Conserv. 119, 421–426 (2004).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Zaninović, K. et al. Climate atlas of Croatia 1961–1990., 1971–2000. (State Hydrometeorological Institute, 2008).
Vos, P. et al. Aflp: a new technique for DNA-fingerprinting. Nucleic Acids Res. 23, 4407–4414 (1995).
Carović-Stanko, K. et al. Molecular and chemical characterization of the most widespread Ocimum species. Plant Syst. Evol. 294, 253–262 (2011).
Herrmann, D. et al. Selection criteria for scoring amplified fragment length polymorphisms (AFLPs) positively affect the reliability of population genetic parameter estimates. Genome 53, 302–310 (2010).
Lewontin, R. C. The apportionment of human diversity. Evol. Biol. 6, 381–398 (1972).
Ehrich, D. AFLPDAT: a collection of R functions for convenient handling of AFLP data. Mol. Ecol. Notes 6, 603–604 (2006).
R Core Development Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2019).
Excoffier, L., Smouse, P. E. & Quattro, J. M. Analysis of Molecular Variance Inferred from Metric Distances among DNA Haplotypes - application to Human Mitochondrial-DNA Restriction Data. Genetics 131, 479–491 (1992).
Excoffier, L. & Lischer, H. E. L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567 (2010).
Huff, D. R. RAPD characterization of heterogeneous perennial ryegrass cultivars. Crop Sci. 37, 557–564 (1997).
Zhivotovsky, L. A. Estimating population structure in diploids with multilocus dominant DNA markers. Mol. Ecol. 8, 907–913 (1999).
Vekemans, X., Beauwens, T., Lemaire, M. & Roldan-Ruiz, I. Data from amplified fragment length polymorphism (AFLP) markers show indication of size homoplasy and of a relationship between degree of homoplasy and fragment size. Mol. Ecol. 11, 139–151 (2002).
Lynch, M. & Milligan, B. G. Analysis of population genetic-structure with Rapd markers. Mol. Ecol. 3, 91–99 (1994).
Felsenstein, J. PHYLIP (Phylogenetic Inference Package, version 3.6b). Dpartement of Genetics, SK, University of Washington, Seattle, WA (1993).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 (2000).
Corander, J., Waldmann, P. & Sillanpaa, M. J. Bayesian analysis of genetic differentiation between populations. Genetics 163, 367–374 (2003).
Corander, J., Waldmann, P., Marttinen, P. & Sillanpaa, M. J. BAPS 2: enhanced possibilities for the analysis of genetic population structure. Bioinformatics 20, 2363–2369 (2004).
Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14, 2611–2620 (2005).
Earl, D. A. & Vonholdt, B. M. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361 (2012).
Kopelman, N. M., Mayzel, J., Jakobsson, M., Rosenberg, N. A. & Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 15, 1179–1191 (2015).
Corander, J., Marttinen, P., Siren, J. & Tang, J. Enhanced Bayesian modelling in BAPS software for learning genetic structures of populations. BMC Bioinformatics 9, (2008).
Corander, J. & Marttinen, P. Bayesian identification of admixture events using multilocus molecular markers. Mol. Ecol. 15, 2833–2843 (2006).
Rousset, F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145, 1219–1228 (1997).
Rohlf, F. J. NTSYS-pc, numerical taxonomy and multivariate system. version 2.1. User Guide. Exet. Publ. (2000).
Beaumont, M. A. & Nichols, R. A. Evaluating loci for use in the genetic analysis of population structure. Proc. R. Soc. B Biol. Sci. 263, 1619–1626 (1996).
Antao, T. & Beaumont, M. A. Mcheza: A workbench to detect selection using dominant markers. Bioinformatics 27, 1717–1718 (2011).
Caye, K., Jumentier, B., Lepeule, J. & François, O. LFMM 2: Fast and Accurate Inference of Gene-Environment Associations in Genome-Wide Studies. Mol. Biol. Evol. 36, 852–860 (2019).
Legendre, P. & Legendre, L. Numerical Ecology, Volume 24. (Elsevier, 2012).
Oksanen, J. et al. vegan: Community ecology package. (2013). Available at: https://cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed 3 Jan 2021
Roesti, M., Salzburger, W. & Berner, D. Uninformative polymorphisms bias genome scans for signatures of selection. BMC Evol. Biol. 12, 94 (2012).
Jeffreys, H. Theory of probability. (Clarendon Press, 1961).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Frichot, E., Schoville, S. D., De Villemereuil, P., Gaggiotti, O. E. & François, O. Detecting adaptive evolution based on association with ecological gradients: orientation matters!. Heredity (Edinb). 115, 22–28 (2015).
Storey, J. D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA. 100, 9440–9445 (2003).
Legendre, P. & Gallagher, E. D. Ecologically meaningful transformations for ordination of species data. Oecologia 129, 271–280 (2001).
Acknowledgements
The research was supported by the Split-Dalmatia County through Support Program for Scientific Research in Agriculture. Publication was supported by the Open Access Publication Fund of the University of Zagreb Faculty of Agriculture.
Author information
Authors and Affiliations
Contributions
T.N.: Conceptualization, sampling, molecular experiments, writing, editing. M.J.D.: Conceptualization, sampling, editing. M.G.: Writing, editing. Z.L.: Molecular experiments, editing. F.V.: Editing. D.P.: Editing. Z.S.: Conceptualization, data analysis, supervision, writing, editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ninčević, T., Jug-Dujaković, M., Grdiša, M. et al. Population structure and adaptive variation of Helichrysum italicum (Roth) G. Don along eastern Adriatic temperature and precipitation gradient. Sci Rep 11, 24333 (2021). https://doi.org/10.1038/s41598-021-03548-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03548-6
This article is cited by
-
Controlled Elicitation and Greenhouse Acclimation Time Effects on Morphological and Biochemical Variables in Collections of Heliopsis longipes from Central México
Journal of Plant Growth Regulation (2023)
-
Plant phylogeography of the Balkan Peninsula: spatiotemporal patterns and processes
Plant Systematics and Evolution (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.