Abstract
Hyaluronic acid (HA) is a key component of the extracellular matrix. HA and its metabolism are suggested to be altered in the lungs of patients with chronic obstructive pulmonary disease (COPD). The present study explored systemic HA, and its metabolic regulators, in patients with clinically stable COPD and smoking and non-smoking controls. Furthermore, associations of HA with acute exacerbations (AECOPD), airway-related hospitalizations, systemic inflammation and cardiovascular risk were studied. In total, 192 patients with moderate to very severe COPD [aged 62.3 y (± SD 7.0)], 84 smoking controls [aged 61.8 y (± 5.7)], and 107 non-smoking controls [aged 60.1 y (± 7.0)] were included. Plasma HA was reduced in patients with COPD compared to non-smoking controls (p = 0.033), but was comparable after adjusting for age and sex. Expression of HAS-3 did not differ between groups, but was substantially less detectable in more patients with COPD than (non)smoking controls (p < 0.001). Expression of HYAL-2 was enhanced in patients with COPD versus smoking (p = 0.019) and non-smoking (p < 0.001) controls, also in the age- and sex- adjusted model (p < 0.001). Plasma HA was not associated with AECOPD, airway-related hospitalizations in the previous year, or systemic inflammation in COPD. Arterial pulse wave velocity explained some of the variance (< 10%) in plasma HA (p = 0.006). Overall, these results indicate that expression of HYAL-2, but not plasma HA nor HAS-3, is enhanced in patients with COPD compared to (non)smoking controls. Furthermore, HA was not associated with clinical outcomes, yet, cardiovascular risk might play a role in its systemic regulation in stable COPD.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous chronic lung disease that is characterized by persistent airflow limitation and respiratory symptoms1,2,3. At present, there is a growing understanding of the essential role of extracellular matrix (ECM) integrity in the pathophysiology of COPD4,5,6,7,8,9,10. Attracting a particular interest is the ECM’s most abundant non-sulphated glycosaminoglycan (GAG) hyaluronic acid (HA), or also referred to as hyaluronan. Depending on its molecular size, HA may exert different biological functions5,6,11. As such, high-molecular weight (HMW) HA (> 500 kDa) has anti-inflammatory and immunosuppressive properties and contributes to tissue hydration and stability, whereas low-molecular weight (LMW) HA (< 250 kDa) is positively associated with inflammation and tissue injury6,12,13,14.
The role of HA in COPD is still largely unknown. Several studies have reported increased HA, in particular LMW, in the lungs of patients with COPD12,15, whereas others showed decreased levels in isolated airway smooth muscle cells (ASMCs)16. Moreover, alterations in the expression and activity of the enzymatic regulators of HA metabolism, HA synthases (HAS) and hyaluronidases (HYAL), have been reported in the lungs15 and ASMCs of patients with COPD16, as well as in cigarette smoke-exposed mice17 and primary human lung-derived models18. The intracellular synthesis of HA is conducted by HAS, whereas HYAL metabolically degrade HA6, each isoform at a distinct catalytic rate, yielding HA of distinct molecular masses19,20. The formation of smaller fragments of HA is now suggested to contribute to the continuation of inflammation6,19,21,22,23.
Indeed, positive associations of pulmonary HA with local inflammation and decreased lung function have been reported in COPD12,13,15. Furthermore, HA seems to be associated with acute exacerbations of COPD (AECOPD). These episodic acute events, that are characterized by increased respiratory symptoms, play a pivotal role in the natural course of COPD and worsen quality of life and physical activity1. Also, they are associated with increased risk of hospitalization, disease progression and mortality, and significantly contribute to healthcare costs3,24,25. During these events, increased HYAL activity and subsequent degradation of HA were observed in the lungs of patients with COPD, and therefore suggested as potential targets to control airway inflammation and remodeling12. These findings were recently also shown, for the first time, in serum of exacerbating patients with COPD11. Hence, the potential of HA to serve as a biomarker of COPD disease severity and/or progression, was suggested. However, the role and clinical usefulness of HA as biomarker remains unknown while case–control studies remain lacking.
Furthermore, although COPD, particularly during AECOPD, is characterized by transiently increased airway- and systemic inflammation1,24, which may result in decreased integrity of the ECM6,26,27,28,29, systemic HA was shown not to be associated with emphysema11,12 and may therefore not originate from degradation of the parenchymal ECM. Instead, a cardiovascular origin seems plausible since cardiovascular pathologies are highly associated with dysfunction and degradation of the HA-rich endothelial glycocalyx30,31. Indeed, individuals at increased cardiovascular risk exhibit increased serum HA32. Though, degradation of the endothelial glycocalyx can also be inflammatory-mediated30,31,33. Moreover, circulating immune cells may be a direct source of HA due to their CD44 dependent pericellular HA-rich coat6,21,34,35,36,37. Finally, immune cells may also exhibit HYAL activity38,39, which might further enhance systemic levels of HA by increased HA fragmentation6,20. Taken together, a cardiovascular and/or systemic inflammatory origin of systemic HA is presumable, yet it is unexplored.
The present study was designed to assess (1) whether, and to what extent, systemic HA and HA metabolism differ between patients with COPD and (non)smoking controls, and (2) to study the associations of HA with AECOPD frequency, airway-related hospitalizations, systemic inflammation and cardiovascular risk in COPD. We hypothesize that systemic HA is increased, and related to the number of past AECOPD and airway-related hospitalizations, in patients with COPD. Moreover, a shared cardiovascular and systemic inflammatory origin is expected.
Methods
Study population
The present study is a post-hoc cross-sectional analysis of baseline data of the “Individualized COPD Evaluation in relation to Ageing” (ICE-Age) study; a single-center, longitudinal, observational study conducted between December 2010 and August 2016 at Ciro, a tertiary care center for patients with chronic respiratory diseases in Horn, the Netherlands. Detailed information about the aims, inclusion and exclusion criteria of the ICE-Age study has previously been described elsewhere40,41.
Clinical characteristics
Demographics and clinical characteristics were collected40,41, please visit the online supplement for an overview. Of note, clinical stability, defined by the absence of respiratory tract infection or exacerbation for < 4 weeks before study entry, had to be met for patients with COPD to be included in the ICE-Age study. The number of AECOPD in the previous 12 months was documented at study entry and relied on self-report40. An exacerbation was defined by the acute need of oral glucocorticosteroids or antibiotics and/or hospitalization, due to acute respiratory worsening. Based on the Global initiative for chronic Obstructive Lung Disease (GOLD) strategy document1 the following subgroups were identified; infrequent exacerbators (i.e. patients experiencing < 2 AECOPD in the past year) and frequent exacerbators (i.e. patients experiencing ≥ 2 AECOPD in the past year). Furthermore, the number of self-reported hospital admissions for airway disease in the last 12 months was recorded. Patients with a moderate disease history (i.e. no hospital admissions in the past year) and patients with a severe disease history (i.e. ≥ 1 hospital admission in the past year) were identified. The control group was divided into smoking (i.e. ≥ 10 pack years) and non-smoking (i.e. < 10 pack years) controls. Arterial pulse wave velocity (APWV)40 was included to study cardiovascular risk. The validated threshold value of 10 m/s was used to discriminate between normal and pathological patterns42. Moreover, a panel of systemic inflammatory markers, including total leukocyte counts, fibrinogen, interleukin (IL) 6 and 8, tumor necrosis factor (TNF) alpha and high-sensitivity C-reactive protein (CRP)41 were included to study the association with systemic inflammation.
Plasma HA measurements
Fasted venous blood samples were collected in ethylene diamine tetra acetic acid (EDTA) containing tubes and stored at − 80 °C until further analysis, as previously described41. Plasma samples obtained at baseline, available for secondary research were used in the present study; only blood samples of subjects who provided written approval for use of body material for secondary research purposes were used. Natural plasma HA was measured using a solid phase HA binding protein-based sandwich enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s protocol (Hyaluronan DuoSet ELISA, R&D Systems, Minneapolis, MN, USA). Samples were measured in duplicate, and phosphate buffered saline samples were included and confirmed as negative controls. Results were analyzed in Excel (Microsoft Excel 2007, Redmond, WA, USA). The average intra-assay coefficient of variation was 12.6%. A power calculation is provided in the online supplement.
mRNA expression of HAS and HYAL
cDNA from peripheral blood mononuclear cells (PBMC) was available from a subset of subjects to measure gene expression of the enzymatic regulators of HA; HAS and HYAL. Specifically, expression levels of HAS-3 and HYAL-2 were assessed, whereas HAS-1, HAS-2 and HYAL-1 were excluded due to their lack of expression in PBMC43. Since HAS-3 is the most active HAS isoform19, and due to its expression in T-cells43, the most prominent cell type in PBMC, HAS-3 was included in the present study. Moreover, HYAL-2 is widely expressed in PBMC, including monocytes, T-cells and natural killer cells, and was therefore included as well43. In total, samples of 143 patients with COPD, 21 smoking controls and 20 non-smoking controls were available for the present analyses. Please see the online supplement for the quantitative polymerase chain reaction (qPCR) procedure, the primer sequences of HAS-3, HYAL-2, and the housekeeping genes ribosomal protein P0, ribosomal protein L13A and beta-globin. Of note, expression of HAS-3 was below the threshold in a substantial number of samples, please see results. However, the low expression of HAS-3 was not related to the quality of these samples since the housekeeping genes were expressed. Expression levels of HAS-3 were therefore extrapolated in these samples. Please see the online supplement for a detailed description. For visual presentation, expression levels of HAS-3 were multiplied by a factor of 1.000.000, and expression levels of HYAL-2 were multiplied by a factor of 1.000. Measurements of plasma HA as well as HAS and HYAL expression were performed in a random order and single-blinded.
Statistical analyses
Statistical analyses and visualization were performed using IBM SPSS Statistics 25 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8.3.5. (GraphPad Software, La Jolla, CA, USA). Categorical variables were expressed as absolute numbers and percentages. Continuous variables were tested for normality using the Shapiro–Wilk test and visual inspection of histograms, and were expressed accordingly as mean and standard deviation (SD), or as median and interquartile range (IQR). The Pearson Chi-Square test was used to assess differences in dichotomous variables between groups. Differences in continuous variables were analyzed using the one-way analysis of variance (ANOVA) test, Mann–Whitney U test and Kruskal–Wallis H-test, as appropriate. Post hoc pairwise comparisons were performed and corrected for multiple comparisons with a Bonferroni correction, adjusted p values were selected. The analysis of covariance (ANCOVA) was performed to adjust for the covariates age and sex. Correlations were assessed using the nonparametric Spearman’s rho correlation test. The magnitude of correlations was interpreted using Cohen’s effect sizes; correlation coefficients of < 0.10 represent a poor correlation, correlation coefficients of 0.30 represent a moderate correlation and correlation coefficients of > 0.50 represent a strong correlation44.
Furthermore, multiple regressions were performed to study the association between HA and APWV, as well as markers of systemic inflammation. The dependent variable HA, and independent variables APWV, total leukocytes, fibrinogen, IL-6, IL-8, TNF-alpha and CRP were added to the regression models. Moreover, age, sex and COPD-specific medications including long-acting β2-agonists (LABA), inhaled corticosteroids (ICS) and a combination thereof were included as covariates. The latter for their known effects on HA metabolism45. Univariate models were performed in model 1. Significant variables were considered for inclusion in the covariate adjusted model 2. A priori, p values ≤ 0.05 were considered statistically significant.
Ethics approval and consent to participate
The ICE-Age study was approved by the local ethics and review board of the Maastricht University Medical Centre (Maastricht, The Netherlands; MEC 10-3-033) and is ISRCTN registered (ISRCTN86049077). The ICE-Age study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. Subjects enrolled in the ICE-Age study provided written informed consent. Only plasma samples of subjects who provided written approval for use of body material for secondary research purposes were used in the present study.
Consent for publication
Results of the ICE-Age study may be published conform the basic principles of the CCMO publication policy.
Results
Baseline clinical characteristics
In total, 192 patients with moderate to severe COPD, 84 smoking and 107 non-smoking controls were analyzed. The majority of patients with COPD and smoking controls was male, whereas non-smoking controls were mainly female and 2 years younger than patients with COPD (Table 1). The vast majority of patients was classified as GOLD II, and used long-acting muscarinic antagonists (LAMA), LABA and/or ICS. Most patients were highly symptomatic as expressed by a medical research council (MRC) dyspnea score of three or higher. Furthermore, almost half of the patients experienced two or more AECOPD in the previous year, while close to one third experienced at least one airway-related hospital admission in the last year. Smoking and non-smoking controls had significantly less pack years than patients with COPD. Finally, groups were similar in body mass index (BMI) and were mainly overweight. Please see the online supplement for baseline cardiovascular- and inflammatory measures (Table S2, online supplement). Briefly, APWV, total leukocyte counts, fibrinogen, IL-8 and CRP were significantly higher in patients with COPD compared to smoking and non-smoking controls. Furthermore, TNF-alpha was significantly higher in smoking controls compared to patients with COPD.
Plasma HA and its enzymatic regulators
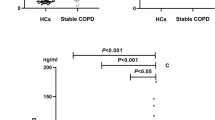
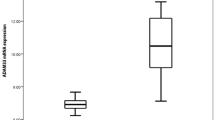
Plasma HA was lower in patients with COPD compared to non-smoking controls, but did not differ compared to smoking controls, neither between both control groups (Fig. 1A). However, after correcting for baseline differences in age and sex, plasma HA was comparable between patients with COPD and non-smoking controls (p = 0.185). Expression of HAS-3 did not differ between the groups (Fig. 1B), yet a below limit detection was observed in significantly more samples of patients with COPD (n = 80, 56.0%) than smoking controls (n = 7, 33.3%) and non-smoking controls (n = 2, 10.0%), p < 0.001. Excluding these samples, i.e. a below limit detection of HAS-3 expression, revealed enhanced expression in patients with COPD compared to smoking controls (Fig. S1, online supplement), yet, no longer when adjusting for age- and sex (p = 0.231). Expression of HYAL-2 was significantly higher in patients with COPD compared to both smoking and non-smoking controls, but did not differ between the control groups (Fig. 1C). These findings did not change when adjusted for age and sex (p < 0.001).
(A) Plasma hyaluronic acid (HA) in patients with COPD (n = 192), smoking controls (SC, n = 84) and non-smoking controls (NSC, n = 107). (B) mRNA expression of HAS-3 and; (C) HYAL-2 in patients with COPD (n = 143), SC (n = 21) and NSC (n = 20). Median and interquartile ranges are presented. *Significant post-hoc pairwise comparison. Figures were created using GraphPad Prism 8.3.5, https://www.graphpad.com/scientific-software/prism/.
Subdividing the COPD group based on plasma HA levels below and above the median of 8.0 ng/ml did not reveal any differences in HAS-3 (p = 0.497) or HYAL-2 (p = 0.219) expression. Yet, HA was significantly correlated with HAS-3 and HYAL-2 in patients with COPD (respectively negative and positive correlations, Table S3, online supplement). Furthermore, a complete-case analysis on plasma HA, HAS-3 and HYAL-2 revealed a slightly reduced median of plasma HA in the patient group (7.18 ng/ml, n = 143), whereas higher medians were found in smoking (9.02 ng/ml, n = 21) and non-smoking (10.4 ng/ml, n = 20) controls (p = 0.040). The previously observed group differences in HAS-3 (p = 0.352) and HYAL-2 expression (p < 0.001) did not change in this complete-case analysis.
Association of HA with previous AECOPD and airway-related hospitalizations
No significant differences were observed in plasma HA between frequent and infrequent exacerbating patients with COPD (Fig. 2A) or between patients with a moderate and severe disease history in the last year (Fig. 2B). In line with these findings, no significant correlations were observed between HA and the number of AECOPD [(r = 0.006, p = 0.935) n = 181] or airway-related hospital admissions [(r = 0.142, p = 0.051) n = 190]. With respect to HAS-3 and HYAL-2, no significant differences were observed between frequent and infrequent exacerbating patients (Fig. 2C and E) or between patients with a moderate and severe disease history in the last year (Fig. 2D and F). Similarly, no significant correlations were observed between HAS-3 and HYAL-2 and the number of AECOPD [(r = -0.072, p = 0.405 and r = -0.070, p = 0.421 respectively) n = 136] or the number of airway-related hospitalizations [(r = -0.035, p = 0.681 and r = -0.008, p = 0.921 respectively) n = 141].
Differences in plasma HA and expression of HAS-3 and HYAL-2 in patients with COPD grouped by AECOPD frequency, and disease severity in the past year*. (A) Plasma HA in infrequent (n = 95) and frequent exacerbating patients (n = 86). (B) Plasma HA in patients with a moderate (n = 131) and severe disease history (n = 59). (C) Expression of HAS-3; and (E) expression of HYAL-2 in infrequent (n = 72) and frequent exacerbating patients (n = 64). (D) Expression of HAS-3; and F) expression of HYAL-2 in patients with a moderate (n = 100) and severe disease history (n = 41). *Infrequent AECOPD were defined by < 2 AECOPD and frequent AECOPD by ≥ 2 AECOPD in the past year. A moderate disease history was defined by no airway-related hospitalizations and a severe disease history by ≥ 1 airway-related hospitalization in the past year. Median concentrations/expression levels and interquartile ranges are presented. Figures were created using GraphPad Prism 8.3.5, https://www.graphpad.com/scientific-software/prism/.
Association of plasma HA with systemic inflammation and cardiovascular risk
Plasma HA was positively correlated with IL-6, and negatively correlated with IL-8, in patients with COPD (Table 2). No significant correlations were observed in the control groups. With respect to cardiovascular risk, a positive correlation with APWV was observed in patients with COPD, but not in smoking and non-smoking controls. Furthermore, except for a positive correlation between HA and age in all groups, no significant correlations with any of the clinical outcomes were observed (Table S3, online supplement).
With respect to our regression models, APWV was significantly associated with plasma HA in patients with COPD, in both the univariate and multivariate model (Table 3). Though, the explained variance (R2) was less than 10% in both models (univariate; 6.8%, multivariate; 8.7%). No significant results were observed in the control groups (data not shown). Of note, subdividing patients with COPD based on APWV scores below and above the cut-off value that discriminates between cardiovascular risk, revealed significantly elevated plasma HA levels in patients at increased cardiovascular risk (9.32 ng/ml, n = 78) compared to patients who were not (7.14 ng/ml, n = 93), p = 0.015. Yet, expression of HAS-3 and HYAL-2 did not differ between the latter groups (data not shown).
Discussion
This study provides novel insights into the alterations of systemic HA and its metabolism in patients with clinically stable COPD. Our results revealed that expression of HYAL-2, but not plasma HA nor HAS-3, was enhanced in patients with COPD compared to (non)smoking controls. Furthermore, while cardiovascular risk was positively associated with plasma HA in COPD, no additional associations with clinical outcomes were found. To the best of our knowledge this is the first study to report plasma levels of HA in patients with COPD and non-COPD controls, and to show that cardiovascular risk might be involved with its systemic regulation in stable COPD.
In contrast to our hypothesis, plasma HA did not differ between patients with COPD and (non)smoking controls. While these results initially may seem to contradict previous findings, it should be noted that patients with stable COPD, defined by the absence of AECOPD at least 4 weeks prior to inclusion, were included in the present study. Previous studies have reported elevated levels of serum HA at exacerbation of COPD compared to a convalescent disease state11. Moreover, to date no controls were included in such studies. Hence, our data cannot be compared. In this respect, to what extent background heterogeneity may have confounded the present results remains unknown. Therefore, case–control studies with longitudinal follow-up are indicated to validate our findings as well as to compare the effects of an exacerbation. Nonetheless, while the present cross-sectional design by no means allows us to study the biomarker potential of HA, we hypothesize that its clinical potential, if any, may be acute rather than predictive/prognostic. Studies are warranted to elaborate further on this. Noteworthy, the observed concentrations of HA in plasma were lower compared to previous findings in serum of patients with COPD11. Whether these differences are of physiological relevance, remains unknown.
Serum levels of HA were previously reported to remain significantly elevated during, and up to 4 weeks after an AECOPD11. Therefore, elevated levels of plasma HA may have been expected in the current frequent exacerbating group. However, plasma HA, nor HAS-3 and HYAL-2, differed between frequent and infrequent exacerbating patients with COPD, or between patients with a moderate and severe disease history in the past year. These findings may further support opposite acute and chronic effects, in which enhanced levels of systemic HA may be observed during AECOPD11, in contrast to lower levels in stable disease. Furthermore, serum HA was reported to associate with the severity of AECOPD11. Indeed, although lacking statistical significance, we observed a close trend towards higher plasma HA levels in patients with a severe disease history (Fig. 2B), as well as a positive association with airway-related hospital admissions. Notwithstanding, plasma HA did not differ between patients with COPD with different GOLD stages (p = 0.952).
Systemic HA is cleared by the lymphatic system, liver and kidney46. As a result, differential clearance rates may have affected the observed differences in plasma HA. In the present study, hepatic function did not differ between the groups. Furthermore, although patients with COPD had a lower estimated glomerular filtration rate (eGFR) than smoking controls, renal dysfunction was not observed. Nevertheless, to ensure that clearance rate variability was accounted for when comparing plasma HA across the groups, we adjusted for these markers of renal- and hepatic function in addition to age and sex. The latter did not reveal any differences in plasma HA between the groups (p = 0.283). Noteworthy, eGFR (p = 0.565) nor alanine aminotransferase (ALT) (p = 0.263) contributed significantly to the model. It is unknown whether renal- and/or hepatic dysfunction may have affected the previously observed concentrations of HA in serum11.
While increased expression of HAS-3 may have been expected in patients with COPD, due to the low-grade systemic inflammation that is associated with the disease47 and known to increase its expression21, no differences in HAS-3 expression were observed between patients with COPD and (non)smoking controls in the present study. However, having included stable patients with COPD may explain these findings due to the absence of acute stress and/or inflammation. Indeed, it may require an acute increase in inflammation, as typically observed during AECOPD24, for increased expression of HAS-3 to be reflected systemically, and thus to yield differences compared to non-COPD controls.
With respect to HYAL-2, increased expression was observed in patients with COPD compared to both smoking and non-smoking controls. These results are in line with previous findings in sputum of stable-15, as well as in serum and the lungs of exacerbating patients with COPD11,12. Although these results may suggest a clinical potential of HYAL-2, rather than HA and HAS-3, no associations with clinical outcomes of COPD were found (data not shown). Nonetheless, the observed group differences in HYAL-2 expression may provide an indication of the molecular size of HA. Indeed, plasma HA was measured by ELISA (R&D Systems), which detects HA of low (15–40 kDa), medium (75–350 kDa) and high (> 950 kDa) MW. However, this assay does not distinguish between these different molecular sizes, and thus biological effects of HA. Therefore, while the net result of HYAL-2 activity is an increase in LMW-HA6,19,20, it is tempting to presume its enhancement in the patient group. In this view, a positive correlation between plasma HA and HYAL-2 was observed in patients with COPD. Thus, while the overall concentration of plasma HA in the patient group may have been low, it is plausible that the increased expression of HYAL-2 elicited an increased pool of the pro-inflammatory LMW-HA in patients with COPD compared to non-COPD controls.
Previous studies reported that HA was not related to markers of emphysema11,12. Although CT-scan parameters were lacking, HA and TLCO were also not correlated in the present study. Therefore, the association of HA with markers of systemic inflammation and cardiovascular risk was explored. In line with others48,49, and as previously published40, we observed that APWV was increased in patients with COPD compared to controls. Yet, the median value did not exceed the cutoff value of 10 m/s that is indicative of increased cardiovascular risk50. Our regression models showed that APWV, but none of the inflammatory markers, explained some of the variance (R2 < 10%) in plasma HA of patients with COPD. Thus, cardiovascular risk, rather than systemic inflammation, may be involved with the regulation of systemic HA in stable COPD. Indeed, increased plasma HA levels were observed in patients with COPD at increased cardiovascular risk. In light of the mentioned reduced eGFR in patients with COPD, the addition of this marker to our regression model was explored next. While eGFR did not contribute significantly to the prediction of plasma HA (p = 0.521), the model improved (p = 0.035) and APWV remained significantly associated with plasma HA (p = 0.007).
Patients with COPD often have (multiple) cardiovascular comorbidities40 that are characterized by increased endothelial dysfunction and turnover51. Increased HA shedding from the glycocalyx may result in aggravated destabilization of the endothelial glycocalyx and subsequent vascular complications such as angiopathy52,53. Bearing in mind the increased expression of HYAL-2, protecting the endothelial glycocalyx from HA shedding, an increasingly recognized goal in the management of sepsis and diabetes mellitus52,53, may warrant attention in patients with COPD as well. Nevertheless, cardiovascular risk only explained a minority of the variance in plasma HA. This emphasizes that our knowledge of its origin still is in its infancy. Indeed, although our data support a systemic origin, it does not provide proof, causal relationships or mechanistic insight, nor does it rule out a pulmonary or different organ origin.
Major strengths of the present study were the comprehensive clinical characterization and the large sample size to study plasma HA. Moreover, plasma samples, as well as PBMC, available for secondary analyses were used in this study. This led to optimum use of biological samples, preventing unnecessary waste. Still, several limitations were encountered. First, the sample size of the available PBMC samples to study mRNA expression of HAS-3 and HYAL-2 was substantially smaller in the control groups. In view of this, these analyses were also performed not dividing the control group into smoking and non-smoking controls. The latter revealed similar results, even after adjustment for age, sex and pack years. Furthermore, the number of AECOPD and airway-related hospital admissions in the previous year relied on self-report. In this respect, patients with COPD included in the study were not matched for disease severity and/or specific clinical features. Hence, the degree to which heterogeneity of disease has confounded the present results remains unknown and warrants further research. Finally, since cross-sectional data was presented, causal relations remain unknown. Therefore, longitudinal case–control studies including different sample types are suggested to provide a better understanding of the differences in local and systemic HA, within and across individuals over time. However, careful attention must be paid to the burden as well as costs of such extensive sampling, keeping future clinical implementation in mind.
Conclusion
Taken together, this study showed that expression of HYAL-2, but not plasma HA nor HAS-3, was enhanced in patients with clinically stable COPD compared to (non)smoking controls. Plasma HA was not associated with the frequency of AECOPD and airway-related hospitalizations in the past year, nor systemic inflammation in COPD. Nevertheless, the results suggested that cardiovascular risk might play a role in the regulation of systemic HA in stable COPD. Future studies are warranted to further increase our understanding of systemic HA, as well as its enzymatic regulators, in patients with COPD to support clinical recommendations.
Data availability
Based on ethical permission and Dutch patient data-protection laws data of the current study is not publicly available. Aggregated data is available from the senior author after formal ethics approval on reasonable request.
Abbreviations
- ASMC:
-
Airway smooth muscle cells
- AECOPD:
-
Acute exacerbation of COPD
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- ECM:
-
Extracellular matrix
- GAG:
-
Glycosaminoglycan
- HA:
-
Hyaluronic acid
- HAS:
-
HA synthases
- HYAL:
-
Hyaluronidases
- HMW:
-
High molecular weight
- LMW:
-
Low molecular weight
- IL:
-
Interleukin
- PBMC:
-
Peripheral blood mononuclear cells
- TNF-alpha:
-
Tumor necrosis factor alpha
References
GOLD. Global strategy for the prevention, diagnosis and management of chronic obstructive pulmonary disease—2021 report.
Celli, B. R. & Agustí, A. COPD: Time to improve its taxonomy?. ERJ Open Res. 4(1), 00132–02017 (2018).
Burge, S. & Wedzicha, J. COPD exacerbations: Definitions and classifications. Eur. Respir. J. 21(41 suppl), 46s–53s (2003).
Zhou, Y. et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 73, 77–104 (2018).
Burgstaller, G. et al. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 50(1), 1601805 (2017).
Papakonstantinou, E. & Karakiulakis, G. The ‘sweet’and ‘bitter’involvement of glycosaminoglycans in lung diseases: Pharmacotherapeutic relevance. Br. J. Pharmacol. 157(7), 1111–1127 (2009).
Karakioulaki, M., Papakonstantinou, E. & Stolz, D. Extracellular matrix remodelling in COPD. European Respiratory Review. 29, 158 (2020).
Dournes, G. et al. Computed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease. Correlation with pulmonary hypertension. Am. J. Respir. Crit. Care Med. 191(1), 63–70 (2015).
Viegi, G. et al. Definition, epidemiology and natural history of COPD. Eur. Respir. J. 30(5), 993–1013 (2007).
Wang, Y., Xu, J., Meng, Y., Adcock, I. M. & Yao, X. Role of inflammatory cells in airway remodeling in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 3341 (2018).
Papakonstantinou, E. et al. Serum levels of hyaluronic acid are associated with COPD severity and predict survival. Eur. Respir. J. 53(3), 1801183 (2019).
Papakonstantinou, E. et al. COPD Exacerbations are associated with proinflammatory degradation of hyaluronic acid. Chest 148(6), 1497–1507 (2015).
Garantziotis, S., Brezina, M., Castelnuovo, P. & Drago, L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 310(9), L785–L795 (2016).
Turino, G. M., Ma, S., Lin, Y. Y. & Cantor, J. O. The therapeutic potential of hyaluronan in COPD. Chest 153(4), 792–798 (2018).
Dentener, M., Vernooy, J., Hendriks, S. & Wouters, E. Enhanced levels of hyaluronan in lungs of patients with COPD: Relationship with lung function and local inflammation. Thorax 60(2), 114–119 (2005).
Klagas, I. et al. Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Eur. Respir. J. 34(3), 616–628 (2009).
Bracke, K. R. et al. Enhanced deposition of low-molecular-weight hyaluronan in lungs of cigarette smoke–exposed mice. Am. J. Respir. Cell Mol. Biol. 42(6), 753–761 (2010).
Papakonstantinou E, Klagas I, Miglino N, Karakiulakis G, Tamm M, Roth M. Cigarette smoke alters hyaluronic acid homeostasis in primary human lung fibroblasts. Eur. Respir. Soc. (2011).
Stern, R. Hyaluronan metabolism: A major paradox in cancer biology. Pathol. Biol. (Paris) 53(7), 372–382 (2005).
Stern, R., Kogan, G., Jedrzejas, M. J. & Šoltés, L. The many ways to cleave hyaluronan. Biotechnol. Adv. 25(6), 537–557 (2007).
Jiang, D., Liang, J. & Noble, P. W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91(1), 221–264 (2011).
Maharjan, A. S., Pilling, D. & Gomer, R. H. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PloS One. 6(10), e26078 (2011).
Rayahin, J. E., Buhrman, J. S., Zhang, Y., Koh, T. J. & Gemeinhart, R. A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 1(7), 481–493 (2015).
Perera, W. R. et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur. Respir. J. 29(3), 527–534 (2007).
Brightling C, Greening N. Airway inflammation in COPD-progress to precision medicine. Eur. Respir. J. 54, 1900651 https://doi.org/10.1183/13993003.00651-2019 (2019).
Schumann, D. M. et al. Collagen degradation and formation are elevated in exacerbated COPD compared with stable disease. Chest 154(4), 798–807 (2018).
Sand, J. M. et al. Accelerated extracellular matrix turnover during exacerbations of COPD. Respir. Res. 16(1), 69 (2015).
Bihlet, A. R. et al. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir. Res. 18(1), 22 (2017).
Stolz, D. et al. Systemic biomarkers of collagen and elastin turnover are associated with clinically relevant outcomes in COPD. Chest 151(1), 47–59 (2017).
Becker, B. F., Jacob, M., Leipert, S., Salmon, A. H. & Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 80(3), 389–402 (2015).
Tarbell, J. M. & Cancel, L. M. The glycocalyx and its significance in human medicine. J. Intern. Med. 280(1), 97–113 (2016).
Papanastasopoulou, C. et al. Cardiovascular risk and serum hyaluronic acid: A preliminary study in a healthy population of low/intermediate risk. J. Clin. Lab. Anal. 31(1), 22010 (2017).
Uchimido, R., Schmidt, E. P. & Shapiro, N. I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 23(1), 16 (2019).
Evanko, S. P., Tammi, M. I., Tammi, R. H. & Wight, T. N. Hyaluronan-dependent pericellular matrix. Adv. Drug Deliv. Rev. 59(13), 1351–1365 (2007).
Lee-Sayer, S. S. et al. The where, when, how, and why of hyaluronan binding by immune cells. Front. Immunol. 6, 150 (2015).
Culty, M., O’Mara, T. E., Underhill, C. B., Yeager, H. Jr. & Swartz, R. P. Hyaluronan receptor (CD44) expression and function in human peripheral blood monocytes and alveolar macrophages. J. Leukoc. Biol. 56(5), 605–611 (1994).
Johnson, P., Arif, A. A., Lee-Sayer, S. S. & Dong, Y. Hyaluronan and its interactions with immune cells in the healthy and inflamed lung. Front. Immunol. 9, 2787 (2018).
Girard, N. et al. Human monocytes synthesize hyaluronidase. Br. J. Haematol. 119(1), 199–203 (2002).
De La Motte, C. et al. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am. J. Pathol. 174(6), 2254–2264 (2009).
Triest, F. J. et al. Disease-specific comorbidity clusters in COPD and accelerated aging. J. Clin. Med. 8(4), 511 (2019).
Rutten, E. P. et al. Various Mechanistic Pathways Representing the Aging Process Are Altered in COPD. Chest 149(1), 53–61 (2016).
Van Bortel, L. M. et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 30(3), 445–448 (2012).
Uhlen, M. et al. Towards a knowledge-based human protein atlas. Nat. Biotechnol. 28(12), 1248–1250 (2010).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences. (Academic Press, Cambridge, 2013).
Papakonstantinou, E. et al. Glucocorticoids and β2-agonists regulate the pathologic metabolism of hyaluronic acid in COPD. Pulm. Pharmacol. Ther. 48, 104–110 (2018).
Garg, H. G. & Hales, C. A. Chemistry and Biology of Hyaluronan (Elsevier, New York, 2004).
Garcia-Rio, F. et al. Systemic inflammation in chronic obstructive pulmonary disease: A population-based study. Respir. Res. 11(1), 63 (2010).
Vanfleteren, L. E. et al. Arterial stiffness in patients with COPD: The role of systemic inflammation and the effects of pulmonary rehabilitation. Eur. Respir. J. 43(5), 1306–1315 (2014).
Gale NS, Albarrati AM, Munnery MM, McDonnell BJ, Benson VS, Singer RMT, et al. Aortic pulse wave velocity as a measure of cardiovascular risk in chronic obstructive pulmonary disease: Two-year follow-up data from the ARCADE Study. Medicina (Kaunas, Lithuania) 55(4), 1–12 (2019).
Van Bortel, L. M. et al. Clinical applications of arterial stiffness, task force III: Recommendations for user procedures. Am. J. Hypertens. 15(5), 445–452 (2002).
Tziomalos, K., Athyros, V. G., Karagiannis, A. & Mikhailidis, D. P. Endothelial dysfunction in metabolic syndrome: Prevalence, pathogenesis and management. Nutr. Metab. Cardiovasc. Dis. 20(2), 140–146 (2010).
Dogné, S. & Flamion, B. Endothelial glycocalyx impairment in disease: Focus on hyaluronan shedding. Am. J. Pathol. 190(4), 768–780 (2020).
Wang, G., Tiemeier, G. L., van den Berg, B. M. & Rabelink, T. J. Endothelial glycocalyx hyaluronan: Regulation and role in prevention of diabetic complications. Am. J. Pathol. 190(4), 781–790 (2020).
Acknowledgements
We thank the research team and participants of the ICE-Age study for their dedication and contribution to the study and availability of the samples and clinical data for the current study.
Funding
The original study (ICE-Age) was funded by the Dutch Asthma Foundation and the Dutch Weijerhorst Foundation (3.2.09.049) without involvement in the planning, execution, drafting or writing of the study. The current study was co-funded by AstraZeneca and the PPP Allowance made available by Health ~ Holland, Top Sector Life Sciences & Health (LSHI19003).
Author information
Authors and Affiliations
Contributions
The conceptualization, laboratory and statistical analyses, interpretation of the data and writing of the report were performed by KWS, initially as part of a Master Thesis, with input and supervision from NLR, RJHCGB and FMEF. Review and editing of the manuscript were performed by NLR, RJHCGB, FMEF, SHW, MAS and SS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Drs. Waeijen-Smit, Dr. Houben-Wilke, Dr. Reynaert, Dr. Beijers and prof. Spruit declare no competing interests. Dr. Franssen reports grants and personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Chiesi, personal fees from GlaxoSmithKline, grants and personal fees from Novartis, personal fees from TEVA, outside the submitted work. Dr. Simons reports grants and personal fees from AstraZeneca, grants from Boehringer Ingelheim, personal fees from Chiesi, grants and personal fees from GlaxoSmithKline, all outside the submitted work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waeijen-Smit, K., Reynaert, N.L., Beijers, R.J.H.C.G. et al. Alterations in plasma hyaluronic acid in patients with clinically stable COPD versus (non)smoking controls. Sci Rep 11, 15883 (2021). https://doi.org/10.1038/s41598-021-95030-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95030-6
This article is cited by
-
Assessment of hyaluronic acid in COPD patients as a prognostic biomarker
The Egyptian Journal of Bronchology (2024)
-
Hyaluronic acid production and characterization by novel Bacillus subtilis harboring truncated Hyaluronan Synthase
AMB Express (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.