Abstract
Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation plays an important role in chronic obstructive pulmonary disease (COPD) pathogenesis and might be involved in ongoing chronic inflammation. This study aimed to determine interleukin-1beta (IL-1β) plasma concentration as well as IL1B, NLRP3 and caspase-1 (CASP1) gene expression in the Croatian COPD patients. 109 patients with stable COPD and age- and sex-matched 95 controls were included in the study. Plasma IL-1β concentration was measured by Luminex technology, and gene expression analysis was performed using TaqMan assays. It was shown that COPD patients had increased concentration of IL-1β and enhanced gene expression of IL1B, NLRP3 and CASP1 compared to controls. There was no difference in IL-1β or IL1B, NLRP3 and CASP1 in patients with COPD regarding airflow obstruction severity and smoking history. Finally, the diagnostic potential of the determined parameters was evaluated, and it was found that IL-1β correctly classified 89% of cases in the combination with common inflammatory biomarkers, white blood cell count and fibrinogen, showing a potential in COPD prediction. In conclusion, up-regulation of IL1B, NLRP3, CASP1 and increased IL-1β concentration suggest the activation of NLRP3 inflammasome in the systemic compartment of patients with stable COPD.
Similar content being viewed by others
Introduction
According to the latest data from the World Health Organization, chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide1, with a projected increase in social, economic and health care burden2. COPD is a progressive lung disease characterized by persistent respiratory symptoms and airflow limitation due to the airway and/or alveolar abnormalities that are usually caused by a significant exposure to noxious particles or gases3. The immune response in COPD is altered, and chronic inflammation is known to be associated with the development of COPD. Regardless numerous studies, further investigation is recommended to elucidate the inflammatory response in COPD.
Activation of inflammasomes is considered to be involved in the pathogenesis of COPD. Among the various inflammasomes, which are activated by different agonists, the most studied one is the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome. The NLRP3 inflammasome is a multimeric complex involved in the release of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18. It consists of the sensor NLRP3 protein, the adaptor named apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC) and the effector, which is in most cases pro-caspase-14. There are different pathways for NLRP3 inflammasome activation described in the literature. Those are classical (canonical and non-canonical) as well as alternative pathways. NLRP3 inflammasome activation is usually considered to be a two-step process, consisting of priming and activation (classical pathways). The first step involves sensing and producing, which begins with the recognition of pathogen-associated molecular patterns and/or damage-associated molecular patterns by pattern recognition receptors, such as Toll-like receptors (TLRs)5. Stimulation of TLRs leads to activation of nuclear factor kappa B (NF-κB)-mediated signalling. Activated NF-κB signalling pathway results in increased transcription and production of NLRP3 protein, pro-IL-1β and pro-IL-18, which finally leads to the priming or initiation of the NLRP3 inflammasome activation6. The second step begins with the assembly of the NLRP3 inflammasome, where ASC acts as a zipper and binds NLRP3 with pro-caspase-1, which in turn undergoes proteolytic cleavage that releases caspase-1. On the other hand, caspases-4/5 in humans and caspase-11 in mice are involved in non-canonical pathway that is triggered by bacterial infection i.e. by the internalization of lipopolysaccharide into the cytosol. Active caspase-1 (or caspase-4/5/11) is able to process pro-IL-1β and pro-IL-18 into their mature forms that induce inflammation and pyroptotic cell death4,5,6,7. In contrast, only one signal provokes NLRP3 inflammasome activation in the alternative pathway that ultimately leads to inflammation without pyroptosis8.
Until now, investigation of the role of NLRP3 inflammasome in COPD has been explored in animal models and human respiratory system samples9,10,11, but there is limited information on the NLRP3 inflammasome activation in the peripheral circulation of COPD patients. Besides, activation of the NLRP3 inflammasome is found to be involved in the immune response to cigarette smoke, which is the major environmental risk factor for the onset of COPD12.
The chronic systemic inflammation in COPD and the altered immune response regulated by the activation of NLRP3 inflammasome might play an important role in COPD pathogenesis. Therefore, this study investigated the plasma concentration of IL-1β as well as IL-1β gene (IL1B), NLRP3 and caspase-1 gene (CASP1) expression in the Croatian COPD patients in stable phase in comparison to the healthy controls. The study also aimed to determine the association between the aforementioned parameters and airflow obstruction severity or smoking history.
Materials and methods
Participants
The current retrospective case–control study included 109 COPD patients in a stable phase of the disease and 95 age- and sex-matched healthy individuals who voluntarily participated in the study and signed an informed consent form. The recruitment of the participants was conducted at Clinic for Respiratory Diseases Jordanovac, University Hospital Centre Zagreb in 2017 and 2018. The study was approved by the Ethics Committee of University Hospital Centre Zagreb (Zagreb, Croatia) and by the Ethics Committee for Experimentation of the University of Zagreb Faculty of Pharmacy and Biochemistry (Zagreb, Croatia) (approval protocol numbers: 02/21/JG and 251-62-03-14-78, respectively). COPD diagnosis was confirmed by pulmonology specialists according to the guidelines of Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD)3, after measuring the spirometry parameters forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC). Healthy individuals were included in the study based on medical history data and spirometry results. Based on FEV1, there were 39 COPD patients in GOLD 2, 36 COPD patients in GOLD 3 and 34 COPD patients in GOLD 4 group. Self-reported smoking history data was collected so that there were 48 healthy non-smokers, 47 healthy smokers, 5 COPD non-smokers, 75 COPD former smokers and 29 COPD smokers. Both COPD patients and healthy subjects were older than 40 years, had no lung disease (except COPD in COPD patients), inflammatory systemic diseases, acute infections, diabetes with severe complications, severe liver disease, severe renal insufficiency, malignant diseases, transplantation, and other specific or non-specific acute inflammation.
Common inflammatory biomarkers
Blood samples were collected by venepuncture of a large antecubital vein after overnight fasting from 7 a.m. to 9 a.m. Tubes containing K3-ethylenediaminetetraacetic acid (K3 EDTA) as an anticoagulant (Greiner Bio-One, Kremsmünster, Austria) were used for white blood cells (WBC) count and determination of IL-1β, and for extraction of buffy coat, which was used for RNA and DNA isolation. A tube containing 3.2% sodium citrate (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) was used for fibrinogen (FIB) measurement, while a tube without additive but with clot activator and gel separator was used for C-reactive protein (CRP) determination (Greiner Bio-One, Kremsmünster, Austria)13. Each sample was further treated according to the recommendations14. Determination of WBC, FIB and CRP was performed at Clinical Institute of Laboratory Diagnostics at University Hospital Centre Zagreb (Zagreb, Croatia), as described previously15.
IL-1β determination
EDTA plasma was used for determination of IL-1β concentration using Procarta Plex High Sensitivity Luminex kit (Thermo Fischer Scientific, Waltham, MA, USA), according to the manufacturer’s recommendations. The results were analysed using a Luminex 200 instrument, and the IL-1β concentration was assessed from a standard curve using the xPONENT software package (Luminex Corporation, Austin, TX, USA).
Gene expression analysis
Total RNA was isolated from buffy coat by the TRIzol/chloroform method16, and the quality of the RNA was assessed by the ratio of the 260/280 nm measurements on microvolume spectrophotometer Nanodrop 8000 (Thermo Fischer Scientific, Wilmington, USA). When the ratio was 1.9–2.1, the RNA was suitable for cDNA synthesis by reverse transcription based on polymerase chain reaction using RevertAid First Strand cDNA Synthesis Kit (Thermo Fischer Scientific, Waltham, Massachusetts, USA) with GeneAmp PCR System 9700 (Applied Biosystems, Foster City, USA)17. The assessment of IL1B, NLRP3 and CASP1 gene expressions were performed by TaqMan Gene Expression Assays (Hs01555410_m1 for IL1B, Hs00918082_m1 for NLRP3, Hs00354836_m1 for CASP1; Applied Biosystems, Foster City, USA) and TaqMan Universal Master Mix (Applied Biosystems, Foster City, USA) according to the manufacturer’s guidelines. Data were normalized using gene expression of beta-2-microglobulin (B2M) and peptidylprolyl isomerase (PPIA) (Hs99999907_m1 for B2M, Hs99999904_m1for PPIA; Applied Biosystems, Foster City, USA) as the endogenous controls. In addition, a randomly selected control sample was included in each plate as a calibrator. Finally, the relative expression of target genes in controls and COPD patients was performed using the 2−∆∆Ct comparison method18.
Statistics
All data failed a normality test performed by Kolmogorov–Smirnov test, so the results were shown as a median with corresponding interquartile range (IQR), beside age that was shown as a median with minimum and maximum and sex that was shown in absolute numbers. Non-parametric Mann–Whitney test and Kruskal–Wallis test were used to test the differences between the groups of interest, while categorical data was tested by Chi-squared test. Univariate and multivariable logistic regression analyses were performed to assess the predictor variables on the risk of COPD, so results were shown as odds ratio (OR) with corresponding 95% confidence interval (CI). Influence of age, sex, comorbidities and therapy was excluded in this investigation. Analysis was performed in MedCalc statistical software version 17.9.2. (MedCalc Software, Ostend, Belgium). Results were statistically significant if P < 0.05.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Results
Characteristics of the study participants
109 COPD patients and 95 healthy volunteers were included in the present study. A brief overview of all participants including basic characteristics, spirometry and common inflammatory parameters are shown in Table 1. There are no differences between controls and COPD patients regarding age and sex distribution, while lung function parameters were decreased in COPD patients (P < 0.001). Markers of systemic inflammation, CRP, FIB and WBC were significantly increased in COPD patients.
IL-1β concentration and IL1B, NLRP3 and CASP1 expression in the systemic compartment
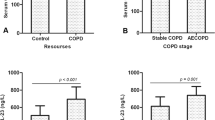
Concentration of IL-1β was also significantly increased in plasma of patients with COPD (6.90 (0.61–23.91) pg/mL) when compared to healthy subjects (0.10 (0.10–0.23) pg/mL), P < 0.001. In addition, expression of IL1B, NLRP3 and CASP1 were increased in COPD patients in comparison to controls (Fig. 1). However, there was no significant difference in IL-1β concentration (Fig. 2a) or IL1B, NLRP3 and CASP1 expression (Fig. 2b) between COPD patients with different severity of the airway obstruction. In addition, the concentration of IL-1β and expressions of IL1B, NLRP3 and CASP1 were assessed based on smoking status in COPD patients and healthy volunteers (Fig. 3). Plasma concentration of IL-1β showed to be increased in all three COPD smoking groups in comparison to all healthy individuals (Fig. 3a). NLRP3 gene expression showed to be increased in healthy smokers compared to healthy non-smokers. On the other hand, all three groups of COPD patients in terms of smoking status showed to have increased NLRP3 mRNA levels in comparison to healthy non-smokers but only former and current COPD smokers had increased NLRP3 expression compared to healthy smokers, while COPD non-smokers did not differ in NLRP3 expression from healthy smokers. In addition, COPD non-smoking group was statistically similar to healthy subjects in terms of IL1B and CASP1 mRNA levels. Expression of IL1B was increased in COPD former smokers in comparison to healthy non-smokers, and in COPD smokers in comparison to both healthy non-smokers and healthy smokers, while CASP1 expression was increased in COPD former smokers and COPD smokers in comparison to both smoking groups of healthy volunteers (Fig. 3b).
Relative gene expression of IL1B, NLRP3 and CASP1 in control and COPD groups. IL1B interleukin-1beta gene, NLRP3 nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 gene, CASP1 caspase-1 gene, COPD chronic obstructive pulmonary disease. Mann–Whitney test was used for comparison of IL1B, NLRP3 and CASP1 relative expression between controls and COPD patients. Results were presented as a median and IQR.
Plasma IL-1β concentration (a) and relative expression of IL1B, NLRP3 and CASP1 (b) in control and COPD subjects according to the severity of airflow obstruction. IL-1β interleukin-1beta, IL1B interleukin-1beta gene, NLRP3 nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 gene, CASP1 caspase-1 gene, COPD chronic obstructive pulmonary disease, GOLD Global Initiative for Chronic Obstructive Pulmonary Disease, C controls, G2 GOLD 2, G3 GOLD 3, G4 GOLD 4. Data were tested by Kruskal–Wallis test, and results were presented as a median and IQR. If P < 0.05, post-hoc test was performed. Connectors are showing the differences with P < 0.05 between the groups after post-hoc analysis.
IL-1β plasma concentration (a) and relative expression of IL1B, NLRP3 and CASP1 (b) in healthy subjects and COPD patients when subdivided into the groups based on smoking status. IL-1β interleukin-1beta, IL1B interleukin-1beta gene, NLRP3 nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 gene, CASP1 caspase-1 gene, COPD chronic obstructive pulmonary disease; 1: control non-smokers; 2: control smokers; 3: COPD non-smokers; 4: COPD former smokers; 5: COPD smokers. Data were tested by Kruskal–Wallis test, and results were presented as a median and IQR. If P < 0.05, post-hoc test was performed. Connectors are showing the differences with P < 0.05 between the groups after post-hoc analysis.
Predictive value of the selected parameters
Next, univariate logistic regression analysis was performed, and it showed that all examined parameters had statistically significant predictive potential for COPD according to the ORs and 95% CIs. Based on the results from univariate logistic regression analysis, multivariable logistic regression analysis was performed, and it showed that the combination of three parameters: IL-1β, FIB and WBC correctly classified 89% of cases. All results are summarized in Table 2.
Discussion
Our study showed significantly increased plasma concentration of IL-1β as well as gene expression of IL1B, NLRP3 and CASP1 in COPD patients in stable phase compared to healthy controls, but there was no association with the severity of airflow obstruction or smoking status. In addition, IL-1β in the combination with WBC and FIB correctly classified 89% of all cases.
Several studies have investigated the systemic and local inflammatory state in COPD19,20,21,22, but the mechanisms of COPD pathogenesis are still poorly understood. Recent evidence suggests the involvement of the multiprotein complex NLRP3 inflammasome in the development of COPD23, but data regarding its activation are still scarce and contradictory. The present study showed that the plasma concentration of IL-1β is elevated in COPD patients compared to healthy controls, and the observed result is consistent with some previous studies that have investigated IL-1β in peripheral blood and in respiratory system samples24,25. However, in the study by Kleniewska et al. IL-1β was increased in the induced sputum of COPD patients, but there was no difference in its concentration in serum in comparison to healthy subjects26.
Faner et al. showed that IL1B and NLRP3 mRNA was upregulated in lung tissue of stable COPD, but caspase-1 was mostly found in an inactive form, suggesting that the NLRP3 inflammasome was primed, but not activated10. On the contrary, in patients in acute exacerbation of COPD significantly increased gene expression of IL1B, NLRP3 and CASP1 as well as plasma concentration of IL-1β in peripheral blood was demonstrated27, whereas our study showed that similar processes also occurred in the stable phase of the disease. Given the lack of data on NLRP3 inflammasome in the systemic compartment of COPD patients, the present study could suggest that its activation may play an important role in the peripheral circulation as well as in the local respiratory compartment.
Regarding the severity of COPD, there was no association between IL1B, NLRP3 and CASP1 expression and the level of airflow obstruction in our study, and the same was observed for the plasma concentration of IL-1β. No association was found between plasma concentration of IL-1β and the severity of airflow obstruction in stable COPD in the study by Zou et al.25.
Cigarette smoke plays an important role in the development of inflammation28. To the best of our knowledge, there are no data on NLRP3 inflammasome activation in the peripheral circulation of COPD patients associated with patients’ smoking status. We found that expression of IL1B, NLRP3 and CASP1 as well as concentration of IL-1β amongst COPD patients were independent of their smoking history. However, results should be interpreted carefully and replicated analysis is suggested due to the small number of COPD non-smokers enrolled in the study.
Finally, we wanted to evaluate the diagnostic potential of the determined parameters. Based on the results of univariate logistic regression analysis, multivariable logistic regression analysis was performed, and it was found that the best model for COPD prediction is composed of IL-1β, FIB and WBC with correctly classified 89% of cases. However, a prospective and longitudinal study of IL-1β, WBC and FIB and of lung function should be performed to confirm potential clinical utility of these biomarkers in early identification of individuals at risk for developing COPD. This is important since COPD is a progressive lung condition with the significant impact on morbidity and mortality worldwide29.
The results of the study should be interpreted considering several limitations. First, a relatively small number of participants were included in the study, which affected the statistical power. Further studies should include more subjects, including those in the GOLD 1 stage of the disease as well as COPD non-smokers. However, COPD patients in GOLD 1 stage rarely contact physicians due to the mild symptoms, while most of the COPD patients are smokers (former or current), and, therefore, it is very difficult to recruit those specific subgroups. Currently, translating the signals into clinical practice and unravelling the complex interactions that lead to disease is challenging. Therefore, further studies are recommended with the aim to explore and explain COPD endotypes.
In conclusion, the results of our study suggest activation of NLRP3 inflammasome in the systemic compartment in patients with stable COPD, and also that the best model for COPD prediction might be the combination of three inflammatory parameters: IL-1β, FIB and WBC. Further investigations need to be designed to fully elucidate the comprehensive heterogeneity of COPD, which will enable to recognise novel diagnostic and/or therapeutic targets.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Global Health Estimates 2020. The Top 10 Causes of Death 2019 (World Health Organization, 2020). Retrieved from https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Miravitlles, M. et al. Prevalence of COPD in Spain: Impact of undiagnosed COPD on quality of life and daily life activities. Thorax 64(10), 863–868 (2019).
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2022 Report) [(accessed on 27th January 2022)]. Available online: https://goldcopd.org/gold-reports/.
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19(8), 477–489 (2019).
Wang, Z. et al. NLRP3 inflammasome and inflammatory diseases. Oxid. Med. Cell. Longev. 2020, 4063562 (2020).
Shao, B. Z. et al. Targeting NLRP3 inflammasome in inflammatory bowel disease: Putting out the fire of inflammation. Inflammation 42(4), 1147–1159 (2019).
Rai, R. C. Host inflammatory responses to intracellular invaders: Review study. Life Sci. 240, 117084 (2020).
Kelley, N., Jeltema, D., Duan, Y. & He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20(13), 3328 (2019).
Pauwels, N. S. et al. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur. Respir. J. 38(5), 1019–1028 (2011).
Faner, R. et al. The inflammasome pathway in stable COPD and acute exacerbations. ERJ Open Res. 2(3), 00002–02016 (2016).
Di Stefano, A. et al. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax 69(6), 516–524 (2014).
Hirota, J. A. et al. The airway epithelium nucleotide-binding domain and leucine-rich repeat protein 3 inflammasome is activated by urban particulate matter. J. Allergy Clin. Immunol. 129(4), 1116–25.e6 (2012).
Simundic, A. M. et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin. Chem. Lab. Med. 56(12), 2015–2038 (2018).
Clinical and Laboratory Standards Institute (CLSI). Collection, Transport, and Processing of Blood Specimens for Testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays; Approved Guideline-Fifth Edition. CLSI Document H21-A5 (CLSI, 2008).
Hlapčić, I. et al. Platelet indices in stable chronic obstructive pulmonary disease—Association with inflammatory markers, comorbidities and therapy. Biochem. Med. 30(1), 010701 (2020).
Rio, D. C., Ares, M., Hannon, G. J. & Nilsen, T. W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, db–prot5439 (2010).
Li, C. et al. Polymorphisms in endoplasmic reticulum aminopeptidase genes are associated with cervical cancer risk in a Chinese Han population. BMC Cancer 20(1), 341 (2020).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4), 402–408 (2001).
Moermans, C. et al. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine 56(2), 298–304 (2011).
Selvarajah, S. et al. Multiple circulating cytokines are coelevated in chronic obstructive pulmonary disease. Mediators Inflamm. 2016, 3604842 (2016).
Hlapčić, I. et al. Combination of systemic inflammatory biomarkers in assessment of chronic obstructive pulmonary disease: Diagnostic performance and identification of networks and clusters. Diagnostics 10(12), 1029 (2020).
Hlapčić, I. et al. Influence of disease severity, smoking status and therapy regimes on leukocyte subsets and their ratios in stable chronic obstructive pulmonary disease. Arch. Med. Sci.18(3), 672–681 https://doi.org/10.5114/aoms.2020.100720 (2022).
Colarusso, C., Terlizzi, M., Molino, A., Pinto, A. & Sorrentino, R. Role of the inflammasome in chronic obstructive pulmonary disease (COPD). Oncotarget 8(47), 81813–81824 (2017).
Botelho, F. M. et al. IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS ONE 6(12), e28457 (2011).
Zou, Y. et al. Serum IL-1β and IL-17 levels in patients with COPD: Associations with clinical parameters. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 1247–1254 (2017).
Kleniewska, A., Walusiak-Skorupa, J., Piotrowski, W., Nowakowska-Świrta, E. & Wiszniewska, M. Comparison of biomarkers in serum and induced sputum of patients with occupational asthma and chronic obstructive pulmonary disease. J. Occup. Health 58(4), 333–339 (2016).
Wang, H. et al. NLRP3 inflammasome involves in the acute exacerbation of patients with chronic obstructive pulmonary disease. Inflammation 41(4), 1321–1333 (2018).
Zhu, B., Wang, Y., Ming, J., Chen, W. & Zhang, L. Disease burden of COPD in China: A systematic review. Int. J. Chron. Obstruct. Pulmon. 13, 1353–1364 (2018).
Matheson, M. C. et al. Prediction models for the development of COPD: A systematic review. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 1927–1935 (2018).
Funding
This work has been fully supported by the Croatian Science Foundation under the project number IP-2014-09-1247. The work of PhD student Iva Hlapčić has been fully supported by the "Young researchers" career development project—training of doctoral students” of the Croatian Science Foundation funded by the European Union from the European Social Fund. This work has been supported in part by project Strengthening the scientific research and innovation capacities of Faculty of Pharmacy and Biochemistry, University of Zagreb (FarmInova; project number KK.01.1.1.02.0021) financed from the European Regional Development Fund, Operational Program Competitiveness and Cohesion for the period 2014–2020.
Author information
Authors and Affiliations
Contributions
I.H. and M.R.A. carried out the experiments. A.V.D., L.R., S.P.-G. and M.S. were involved in planning and supervised the work. I.H. and L.R. processed the experimental data and performed the analysis, while I.M. and I.H. drafted the manuscript, designed the figure and prepared the tables. I.M., A.V.D., S.P-G. and M.S. collected the samples, while M.R.A. and A.Č. contributed to the sample preparation. A.Č. contributed to the implementation of the research and the experiments. All authors provided critical feedback and helped shape the research, analysis and manuscript. I.M. and I.H. contributed equally, while A.V.D. and L.R. share the senior authorship.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Markelić, I., Hlapčić, I., Čeri, A. et al. Activation of NLRP3 inflammasome in stable chronic obstructive pulmonary disease. Sci Rep 12, 7544 (2022). https://doi.org/10.1038/s41598-022-11164-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11164-1
This article is cited by
-
Linking NLRP3 inflammasome and pulmonary fibrosis: mechanistic insights and promising therapeutic avenues
Inflammopharmacology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.