Abstract
Airway inflammation in patients with chronic obstructive pulmonary disease (COPD) is an amplified response of the normal immune system that occurs as a result of chronic irritation by toxic substances, such as cigarette smoke. This leads to the characteristic pathological changes in the inflammatory cells of COPD patients. ADAM33 has been reported to be involved in the pathogenesis of COPD in East Asia by affecting airway inflammation and other immune responses. The aim of this study was to determine the potential role of ADAM33 (mRNA and soluble levels) as a biomarker of inflammation in COPD patients. This is a case control study using consecutive sampling. The COPD case and control (non-COPD) groups comprised 37 and 29 patients, respectively. We used univariate analysis to assess differences in the parameters between the groups and bivariate analysis to non-parametrically compare these parameters between the two groups. We observed significantly higher mRNA levels of ADAM33 in the COPD patients (10.39 ± 1.76) as compared to that in the non-COPD individuals (6.93 ± 0.39; P < 0.001). The levels of soluble ADAM33 were also significantly higher in the COPD patients (2.188 ± 1.142 ng/ml) compared to the non-COPD individuals (0.487 ± 0.105 ng/ml; P < 0.001). The mRNA and soluble ADAM33 levels were significantly higher in COPD patients compared to those in the parameter-matched non-COPD individuals. Thus, ADAM33 is a potential biomarker and treatment for inflammation in COPD patients.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic lung disease that manifests with persistent respiratory symptoms and expiratory airflow limitation. Exposure to harmful particles or toxic gases results in abnormalities in the airways or alveoli that lead to COPD1,2. Airway inflammation in COPD patients is an amplified inflammatory response to chronic irritation, such as cigarette smoke. Among several studies, Wang et al. reported the involvement of ADAM33 in the pathogenesis of COPD in the East Asian population: ADAM33 regulates airway inflammation and immune response in COPD patients. Laxmi et al. showed a significant correlation between the genetic polymorphisms in ADAM33 S1-A/G and S2-C/G and pathophysiology of COPD in the South Indian population. The inflammatory response associated with COPD includes changes in pro-inflammatory or anti-inflammatory cytokines and occurs predominantly in the presence of ADAM33 polymorphisms3,4,5.
Kim et al. demonstrated that active vitamin D3 regulates vascular endothelial growth factor (VEGF) that stimulates ADAM33 expression and proliferation in smooth muscle cells; this can be used to influence the treatment outcome in patients with asthma6. Zhang et al. showed that lipopolysaccharide decreases the proliferation and functionality of human primary lung fibroblasts (important targets in patients with COPD)7. Increased mRNA and soluble levels of ADAM33 can serve as bridges for these cytokines that results in damage to the matrix. In this study, we investigated the role of ADAM33 (mRNA and soluble form) in matrix damage as part of the pathomechanism involved in COPD.
Methods
Study design and population
This is a case control study using consecutive sampling. The COPD case group included 37 patients, and the control group consisted of 29 non-COPD patients who were healthy people living near the hospital. Inclusion Criteria The control group (non-COPD) is non-COPD patients aged over 40 years who live in the vicinity of the Pondok Kopi and Sukapura Islamic Hospitals in Jakarta and are willing to voluntarily participate in the entire research program by signing an informed consent form. Exclusion Criteria Control group (non-COPD), i.e not COPD patients with other lung diseases (e.g.: asthma, pulmonary TB, pneumonia and lung tumors). The inclusion criteria for the subject group of COPD patients are stable COPD patients aged over 40 years who come to the pulmonary polyclinic of the Jakarta Islamic Hospital Pondok Kopi and Sukapura and are willing to voluntarily participate in the entire research program by signing an informed consent form. Exclusion Criteria Subject group COPD patients, namely COPD patients with other lung diseases (e.g.: asthma, pulmonary TB, pneumonia and lung tumors). We used IBM SPSS Statistics version 23 in this study. Univariate analysis was used to evaluate the differences in the parameters between groups. Bivariate analysis was performed to compare these parameters between two groups by non-parametric statistics. The study was performed in accordance with the ethical standards of the Declaration of Helsinki (1964) and its subsequent amendments.
COPD patients
COPD is a preventable and treatable chronic lung disease characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities usually caused by exposure to noxious particles or noxious gases1,2.
The diagnosis of COPD was based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria:
Stage I: mild, FEV1/FVC < 70%, FEV1 ≥ 80% predicted (post bronchodilator test).
Stage II: moderate, FEV1/FVC < 70%, 50% < FEV1 < 80% predicted (post bronchodilator test).
Stage III: severe, FEV1/FVC < 70%, 30% < FEV1 ≤ 50% predicted (post bronchodilator test).
Stage IV: very severe, FEV1/FVC < 70%, FEV1 ≤ 30% predicted (post bronchodilator test).
Chronic Obstructive Pulmonary Disease (COPD) in this study were all COPD patients with at least Stage I GOLD COPD. The diagnosis of COPD was based on lung function in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria. Meanwhile, the non-COPD control group had lung function within normal limits1,2.
ADAM33 mRNA levels
The mRNA levels of ADAM33 were determined by extracting nucleic acids using the method described in previous study8,9. Briefly, ~ 100 µl of peripheral blood was incubated with 900 μl of a solution consisting of guanidium thiocyanate. Subsequently, a 20-μl suspension consisting of 50 ml of H2O and 500 μl of 32% (w/v) diatom was added to the solution. We removed the supernatant and washed the sediment with a buffer followed by two washes with 1 ml of 70% ethanol and 1 ml of acetone. The resulting solution was heated in a water bath at 56 °C for 10 min and added to 60 μl of TE buffer consisting of 1 mM EDTA in 10 mM Tris HCl (pH 8.0). The supernatant from this mixture was transferred into a fresh tube for nucleic acid isolation and stored at − 80 °C until subjected to polymerase chain reaction (PCR)8,9.
The expression profile of target genes was determined using real-time PCR (qRT-PCR). Expression was represented as a ratio of expression of the primary oligonucleotide-specific gene to that of GAPDH (housekeeping gene). ADAM33 mRNA was detected using specific forward (5′-CAGGAATGCCAGCTATTATC-3′) and reverse (5′-GTTTGGTGTGGTTCAAGTTT-3′) primers. GAPDH was detected using specific forward (5′-GGCCAAAAGGGTCATCATC-3′) and reverse (5′-GTGATGGCATGGACTGTGG-3′) primers. The PCR thermal protocol was as follows: for ADAM33, 38 cycles of 94 °C for 3 min and 54 °C for 30 s; for GAPDH, 32 cycles of 94 °C for 10 s and 54 °C for 30 s according to the protocol described by Kim et al.6. qRT-PCR was performed with the One-Step SYBR Green qRT-PCR kit optimized for the Real-Time PCR CFX 6400 thermal cycler [6.10]. The total reaction volume was 25 µl (including experimental RNA) with 12.5 µl of the 2 × SYBR Green qRT-PCR master mix and “x” µl of the concentration-optimized primer stocks. Subsequently, “x” µl of nuclease-free water along with concentration-optimized final primers, 0.375 µl of the reference dye solution from stage 1 (optional), and 1 µl of the RT/RNase block enzyme mixture were added to a total reaction volume of 50 µl. The reaction was mixed slowly to avoid frothing (without rotating) and “x” µl of the RNA-solution mixture was added to individual experimental PCR tubes. The reaction was mixed slowly, briefly centrifuged, and placed in the instrument. The PCR program was run using a real-time PCR machine (CFX Connect system, Bio-Rad Laboratories, Real-Time PCR, 96 wells, 0.1 ml, USA)6,9,10,11.
Levels of soluble ADAM33, Interleukin (IL)-6, IL-8, IL-10, matrix metalloproteinase (MMP)-9
Levels of soluble ADAM33, IL-6, IL-8, IL-10 and MMP-9 were measured by enzyme-linked immunosorbent assay (ELISA). Patient serum samples were prepared using an ADAM33, IL-6, IL-8, IL-10 and MMP-9 kit at room temperature. Each sample was analyzed in duplicates to ensure the validity of the data obtained by ELISA. Initially, 100 µl of the Assay Diluent-containing protein buffer was added to each well. Next, 100 µl of Standard fluid-containing recombinant human target from a predetermined kit or diluted patient serum samples (1:10) was added to each well. The plate was then incubated for 2 h at room temperature. The liquid was removed and each well was washed four times with sterile phosphate-buffered saline. Then, 200 µl of Conjugate buffer with horseradish peroxidase-streptavidin was added to each well, the plate was covered with a plastic lid, and incubated at room temperature for 2 h. The liquid was removed and plate was washed four times with sterile phosphate-buffered saline. Next, 200 µl of Substrate Solution containing TMB was added to each well. The plate was incubated at room temperature for 20 min in the dark. After incubation, the reaction was stopped by adding 50 µl of Stop Solution containing H2SO4 to each well following which levels were measured within 30 min using the ELISA Reader 270 (Biomerieux, France) at a wavelength of 450 nm. The target soluble protein concentration was represented in ng/ml7,12,13,14.
Ethics approval and consent to participate
This research was submitted to the ethics committee of the Faculty of Medicine, Hasanuddin University, Makassar, Indonesia (No. 1006/H4.8.4.5.31/PP36-KOMETIK/2017, November 27, 2017) to obtain approval for ethical studies. Written informed consent was obtained from all participants.
Results
Table 1 shows that there were more males with COPD as compared to females with COPD. Patients with COPD were between 60 and 80 years and were presented to our hospital at an average age of 65.68 years. In comparison, the non-COPD patient controls were between 60 and 81 years with an average age of 67 years. The highest Brinkmann index (3) was found in patients with COPD and non-smokers (0) in non-COPD control individuals. There were no differences in patient characteristics between groups (P > 0.05). Individuals in both groups were homogeneous based on sex, age, and Brinkmann index.
This study also looked at the characteristics of the COPD patient group which was divided based on COPD patients based on the stage of GOLD obstruction, based on the value of post bronchodilator obstruction can be seen in the summary analysis in the table below showing the highest number of COPD obstruction stages GOLD II with a total of 12 COPD patients (32.43%). in the Table 2 below is shown in full.
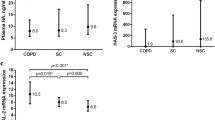
The mRNA levels of ADAM33 were significantly higher in COPD patients (10.39 ± 1.76 fold change; 95% confidence interval (CI) 9.802–10.981) as compared to that in non-COPD individuals (6.93 ± 0.39 fold change; 95% CI 6.780–7.079; P < 0.001; Fig. 1). Similarly, the levels of soluble ADAM33 were also significantly higher in COPD patients (2.188 ± 1.142 ng/ml; 95% CI 1.807–2.569) as compared to that in non-COPD individuals (0.487 ± 0.105 ng/ml; 95% CI 0.447–0.526; P < 0.001; Fig. 2).
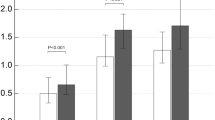
The role of pro-inflammatory cytokines (IL-6 and IL-8) and anti-inflammatory cytokines (IL-10) in COPD patients with differences in soluble IL-6 and IL-8 levels being higher in COPD patients than in non-COPD patients, while Lower IL-10 in COPD patients than in non-COPD patients; each of which found a statistically significant difference (P < 0.01). This is shown in the Table 3 below.
Matrix damage in COPD patients was found to be consistent with the presence of increased levels of soluble MMP-9, the matrix marker studied in this study was MMP-9. Table 4 below shows that there is matrix damage in COPD patients with differences in soluble MMP-9 levels being higher in COPD patients than in non-COPD patients; which were statistically significant (P < 0.01).
Factors associated with ADAM33 that have changed in COPD patients: MMP-9 and Cytokines (IL-6, IL-8 and IL-10). Table 5 below shows that there is a significant linear correlation between soluble ADAM33 levels and ADAM33 mRNA expression with soluble MMP-9 levels in COPD patients through the Spearman Correlation test (P < 0.001), so the higher the soluble ADAM33 level, the higher the soluble MMP-9 level. Serum levels, as well as the expression of ADAM33 mRNA on soluble MMP-9 levels. However, there was no correlation between soluble ADAM33 levels and ADAM33 mRNA expression with soluble MMP-9 levels in non-COPD patients (P > 0.05). This proves that there is a relationship between ADAM33 and MMP-9 in COPD pathomechanism.
This study also showed that there was a significant linear correlation, levels of soluble cytokines IL-6, IL-8 and IL-10 and mRNA expression of the ADAM33 gene with soluble ADAM33 levels in COPD patients through the Spearman Correlation test (P < 0.001). And there was no correlation between levels of soluble cytokines IL-6, IL-8, IL-10 and ADAM33 gene mRNA expression with soluble ADAM33 levels in non-COPD patients (P > 0.05). As shown in Table 6 below, it proves that there is a relationship between IL-6, IL-8 and IL-10 cytokines with ADAM33 in COPD pathomechanism.
This study also showed that there was a linear correlation in the direction of soluble cytokine levels IL-6, IL-8 and IL-10 with soluble MMP-9 levels which was significant in COPD patients through the Spearman Correlation test (P < 0.001). There was also a correlation between soluble cytokine levels IL-8 and IL-10 with soluble MMP-9 levels in non-COPD patients (P < 0.001). And there was no correlation between the levels of soluble cytokine IL-6 with soluble levels of MMP-9 in non-COPD patients (P > 0.05). As in Table 7 below, it explains that there is a relationship between IL-6, IL-8 and IL-10 cytokines with MMP-9 in COPD pathomechanism.
There is an association between ADAM33 and inflammation in COPD patients as indicated by the correlation of ADAM33 with MMP-9 and cytokine levels (IL-6, IL-8 and IL-10) in COPD patients. On Fig. 3 shows a linear regression graph between soluble levels of IL-6 and soluble levels of ADAM33 with the determinant R2 of 0.971; it means that the contribution of soluble IL-6 levels to the soluble levels of ADAM33 is 97.1%. The higher the soluble IL-6 level, the higher the serum soluble ADAM33 level, which is the relationship between IL-6 cytokines and ADAM33 in COPD pathomechanism.
On Fig. 4 shows a linear regression graph between soluble levels of IL-8 and soluble levels of ADAM33 with the determinant R2 of 0.952; it means that the contribution of soluble IL-8 levels to the soluble levels of ADAM33 is 95.2%. The higher the soluble IL-8 level, the higher the serum soluble ADAM33 level, which is the relationship between IL-8 cytokines and ADAM33 in COPD pathomechanism.
Figure 5 shows a linear regression graph between soluble levels of IL-10 and soluble levels of ADAM33 with the determinant R2 of 0.797; it means that the contribution of soluble IL-10 levels to the soluble levels of ADAM33 is 79.7%. The lower the soluble IL-10 level, the higher the serum soluble ADAM33 level, which is the relationship between IL-10 cytokines and ADAM33 in COPD pathomechanism.
Figure 6 below shows a linear regression graph between the soluble levels of MMP-9 and the soluble levels of ADAM33 with the determinant R2 of 0.965; it means that the contribution of the soluble content of MMP-9 to the soluble content of ADAM33 is 96.5%. The higher the soluble MMP-9 level, the higher the serum soluble ADAM33 level, which is the relationship between MMP-9 cytokines and ADAM33 in COPD pathomechanism.
Figure 7 below shows a linear regression graph between ADAM33 gene mRNA expression and ADAM33 soluble content with a R2 determinant of 0.898; it means that the contribution of ADAM33 gene mRNA expression to ADAM33 soluble content is 89.8%. The higher the ADAM33 gene mRNA expression, the higher the serum ADAM33 soluble level which is the relationship between ADAM33 gene mRNA expression and ADAM33 soluble levels in COPD pathomechanism.
Table 8 shows the correlation of ADAM33 level between COPD and non-COPD patients according to GOLD COPD stage and their inter GOLD COPD stage. ADAM33 levels at each level of GOLD COPD stage have a high deviation value as well as ADAM33 levels in non-COPD patients, so that if ADAM33 levels at each level of GOLD COPD stage, the significance value is low. This is the same as the relationship between ADAM33 levels of inter-GOLD COPD stage, except for the statistically significant correlation of ADAM33 levels in GOLD COPD stage II and IV.
In Fig. 8 shows the correlation between lung function, ADAM33 and GOLD COPD stage in COPD and non-COPD patients. In Fig. 8A the lung function (FEV1%) and GOLD COPD stage in COPD and non-COPD patients. The mean value of lung function (FEV1%) decreases according to a range of lung function values (FEV1%) of GOLD COPD stage I to IV, except for GOLD COPD stage II the tendency for the average lung function value (FEV1%) to approach the lowest limit of the range of lung function values (FEV1%) of GOLD COPD stage II. In GOLD COPD stage III, the mean value of lung function tends to approach the highest limit of the range of lung function values (FEV1%) of GOLD COPD stage III. In Fig. 8B shows ADAM33 levels (ng/ml) and GOLD COPD stage I to IV in COPD and non-COPD patients tend to be lower, except for the average ADAM33 levels in GOLD COPD stage II which tends to be lower when associated with the average ADAM33 levels in GOLD COPD stage III.
Discussion
The results of this study were in accordance with those reported by Shamara and Fachri (2014): majority of COPD patients were stable (37 people, 86%) and 60–69 years (16 people, 37.2%). Moreover, 19 stable COPD patients (44.2%) each manifested with a moderate (2) or severe (3) Brinkmann Index owing to cigarette smoking15,16. The results of the present study were also in accordance with those seen in COPD patients treated in the Department of Pulmonology, Faculty of Medicine, Persahabatan Hospital, Indonesia: majority of the COPD patients were males (86.2%) and this can be attributed to the difference in the percentage of male and female smokers17. Similarly, Suradi et al. showed that the prevalence of COPD was greater in men than in women comprising a cohort of 49 men and 16 women of which 47 men (72%) were smokers with acute exacerbation of COPD and positive sputum cultures of Mycobacterium tuberculosis in both DM and without type 2 DM18,19,20,21]. The present study also revealed a role for ADAM33 as a biomarker of inflammation in COPD patients. We found higher levels of ADAM33 mRNA and soluble ADAM33 in COPD patients than that in non-COPD individuals (P < 0.01).
Among the numerous studies that have examined the role of ADAM33 mRNA and soluble ADAM33 levels, most are limited to patients with asthma. Foley et al. showed that the mRNA levels of ADAM33 in in vitro cultured primary bronchial epithelial cells from asthma patients were higher than that in donor epithelial cells from asthma patients and cells from normal individuals22. Ito et al. compared ADAM33 mRNA levels in smooth muscle cells from asthma patients to that in controls by determining the percentage of the total smooth muscle cells both groups; the ratio of positive/total smooth muscle cells was higher in patients with asthma than that in control individuals. The mRNA levels of ADAM33 were higher in smooth muscle cells from patients with asthma than that in control individuals (P = 0.002)23. Puxeddu et al. demonstrated an increase in the levels of soluble ADAM33 and its role in angiogenesis; ADAM33 acts as a remodeling gene that functions independent of airway inflammation in airway obstruction through an inflammatory mechanism. Thus, ADAM33 contributes to the pathogenesis of asthma and COPD by interactions between genetic and environmental factors24. Taken together, to the best of our knowledge, this is the first report of the mRNA and soluble ADAM33 levels in COPD patients and control individuals.
In the study of Moraes et al., there was agreement with this study, the relationship between biomarkers of the inflammatory reaction of COPD patients and the control group, indicated by higher soluble IL-6 levels in the COPD patient group (32.97 ± 31.08 pg/ml) than the control group. control (5.42 ± 3.72 pg/ml; P = 0.0110). And indicated by higher soluble IL-8 levels in COPD patients (23.63 ± 14.58 pg/ml) than in the control group (13.05 ± 6.73 pg/ml; P = 0.0217)25. Soluble IL-10 levels of COPD GOLD I (mean = 8.5 ± 2.7 pg/mg tissue) and COPD GOLD II patients (mean = 7.8 ± 1.8 pg/mg tissue) were actually higher lower than patients with normal lung function (mean = 17.9 ± 3.1 pg/mg tissue, P < 0.05). Hackett et al.'s study is in accordance with this study, namely COPD patients with mild to moderate airflow obstruction (COPD GOLD I and II) levels of soluble IL-10 as an anti-inflammatory cytokine were lower in COPD patients than non-COPD patients26.
In a review article by Katarzyna Grzela et al. based on some research from Cataldo et al. 2002, Gagliardo et al. 2009, Lee et al. 2001 and Lem-jabbar et al. 1999, there were elevated levels of MMP-9 found in serum, sputum and bronchoalveolarlavage from patients with asthma exacerbations. In COPD patients, this review article is based on a study by Brajer et al. 2008 and Erlewyn-La-jeunesse et al. 2008 also showed an increase in serum MMP-9 levels, which was more negatively correlated with the Tiffeneau-Pinelli index (FEV1/FVC ratio). The level and activity of MMP-9 from sputum samples of COPD patients according to the study of Culpitt et al. 2005, found to be 12 times higher than the control group. So, the increase in serum MMP-9 levels in asthmatic patients is not as high as in COPD patients27. A study conducted by Jie Ji et al. revealed that serum MMP-9 levels (ng/ml) were higher in COPD patients with smokers (757 (557–1000)) and COPD patients who did not smoke (490 (382–801)) compared to controls who did not smoke. healthy (430 (251–577)) (P = 0.006). Elevated levels of MMP-9 in the extracellular matrix are essential for the remodeling process of COPD patients and their expression is regulated by specific inhibitors, such as tissue inhibitor of metalloproteinases-1 (TIMP-1). Increased TIMP-1 levels in BAL fluid from both groups of smokers compared with healthy non-smokers and increased serum levels of MMP-9 in the COPD group. Increased levels of soluble MMP-9 and TIMP-1 have been observed in serum, sputum and LAB fluid in COPD. The decrease in plasma MMP-9 levels and the inconsistent increase in TIMP-1 in COPD may be due to the fact that MMP-9 levels can vary over time in relation to the severity of COPD and smoking habits in COPD patients28.
In Ji et al.'s study it was shown that smokers have ongoing inflammation in the central airways (sputum), peripheral airways (BAL fluid), and systemically (blood) and that this inflammatory response is more associated with smoking than with the presence or absence of smoking. chronic airflow limitation. Although IL-8 and MMP-9 levels did not differ between the two groups, there was a significant negative relationship between salivary IL-8 and MMP-9 levels and lung function in COPD. Inflammatory markers in saliva may be associated with disease severity in COPD. A similar relationship was shown between these biomarkers in serum and lung function. This study found a very strong correlation between IL-8 and MMP-9 in saliva and periodontal inflammation as assessed by gingival bleeding in healthy non-smokers but not in the two groups of smokers. These findings suggest that these markers of inflammation in saliva are associated with periodontal inflammation under normal circumstances and this association was not seen in smokers when inflammatory activity is triggered by strong proinflammatory stimuli such as cigarette smoke28. Elevated levels of MMP-9 in the extracellular matrix are important for the remodeling process in COPD and its expression is thought to be regulated by specific inhibitors, such as TIMP-1. Ji et al. found increased levels of TIMP-1 in BAL fluid from both groups of smokers compared with nonsmokers and increased levels of MMP-9 in serum in the COPD group. Elevated levels of MMP-9 and TIMP-1 have been observed in serum, sputum and BAL fluid in COPD patients. However, there are conflicting results showing decreased plasma MMP-9 and TIMP-1 levels in COPD. These inconsistent results may be because MMP levels can vary over time in COPD. Differences in disease severity and smoking habits in the study population may also explain differences in results between studies28.
Soluble ADAM33 and MMPs are important enzymes in processes involved in remodeling and degradation of the extracellular matrix. Increased MMP-9 activity is an important part of the progression of COPD patients, associated with other components in the pathogenesis of COPD. Thus ADAM33 is responsible for airway remodeling and bronchial hyperresponsiveness in the early years of life. The genetic heterogeneity found in this study suggests that asthma is caused by multiple mutations in the same gene. On the other hand, different patterns of linkage disequilibrium (LD) among different populations reveal that the unprocessed causative factors may be the same, but the assortment of SNPs in strong LD with variance differs among ethnic groups. Future research into the genetics of asthma and COPD should evaluate the genetic background of patients with a history of asthma progressing to irreversible airway obstruction with a COPD-like phenotype. All basic science studies explaining genetic changes will have little meaning for doctors if we cannot put this knowledge to use in clinical practice. The genetic profile of each individual and the consequent pattern of enzymatic expression can lead us to establish a rapid diagnosis of potential severe asthma, airway improvement and the gradual and rapid establishment of COPD cases, in smokers and no smokers. It is well known that subjects with lower impairment in pulmonary function or with normal limits on function tests exhibit a greater risk of death and the need for hospitalization. This will benefit all candidate patients and healthcare providers29.
The research of Fei Xu et al., in accordance with this study, can prove that in chronic inflammation there is damage and metaplasia of the respiratory epithelium. Inflammation that occurs in a long time causes the formation of connective tissue in the walls of the airways. The airway wall epithelium produces epidermal growth factors (EGFs/epidermal growth factors) and various protease enzymes, especially MMP-9, which inhibits the degradation of the extracellular matrix so that changes in composition and quantity are associated with thickening of the airway wall epithelium. Smoking habits inhale many chemical compounds that induce chronic inflammation and damage to the airways. Chronic inflammation contributes to structural and cellular damage to the airway wall epithelium, resulting in fibrosis and damage to small airways. Cytokines on airway inflammation in COPD patients, such as IL-6, IL-8 as an increased proinflammatory cytokine and IL-10 as a decreased anti-inflammatory cytokine. Smoking habits cause endothelial cell damage and dysfunction, deposition of the extracellular matrix occurs where the matrix protein profile has changed, resulting in airway remodelling. So in the airways of COPD patients who have undergone remodelling there is an increase in MMP-930.
As members of the zinc-dependent ADAM-metalloproteinase superfamily, ADAM33 regulate their own function and that of a variety of other proteins by proteolytic cleavage. ADAM33 has multiple domains including prodomain, catalytic, metalloprotease, disintegrin (binding integrin), cysteine-rich/epidermal growth factor (cell–cell contact), transmembrane and cytoplasmic domains, and has multiple forms containing various combinations of these domains. ADAM33 is predominantly expressed in airway structural cells, including airway epithelium, airway smooth muscle, myofibroblasts and fibroblasts, thought to have a broad spectrum of functions, such as protease dependent and independent mechanisms. ADAM33 cleaves proteins from the cell surface and may facilitate the release of cytokines and growth factors. The pathogenesis of COPD involves recruitment and regulation of neutrophils, macrophages and lymphocytes to the lung, as well as an induced imbalance between proteinases and antiproteinases, all of which result in lung parenchymal destruction and airway remodelling. Based on the expression profile and function, ADAM33 is involved in the pathogenesis of the COPD. The study of Xinyan Wang et al. showed that the (single nucleotide polymorphisms) SNP Q-1 in the ADAM33 gene was statistically significant (P > 0.01) associated with the release of IL-8 in sputum samples from COPD patients. Therefore, ADAM33 can affect the release of cytokines and growth factors, causing inflammatory cell infiltration in the airways3.
In the data of this study, the correlation of the mean lung function and the GOLD COPD stage and non-COPD as shown by the boxplot Fig. 8A diagram, there is a tendency for the GOLD COPD stage II to approach the lowest limit of the lung function value range (FEV1%) GOLD COPD stage II, whereas in GOLD COPD stage III the mean lung function value (FEV1%) tends to approach the highest limit of the lung function range (FEV1%) of GOLD COPD stage III. In the correlation level of ADAM33 as shown in the boxplot diagram Fig. 8B, there is an average ADAM33 level in GOLD COPD stage II which tends to be lower when associated with an average ADAM33 level in GOLD COPD stage III. This is because in GOLD COPD stage II the tendency of the lung function average value to approach the lowest limit of the lung function value range (FEV1%) GOLD COPD stage II, while in GOLD COPD stage III the lung function average value tends to approach the highest limit of the lung function value range (FEV1%) GOLD COPD stage III.
The fibrotic extent of lung tissue is not directly related to oxygen consumption. The lung function (FEV1%) and ADAM33 level not directly related because ADAM33 has involved in fibrotic of lung tissue mechanism, however the lung function determined by oxygen level. Thus, we did not find directly the relation between ADAM33 level and Lung Function (FEV1%)3,23,24,29,30.
Conclusion
COPD patients showed significantly higher mRNA and soluble ADAM33 levels as compared to that in non-COPD individuals. Thus, ADAM33 may serve as an inflammatory biomarker in COPD patients.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Global Initiative for Chronic Obstructive Pulmonary Disease, Diagnosis and assessment. In: Global strategy for diagnosis management and prevention of Chronic Obstructive Lung Disease. NHLBI Publications and Resources, 1–56 (2017).
Amin, M., Yunus, F. & Antariksa, B. PPOK (Penyakit Paru Obstruktif Kronik) Diagnosis dan Penatalaksanaan Perhimpunan Dokter Paru Indonesia (PDPI) 1–91 (Penerbit Universitas Indonesia, 2016).
Wang, X. et al. Genetic variants in ADAM33 are associated with airway inflammation and lung function in COPD. BMC Pulm. Med. 14, 173 (2014).
Wang, X. et al. Association of ADAM 33 gene polymorphisms with COPD in a northeastern Chinese population. BMC Med. Genet. 10, 132 (2009).
Zhou, D. C. et al. Association of a disintegrin and metalloprotease 33 (ADAM33) gene polymorphisms with the risk of COPD: An updated meta-analysis of 2,644 cases and 4,804 controls. Mol. Biol. Rep. 42(2), 409–422 (2015).
Kim, S. H. et al. Effect of active vitamin D3 on VEGF-induced ADAM33 expression and proliferation in human airway smooth muscle cells: Implications for asthma treatment. Respir. Res. 18(1), 7 (2017).
Wardani, I. S. et al. Serum vitamin D receptor and High Mobility Group Box-1 (HMGB1) levels in HIV-infected patients with different immunodeficiency status: A cross-sectional study. Ann. Med. Surg. 63(2021), 102174. https://doi.org/10.1016/j.amsu.2021.02.020 (2021).
Rusyati, L. M. M. et al. Higher Treg FoxP3 and TGF-β mRNA expression in Type 2 reaction ENL (erythema nodosum leprosum) patients in mycobacterium leprae infection. Open Microbiol. J. 14, 304–309. https://doi.org/10.2174/1874434602014010304 (2020).
Hatta, M., Surachmanto, E. E., Islam, A. A. & Wahid, S. Expression of mRNA IL-17F and sIL-17F in atopic asthma patients. BMC Res. Notes 10(1), 202 (2017).
Prihantono, P. et al. Ki-67 expression by immunohistochemistry and quantitative real-time polymerase chain reaction as predictor of clinical response to neoadjuvant chemotherapy in locally advanced breast cancer. J. Oncol. 2017, 6209849 (2017).
Sirait, R. H., Hatta, M., Ramli, M., Islam, A. A. & Arief, S. K. Systemic lidocaine inhibits high-mobility group box 1 messenger ribonucleic acid expression and protein in BALB/c mice after closed fracture musculoskeletal injury. Saudi J. Anaesth. 12(3), 395–298. https://doi.org/10.4103/sja.SJA_685_17 (2018).
Wahyuni, T. D., Hatta, M., Bukhari, A., Santoso, A. & Massi, M. N. Increasing natural resistance associated macrophage protein 1 SErum level after Miana treatment in BALB/c induced Klebsiella pneumoniae experimental research. Ann. Med. Surg. 65(2021), 102262. https://doi.org/10.1016/j.amsu.2021.102262 (2021).
Farsida, I. et al. Relationship between expression mRNA gene Treg, Treg, CD4+, and CD8+ protein levels with TST in tuberculosis children: A nested case–control. Ann. Med. Surg. 61, 44–47. https://doi.org/10.1016/j.amsu.2020.12.011 (2021).
Oley, M. H. et al. Hyperbaric oxygen therapy in the healing process of foot ulcers in diabetic type 2 patients marked by interleukin 6, vascular endothelial growth factor, and PEDIS score: A randomized controlled trial study. Int. J. Surg. Open 27, 154–161. https://doi.org/10.1016/j.ijso.2020.11.012 (2020).
Shamara, F. & Fachri, M. Karakteristik pasien PPOK stabil dikaitkan dengan kebiasaan merokok berdasarkan nilai indeks brinkman di RS Islam Sukapura. J. Indonesian Med. Assoc. 64(12), 564–569 (2014).
Brinkmann, G. L. & Coates, E. O. The effect of bronchitis, smoking and occupation on ventilation. Am. Rev. Respir. Dis. 87, 684–693 (1963).
Yunus, F. Gambaran penderita PPOK yang dirawat di bagian Pulmonologi FKUI/RSUP Persahabatan Jakarta. J. Respirol. Indonesia 20, 64–68 (2000).
Suradi, Y., Sutanto, S., Harsini, R. & Marhendra, D. Hubungan antara Penyakit Paru Obstruktif Kronik Eksaserbasi Akut dengan Hasil Kultur Sputum Bakteri pada Rumah Sakit R Moewardi Surakarta. J. Respirol. Indonesia 32(4), 218–222 (2012).
Hatta, M., Sultan, A. R., Tandirogang, N. & Yadi, M. Detection and identification of mycobacteria in sputum from suspected tuberculosis patients. BMC. Res. Notes 3, 72. https://doi.org/10.1186/1756-0500-3-72 (2010).
Wikanningtyas, T. A. et al. Hematologic parameters in pulmonary tuberculosis patients based on the microscopic sputum examination. Enfermería Clín. 30(Suppl 2), 243–246. https://doi.org/10.1016/j.enfcli.2019.07.098 (2020).
Fachri, M. et al. Comparison of acid fast Bacilli (AFB) smear for Mycobacterium tuberculosis on adult pulmonary tuberculosis (TB) patients with type 2 diabetes mellitus (DM) and without type 2 DM. Respir. Med. Case Rep. 23, 158–162. https://doi.org/10.1016/j.rmcr.2018.02.008 (2018).
Foley, S. C. et al. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J. Allergy Clin. Immunol. 119(4), 863–871 (2007).
Ito, I. et al. Downregulation of a disintegrin and metalloproteinase 33 by IFN-gamma in human airway smooth muscle cells. J. Allergy Clin. Immunol. 119(1), 90–97 (2007).
Puxeddu, I. et al. The soluble form of a disintegrin and metalloprotease 33 promotes angiogenesis: Implications for airway remodeling in asthma. J. Allergy Clin. Immunol. 121(6), 1400–6.e4 (2008).
Moraes, X. et al. Interleukin-6 and interleukin-8 blood levels’ poor association with the severity and clinical profile of ex-smokers with COPD. Int. J. COPD 9, 735–743 (2014).
Hackett, T. L., Holloway, R., Holgate, S. T. & Warner, J. A. Dynamics of pro-inflammatory and anti inflammatory cytokine release during zcute inflammation in chronic obstructive pulmonary disease: An ex vivo study. Respir. Res. 9, 47 (2008).
Grzela, K., Litwiniuk, M., Zagorska, W. & Grzela, T. Airway remodeling in chronic obstructive pulmonary disease and asthma: The role of matrix metalloproteinase-9. Arch. Immunol. Ther. Exp 64, 47–55 (2016).
Ji, J. et al. Compartment differences of inflammatory activity in chronic obstructive pulmonary disease. Respir. Res. 15, 104 (2014).
Sampsonas, F. et al. DNA sequence variations of metalloproteinases: Their role in asthma and COPD. Postgrad. Med. J. 83, 244–250 (2007).
Xu, F. et al. Scutellaria baicalensis attenuates airway remodeling via PI3K/Akt/NF-kB pathway in cigarette smoke mediated-COPD rats model. Evid. Based Complementary Altern. Med. 20, 1–12 (2018).
Acknowledgements
We would like to thank the staff in the Molecular Biology and Immunology Laboratory at the Medical Faculty, Hasanuddin University, Makassar, Indonesia for their technical support for this study. We would like to thank secure.authorservices.springernature.com for English language editing.
Funding
The research was funded by BPPDN scholarship [No. 1405.20/E4.4/2015, August 31, 2015]. The funding bodies had no role in the design of this study, collection, analysis, and interpretation of the data, and writing this manuscript.
Author information
Authors and Affiliations
Contributions
M.F. and M.H. designed the study. M.H., M.F., R.D., A.R.J. and M.R.P. carried out the laboratory analyses. M.F., M.H., M.N.M., A.R.J., M.R.P. and T.A.W., R.D. and M.S. reviewed the data, conducted the statistical analyses and interpreted the results. M.F., M.H., M.N.N., A.S., T.A.W., R.D., A.R.J., M.R.P. and M.S. wrote the first draft of the paper, which all authors critically reviewed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fachri, M., Hatta, M., Massi, M.N. et al. The strong correlation between ADAM33 expression and airway inflammation in chronic obstructive pulmonary disease and candidate for biomarker and treatment of COPD. Sci Rep 11, 23162 (2021). https://doi.org/10.1038/s41598-021-02615-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02615-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.