Abstract
Intestinal mucositis (IM) is a common side effect of 5-fluorouracil (5-FU)-based chemotherapy, which negatively impacts therapeutic outcomes and delays subsequent cycles of chemotherapy resulting in dose reductions and treatment discontinuation. In search of new pharmacological alternatives that minimize your symptoms, this work set out to study the effect of losartan (LOS), a receptor type I (AT1) angiotensin II antagonist, on intestinal mucositis induced by 5-FU. Intestinal mucositis was induced by a single intraperitoneal administration of 5-FU (450 mg/kg) in Swiss mice. Losartan (5, 25 or 50 mg/kg) or saline was orally administered 30 min before 5-FU and daily for 4 days. On 4th day, the animals were euthanized and segments of small intestine were collected to evaluate histopathological alterations (morphometric analysis), concentration of inflammatory cytokines, oxidative stress markers and genic expression of NF-κB p65, Fn-14 and TWEAK. Weight evaluation and changes in leukogram were also analyzed. 5-FU induced intense weight loss, leukopenia and reduction in villus height compared to saline group. Losartan (50 mg/kg) prevented 5-FU-induced inflammation by decreasing in the analyzed parameters compared to the 5-FU group. Our findings suggest that 50 mg/kg of losartan prevents the effects of 5-FU on intestinal mucosa in mice.
Similar content being viewed by others
Introduction
Intestinal mucositis (IM), a common side effect of 5-Fluorouracil (5-FU)-based anticancer regimens, is a complex process that leads to inflammatory and/or ulcerative lesions1,2,3,4. This inflammatory condition may limit the patient’s ability to tolerate chemotherapy, compromising cancer therapy and also contributes to higher hospitalization costs5.
Chemotherapy-induced IM has been linked to symptoms such as nausea, dyspepsia, dysphagia, diarrhea, loss of appetite, malnutrition and pain. Intestinal damage is microscopically characterized by shorted villi, loss of crypt architecture and inflammatory cell infiltration along the intestinal wall resulting in loss of mucosal integrity and bacterial colonization6,7,8,9. Its physiopathology has been described as a sequence of interrelated biological events compromising epithelial integrity and gastrointestinal dysmotility. The last phase is the recomposition of the structure of the epithelium10,11.
5-Fluorouracil is a pyrimidine antimetabolite, widely prescribed against gastric and colorectal cancer, which interferes with the synthesis of RNA and DNA, leading to cell apoptosis and tissue damage12. In addition to the direct cell damage by chemotherapy, there is the release of mediators of oxidative stress and the nuclear factor kappa B (NF-κB), the most studied signaling pathway of IM, which induces the expression of cytokines proinflammatory drugs such as interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) that promote damage and rupture in the intestinal epithelium barrier.
Losartan, a AT1 angiotensin 2 receptor blockers (ARB), used clinically for antihypertensive purposes, has anti-inflammatory effects widely described in the literature13,14,15,16,17. Losartan has been shown to reduce pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and the activation of nuclear transcription factor (NF-κB)18,19, in addition to an antioxidant effect13,20 and the ability to attenuate gastric mucosal damage when used in combination with non-steroidal anti-inflammatory drugs (NSAIDs)21.
Despite its impact on patients, there are currently no effective treatment options to prevent or treat intestinal mucositis associated to chemotherapy22, so the search for new therapeutic targets is relevant. Our research group had already shown that angiotensin 2 pathway modulators have a protective effect on oral23 and intestinal mucositis in rats24. However, as far as we know, the effect of losartan on 5-fluorouracil-induced intestinal mucositis has not yet been described. Thus, the aim of the present study was to investigate the effect of losartan in the prevention of intestinal mucositis, evaluating morphological changes in the intestine and parameters related to oxidative stress and inflammation.
Results
Histopathological evaluation and intestinal morphometry
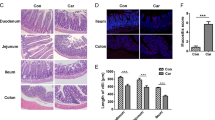
5-FU induced intestinal damage in all intestinal segments investigated, and this damage involved shortened villi, loss of crypt architecture, vacuolization and edema in the mucosal, submucosal and muscle layer and a pronounced inflammatory cell infiltrate compared to the control group (Fig. 1). Losartan 50 mg/kg significantly prevented intestinal injury (Fig. 1). Intestinal damage was also evaluated by blind semi-quantitative analysis (Table 1). All intestinal segments showed moderate to severe intestinal damage in 5-FU administered mice and losartan (50 mg/kg) significantly attenuated this injury in the jejunum and colon (Table 1). The deleterious effects of 5-FU were also visible in the morphometry of small intestine (Fig. 2A), which showed a significant reduction in the villus height (Fig. 2B) and crypt depth (Fig. 2C) in the duodenum and jejunum compared to the control group. Losartan (50 mg/kg) significantly prevented intestinal injury (Fig. 2A–C).
Histopathological analysis of intestinal segments of the duodenum, jejunum, ileum and colon of animals submitted to intestinal mucositis (IM) induced by 5-fluorouracil. Photomicrographs on a 100 μm scale demonstrated that the animals in the saline group maintained the integrity of the intestinal mucosa, with villi and crypts without damage, as well as the absence of inflammatory infiltrate. The 5-FU caused the loss of integrity, structure and shortening of the villi (black arrow), injury and disruption of the crypts with the presence of vacuolization (green arrow) and the presence of inflammatory infiltrate (red arrow). Losartan treatment at a dose of 50 mg/kg decreased the effects caused by 5-FU, improving the appearance of villi (red arrow) and crypts (black arrow).
(A) Images of histological slides stained in H&E, of fragments of the jejunum of mice submitted to intestinal mucositis. Animals saline without IM (a), with intestinal mucositis induced by 5-FU, without treatment (b) and animals with IM, treated with losartan at a dose of 50 mg/kg. The measurement of villus integrity is represented by dashed lines in yellow and the size of crypts is represented by dashed lines in blue. Segments of the duodenum, jejunum and ileum were collected to measure the villus height (10 villi/lamina). Scale bars = 100 µm (B) and crypt depth measurements (C). The bars represent the mean ± SEM of 5 mice in each group. #p < 0.001 vs. saline group, **p < 0.001 vs. 5-FU group. One way ANOVA with Bonferroni post-test.
Percentage change in body mass and total leukocyte count
5-FU caused a pronounced loss of body weight compared to the saline control group (Fig. 3A), which was partially rescued by losartan (50 mg/kg).
(A) Effect of losartan on weight loss and (B) total leukocyte count in mice with 5-FU-induced intestinal mucositis (IM). (A) Weight assessment is shown as a percentage. The bars represent the mean ± SEM (n = 5) in each group. #p < 0.01 versus Saline group; *p < 0.001 versus group 5-FU (Two-way ANOVA analysis of variance followed by the Tukey test). (B) Animals with IM, group 5-FU, reduced the number of leukocytes compared to the saline group (**p < 0.0001). LOS 50 mg/kg significantly increased the number of cells when compared to the 5-FU group. *p < 0.0001 vs. group 5-FU; **p < 0.001 versus 5-FU group and ***p < 0.05 versus 5-FU group. ANOVA one-way test followed by the Tukey test.
5-Fluorouracil significantly decreased the number of leukocytes in the circulating blood compared to the saline group (animals not submitted to IM) (Fig. 3B). This effect was prevented by the three different evaluated doses of losartan (Fig. 3B).
Cytokine assay (IL-1β and TNF-α)
Inflammatory cytokines levels were markedly increased in the jejunum of 5-FU group compared to saline group (Fig. 4A,B). Losartan, at all doses evaluated, significantly prevented the increase in TNF-α levels (Fig. 4A) and IL-1β levels in the jejunal tissue (Fig. 4B) caused by 5-FU-induced intestinal mucositis.
Cytokines tumor necrosis factor alpha (TNF-α) (A) and interleukin 1 beta (IL-1β) (B) in the jejunal portion of mice submitted to the experimental model of intestinal mucositis (IM). The saline group comprised animals without IM; In the 5-FU group, the animals received 5-FU and without treatment. The LOS groups received 5-FU, underwent IM and were treated with losartan (orally), in doses (5, 25 or 50 mg/kg) (n = 5/group). The results are presented as mean ± standard error of the mean (n = 5/group). *p < 0.001 (compared to saline); **p < 0.0001 (compared to 5-FU) (ANOVA analysis of variance with the Tukey post-test).
Malondialdehyde (MDA) and glutathione (GSH) assays
5-FU caused a significant increase in MDA levels (Fig. 5A) associated with a significant reduction in glutathione (GSH) levels (Fig. 5B) in the jejunal tissue, when compared to the saline group (p < 0.05). These effects were prevented by all doses of losartan (Fig. 5A,B).
Malondialdehyde (MDA) (A) and glutathione (GSH) (B) levels in the IM model. The experimental groups saline, 5-FU and losartan are presented in doses of 5, 25 or 50 mg/kg, the values are shown as mean ± SEM. For statistical analysis, ANOVA was used, followed by the Tukey test. *p < 0.01 vs. saline group and **p < 0.01 vs. group 5-FU.
Reverse transcription polymerase chain reaction (RT-PCR)
The 5-FU increased the mRNA expression of TWEAK, Fn14 and NF-κB p65, compared to the Saline group (Fig. 6). Losartan prevented the mRNA genic expression of these three markers, mainly at doses of 25 or 50 mg/kg, compared to animals with IM, without treatment (5-FU) (p < 0.05).
Polymerase chain reaction in real time for TWEAK, Weak apoptosis inducer like cytokine tumor necrosis factor (A); FN-14, inducible fibroblast growth factor 14 (B); NF-κB p65, the nuclear transcription factor kappa B (C). 5-Fluorouracil (5-FU) increased the expression of these genes compared to the Saline group. Losartan decreased the expression of TWEAK, FN-14 and NF-κB p65, compared to the 5-FU group (n = 5); *p < 0.05 compared with the Saline group; **p < 0.05 compared with the group 5-FU; analysis of variance with Tukey's post-test).
Discussion
We found that 5-FU induced intestinal damage in all intestinal segments investigated, and this damage involved shortened villi, loss of crypt architecture and a pronounced inflammatory cell infiltrate in the lamina propria, associated with weight loss compared to the control group. The body weight loss might be related to malabsorption due to intestinal lesions, resulting in loss of the intestinal absorptive surface. We first investigated whether losartan affected 5-FU-induced weight loss and histological alterations. The highest dose of losartan significantly attenuated this injury in the jejunum and colon and partially rescued the pronounced loss of body weight caused by 5-FU.
According to the literature, 5-FU causes apoptosis of intestinal epithelial cells, leading to structural and inflammatory changes, well described characteristic of mucositis25,26. Bacterial colonization at the mucosal ulcers further induces inflammation by stimulating infiltration and activation of proinflammatory macrophages, leading to a vicious cycle of inflammation in the gastrointestinal tract27. Intestinal damage interferes with patient’s quality of life and diet, leading to weight loss and may compromise the delivery of cancer therapy and the overall prognosis28,29,30.
Losartan (LOS) at a dose of 50 mg/kg prevented the histopathological and morphometric changes induced by 5-FU in the jejunum and colon. In addition, LOS 50 mg/kg partially rescued the animals' weight loss induced by 5-FU-induced intestinal mucositis. Losartan is an angiotensin II AT1 receptor blocker. Although the main role of the renin-angiotensin system (RAAS) in the cardiovascular and renal systems, it has been described different subtypes of angiotensin receptors in the mucosal and muscular layers of the intestine, regulating intestinal function31,32,33. SARS plays a role in inflammation, specifically due to the action of angiotensin II. Thus, blocking the AT1 receptor promotes anti-inflammatory action34,35. Corroborating our findings, authors demonstrated the protective effect of losartan in inflammatory bowel diseases36,37,38.
In this investigation, 5-FU reduced the total number of leukocytes in the circulating blood of the animals, compared to the saline group. Leukopenia is an expected effect of chemotherapeutic agents and has been described in previous work on experimental mucositis25,39,40. The three evaluated doses of LOS prevented leukopenia, compared to the 5-FU group. This data is crucial, since leukocytes represent defense cells and can protect mice against bacterial and fungal infections, commonly associated with mucositis41. We believe that the effect of losartan in preventing 5-FU-induced leucopenia is not associate with its antiinflammatory effect. A recent study42 has shown that losartan (0.71 mg/kg, oral treatments, once a day for 50 days) caused significant increments in total white blood cells, neutrophil and monocyte values, in male rats (not treated with any chemotherapeutic agent). This effect, which probably indicates an enhancement on ability of the body to attack and destroy invading bacteria, viruses and other injurious agents, may be partially associated with its positive effect by preventing the rapid colonization of ulcers. The experimental model used in the present study does not allow us to assess whether losartan has a negative impact on 5-FU efficacy. A recent study, however, using a xenograft model of colon cancer, demonstrated that the combination of Losartan and 5-FU revealed synergistic and additive anti-tumorigenic properties43.
In this study, losartan reduced the levels of the pro-inflammatory cytokine’s TNF-α and IL-1β. These results are in accordance with previous data in the literature, where losartan decreased the levels of IL-1β and TNF-α in an animal model of arthritis44 and suppressed serum levels of pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α and MCP-1 in perivasculitis, attenuating coronary inflammation in a murine model of Kawasaki’s disease45.
The literature have demonstrated that the role of LOS in decreasing local production of cytokines in animal models is due to the regulation of the nuclear transcription factor kappa B (NF-κB) and correlated genes, which act in the modulation of important pro-inflammatory cytokines and oxidative stress44. In the present study, 5-FU increased the gene expression of NF-κB p65 and LOS prevented this effect. It was observed a decreased expression of NF-κB p65 in the LOS group when compared to untreated group (5-FU group). NF-κB represents one of the most important signaling pathways in the pathophysiology of mucositis (5, 41). Chemotherapy with 5-FU promotes direct apoptosis in the basal epithelial cells, contributing to NF-κB activation, resulting in the subsequent production of the pro-inflammatory cytokines TNF-α and IL-1β. TNF-α interacts synergistically with NF-κB, amplifying its activation, also contributing to the increase in the expression of other pro-inflammatory factors, which act in tissue damage29,30.
In this study, LOS prevented the oxidative stress caused by lipid peroxidation, evidenced by a reduction in MDA levels and an increase in sulfhydryl groups (GSH), a classical example of a scavenging antioxidant. In other experimental models, losartan reduced inflammation and the release of free radicals, modulating antioxidant enzymes, including catalase and glutathione peroxidase20,46. We demonstrated that 5-FU interfered with NF-κB p65 pathway and increased gene expression of TWEAK (weak inducer of tumor necrosis factor-like apoptosis) and Fn14 (fibroblast growth factor-inducible 14), compared to the saline group. Losartan significantly prevented the increase of TWEAK and Fn14 gene expressions, induced by 5-FU. TWEAK is a cytokine of the TNF family that signals by the Fn14 receptor, participating in biological activities, including proliferation, migration, differentiation, apoptosis, angiogenesis and inflammation47. Angiotensin II modulates the TWEAK/Fn14 pathway in response to inflammation and myocardial injury in rats48. Authors suggest that blocking the TWEAK/Fn14 pathway attenuates chronic intestinal inflammation and promotes epithelial repair49,50,51. Thus, we demonstrated a important effect of losartan on 5-FU-induced intestinal mucositis, preventing histopathological and morphometric changes, reducing the oxidative stress and inflammation and modulating the NF-κB p65, TWEAK/Fn14 pathway, as illustrated in Fig. 7.
Representation of pharmacological modulation of the intestinal mucositis induced by 5-fluorouracil (5-FU) treated with the losartan. DNA damage due to 5-FU promotes the release of reactive oxygen species (ROS) and activates NF-κB that induces the expression of proinflammatory cytokines such as IL-1β and TNF-α, in addition, occurs the oxidative stress, which that together promote inflammation in the intestinal mucosa. Losartan was reducing NF-κB p65 in response the decreased of gene expression TWEAK/Fn14, reducing IL-1β and TNF-α and decreasing oxidative stress, therefore improving intestinal mucositis. TWEAK, Tumor necrosis factor (TNF)-like weak inducer of apoptosis; Fn14, Fibroblast growth factor-inducible 14; NF-κB p65, nuclear transcription factor kappa B.
Conclusions
Losartan 50 mg/kg partially prevented the effects of 5-FU-induced intestinal mucositis in mice. The findings of this study have to be seen in light of some limitations. More research is needed to understand the immunomodulatory effects of losartan and assess the benefits and harms (side effects) of losartan for patients undergoing 5-FU chemotherapy.
Methods
Animals
Female Swiss mice (Mus musculus), weighing 25–30 g (mean age of 8 weeks) were housed in polypropylene boxes and kept in controlled conditions of temperature (24 ± 2 °C), relative humidity of the air (50 ± 5%), 12 h light/dark cycle and access to food and water ad libitum.
Ethical statement
All experimental protocols were approved by the Federal University of Rio Grande do Norte Ethics Committee on the use of Animals (CEUA. No. 132.059/2018) and performed in accordance with the ARRIVE ethical guidelines. All methods were performed in accordance with relevant guidelines and regulations.
Induction of experimental intestinal mucositis (MI)
Intestinal mucositis was induced by a single administration of 5-fluorouracil (5-FU; 450 mg/kg; i.p.), as previously described9. On day 4, the mice were euthanized with an overdose of thiopental (90 mg/kg, i.p.).
Experimental groups
After two weeks of acclimation to the laboratory environment. Thirty five swiss mice were divided into 5 groups (n = 7 per group): saline (healthy mice, not submitted to intestinal mucositis, that received a single intraperitoneal administration of 0.9% saline solution on the first day and oral administrations of saline, starting 30 min before the saline intraperitoneal injection, and daily, once a day, until euthanasia), 5-FU (group submitted to intestinal mucositis by a single intraperitoneal administration of 5-fluorouracil (450 mg/kg) and received oral administrations of saline, starting 30 min before the 5-FU injection, and daily, once a day, until euthanasia; Losartan—LOS 5, 25 or 50 (animals submitted to 5-FU-induced intestinal mucositis and treated with oral administrations of 5, 25 or 50 mg/kg of losartan, starting 30 min before 5-FU administration and daily, once a day, until euthanasia.
Tablets of losartan were macerated using a pestle and a porcelain mortar and dissolved in of saline to obtain a concentrated solution from which the different concentrations of losartan evaluated in the present study were prepared.
Histopathological analysis
After euthanasia, on day 4, the entire intestine was removed, washed with 0.9% saline and divided into segments (duodenum, jejunum, ileum and colon). A portion of each segment was fixed in 10% buffered formaldehyde, dehydrated and embedded in paraffin. Sections (5 µm thick) were obtained for hematoxylin and eosin (HE) staining and subsequent evaluation using light microscope (Olympus BH-2) at 200 × magnification, by an experienced observer, in a blinded manner. The severity of mucositis was graded using a modification of Leitão et al. (2011) histopathological grading system previously described52, considering microscopic findings such as villus and crypt integrity, inflammatory cell influx, vacuolization and edema in the mucous layer and submucosal layer (Table 2).
Intestinal morphometry
For the morphometric analyses, the length of intestinal villi, depth of the Lieberkuhn crypts and villus/crypt ratios were determined, as previously described53 from HE slides using a light microscope equipped with a high-resolution digital camera (LEICA®), connected to the computer with an image capture program. Between 5 and 10 villi and crypts were measured per slice (seven samples for each group). All morphometric measurements were performed blindly with the ImageJ® software (NIH, Bethesda, MD, USA). The conversion of the measurements obtained, of villi and crypts, into pixels were converted into micrometers (μm), using the ratio 100 μm.
Percentage change in body mass and total leukocyte count
The animals were weighed daily until the 4th day of the experimental protocol and the variation in body mass was determined, as previously described. The values obtained were expressed as a weight variable in relation to the initial weight54.
Immediately before euthanasia, blood samples were collected from the heart after anesthesia with an overdose of thiopental (90 mg/kg, i.p.), and diluted (1:20) in Turk’s solution (380 μL). The leukocyte count per mm3 of blood was determined using a Neubauer chamber. The results were expressed in number of leukocytes per mm3 in blood55.
Cytokine assay (IL-1β and TNF-α)
Jejunum samples removed on day 4 were stored at − 80 °C until required for analysis of cytokines. The samples were homogenized and processed as previously described29. The concentrations of IL-1β and TNF-α in the samples were determined using a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA). Briefly, microtiter plates were coated overnight at 4 °C with antibodies against IL-1β (detection range: 62.5–4000 pg/mL; sensibility or lower limit of detection: 12.5 ng/mL of recombinant mouse IL-1β) and TNF-α (detection range: 62.5–4000 pg/mL; sensibility or lower limit of detection: 50 ng/mL of recombinant mouse TNF-α). After blocking the plates, the samples and standard at various dilutions were added in duplicate and incubate at 4 °C for 24 h. After washing the plates (three times with buffer) biotinylated polyclonal anti-IL-1β or anti-TNF-α, diluted 1:1000 with assay buffer 1% BSA, was added to the wells. After further incubation at room temperature for 1 h, the plates were washed and streptavidin-HRP, diluted 1:5000, was added to each well. The chromogenic reagent O-phenylenediamine was added 15 min later and the plates were incubated in the dark for 15 min. The enzymatic reaction was interrupted with H2SO4 and the absorbance was measured at 490 nm using UV–VIS spectrophotometry. The results are expressed as pg/mL56.
Malondialdehyde (MDA) and glutathione (GSH) assays
Lipid peroxidation was measured by the indirect method of quantification of malondialdehyde, based on the reaction with the chromogenic agent 1-methyl-2-phenylindole. Briefly, the jejunum tissues (5 per group) were homogenized in Tris–HCl buffer 1:5 (weight/volume) and, subsequently, centrifuged at 4 °C, in 2500g for 10 min. Finally, the samples were submitted to the spectrophotometer, using an absorbance of 586 nm for reading. The results are expressed in nanomoles of MDA per gram of intestinal tissue57.
Jejunum tissue samples were collected on day 4 and homogenized in 1 mL of 0.02 M EDTA to measure glutathione (GSH) levels, as previously described58. Then, supernatants (400 µL) were removed and added in 320 µL of distilled water, plus 80 µL of trichloroacetic acid (50%, w/v). The eppendorfs were centrifuged at 3000 rpm for 15 min and supernatants were transferred (100 µL) and mixed with 200 µL of Tris buffer (0.4 M, pH 8.9), followed by the addition of DTNB (5,5-dithiobis (2-nitrobenzoic acid) (0.01 M). The absorbance of GSH was read at 412 nm and the concentration was expressed in pg/mL of tissue59.
Reverse transcription polymerase chain reaction (RT-PCR)
A portion of the jejunum was stored in eppendorf vials at − 80 °C for further analysis of biological and molecular markers.
The RNA was extracted from jejunum samples (n = 5) collected on day 4 using TRIzol™ reagent (Invitrogen Co., USA) and the Total RNA Isolation System S.V. (Promega, Madison, WI), resulting in the cDNA. For synthesis of the first strand cDNA, the total RNA kit was used for reaction with the ImProm-IITM reverse transcriptase system for RT-PCR (Promega) according to the manufacturer's protocol. Quantitative real-time PCR analyzes of NF-κB p65, Fn-14 and TWEAK (Table 3) mRNAs were performed using SYBR Green Mix in the Applied Biosystems® 7500 FAST system (Applied Biosystems, Foster City, CA), according to a standard protocol for use with Primers (Table 3). The expression data were standardized using the reference gene Beta (β)-actin in the formula 2−ΔΔCt60.
Abbreviations
- 5-FU:
-
5-Fluorouracil
- Fn-14:
-
Fibroblast growth factor-inducible 14
- GSH:
-
Glutathione
- HE:
-
Hematoxylin and eosin
- IL-1β:
-
Interleukin 1 beta
- IM:
-
Intestinal mucositis
- LOS:
-
Losartan
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear transcription factor
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- TNF-α:
-
Tumor necrosis factor alpha
- TWEAK:
-
Tumor necrosis factor (TNF)-like weak inducer of apoptosis
References
Chen, H. et al. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 124, 109829 (2020).
Li, H. L. et al. Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Front. Cell Infect. Microbiol. 7, 455 (2017).
Yeung, C. Y. et al. Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS One 10(9), e0138746 (2015).
Arends, J. How to feed patients with gastrointestinal mucositis. Curr. Opin. Support. Palliat. Care 12(2), 168–173 (2018).
Sonis, S. T. The pathobiology of mucositis. Nat. Rev. Cancer 4(4), 277–284 (2004).
Chang, C. T. et al. 5-Fluorouracil induced intestinal mucositis via nuclear factor-kappaB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One 7(3), e31808 (2012).
Wu, J. et al. Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomed. Pharmacother. 124, 109883 (2020).
Peterson, D. E., Boers-Doets, C. B., Bensadoun, R. J., Herrstedt, J. & Committee, E. G. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 26(Suppl 5), v139–v151 (2015).
Justino, P. F. et al. Regulatory role of Lactobacillus acidophilus on inflammation and gastric dysmotility in intestinal mucositis induced by 5-fluorouracil in mice. Cancer Chemother. Pharmacol. 75(3), 559–567 (2015).
Wardill, H. R. & Bowen, J. M. Chemotherapy-induced mucosal barrier dysfunction: An updated review on the role of intestinal tight junctions. Curr. Opin. Support. Palliat. Care 7(2), 155–161 (2013).
Bowen, J. et al. The pathogenesis of mucositis: Updated perspectives and emerging targets. Support. Care Cancer 27(10), 4023–4033 (2019).
Focaccetti, C. et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS One 10(2), e0115686 (2015).
Taguchi, I. et al. Irbesartan, an angiotensin receptor blocker, exhibits metabolic, anti-inflammatory and antioxidative effects in patients with high-risk hypertension. Hypertens. Res. 36(7), 608–613 (2013).
Dandona, P., Dhindsa, S., Ghanim, H. & Chaudhuri, A. Angiotensin II and inflammation: The effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J. Hum. Hypertens. 21(1), 20–27 (2006).
Horiuchi, M., Iwanami, J. & Mogi, M. Regulation of angiotensin II receptors beyond the classical pathway. Clin. Sci. (Lond.) 123(4), 193–203 (2012).
Ranjbar, R. et al. The potential therapeutic use of renin-angiotensin system inhibitors in the treatment of inflammatory diseases. J. Cell. Physiol. 234(3), 2277–2295 (2019).
Lee, H. W., Suh, J. K., Jang, E. & Lee, S. M. Effect of angiotensin converting enzyme inhibitor and angiotensin II receptor blocker on the patients with sepsis. Korean J. Intern. Med. 36, 371–381 (2021).
Padda, R. S., Shi, Y., Lo, C.-S., Zhang, S.-L. & Chan, J. S. D. Angiotensin-(1–7): A novel peptide to treat hypertension and nephropathy in diabetes?. J. Diabetes Metab. https://doi.org/10.4172/2155-6156.1000615 (2015).
Hasanin, A. H. & Mohamed, R. H. Telmisartan alone or in combination with etanercept improves anemia associated with rheumatoid arthritis in rats: A possible role of anti-inflammatory and reno-protective effects. Pharmacol. Rep. 72(2), 379–388 (2020).
Lin, C. H. et al. Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp. Gerontol. 58, 174–178 (2014).
Duan, L. et al. Increased risk of aspirin-induced gastric mucosal erosion in elderly Chinese men harboring SLCO1B1*1b/*1b while using aspirin and an ACEI or ARB concomitantly. BMC Med. Genet. 20(1), 183 (2019).
Thomsen, M. & Vitetta, L. Adjunctive treatments for the prevention of chemotherapy- and radiotherapy-induced mucositis. Integr. Cancer Ther. 17(4), 1027–1047 (2018).
Barbosa, M. M. et al. Telmisartan modulates the oral mucositis induced by 5-fluorouracil in hamsters. Front. Physiol. 9, 1204 (2018).
de Araujo, R. F. Jr. et al. Olmesartan decreased levels of IL-1beta and TNF-alpha, down-regulated MMP-2, MMP-9, COX-2, RANK/RANKL and up-regulated SOCs-1 in an intestinal mucositis model. PLoS One 9(12), e114923 (2014).
de Miranda, J. A. L. et al. Protective effect of cashew gum (Anacardium occidentale L.) on 5-fluorouracil-induced intestinal mucositis. Pharmaceuticals (Basel) 12(2), 51 (2019).
Zhang, L. et al. Qingjie Fuzheng Granule attenuates 5-fluorouracil-induced intestinal mucosal damage. Biomed. Pharmacother. 118, 109223 (2019).
Sougiannis, A. T., VanderVeen, B. N., Davis, J. M., Fan, D. & Murphy, E. A. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 320(5), G712–G719 (2021).
Dos Santos Filho, E. X. et al. Curcuminoids from Curcuma longa L. reduced intestinal mucositis induced by 5-fluorouracil in mice: Bioadhesive, proliferative, anti-inflammatory and antioxidant effects. Toxicol. Rep. 3, 55–62 (2016).
Costa, D. V. S. et al. 5-Fluorouracil induces enteric neuron death and glial activation during intestinal mucositis via a S100B-RAGE-NFkappaB-dependent pathway. Sci. Rep. 9(1), 665 (2019).
Basile, D. et al. Mucosal injury during anti-cancer treatment: From pathobiology to bedside. Cancers (Basel) 11(6), 857 (2019).
Patten, G. S. & Abeywardena, M. Y. Effects of antihypertensive agents on intestinal contractility in the spontaneously hypertensive rat: Angiotensin receptor system downregulation by losartan. J. Pharmacol. Exp. Ther. 360(2), 260–266 (2017).
Koga, H., Yang, H., Haxhija, E. Q. & Teitelbaum, D. H. The role of angiotensin II type 1a receptor on intestinal epithelial cells following small bowel resection in a mouse model. Pediatr. Surg. Int. 24(12), 1279–1286 (2008).
Fandriks, L. The renin-angiotensin system and the gastrointestinal mucosa. Acta Physiol. (Oxf.) 201(1), 157–167 (2011).
Simoes e Silva, A. C., Silveira, K. D., Ferreira, A. J. & Teixeira, M. M. ACE2, angiotensin-(1–7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 169(3), 477–492 (2013).
Simoes, E. S. A. C. & Teixeira, M. M. ACE inhibition, ACE2 and angiotensin-(1–7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol. Res. 107, 154–162 (2016).
Liu, T. J., Shi, Y. Y., Wang, E. B., Zhu, T. & Zhao, Q. AT1R blocker losartan attenuates intestinal epithelial cell apoptosis in a mouse model of Crohn’s disease. Mol. Med. Rep. 13(2), 1156–1162 (2016).
Shi, Y. et al. Activation of the renin–angiotensin system promotes colitis development. Sci. Rep. 6(1), 27552 (2016).
Khajah, M. A., Fateel, M. M., Ananthalakshmi, K. V. & Luqmani, Y. A. Anti-inflammatory action of angiotensin 1–7 in experimental colitis. PLoS One 11(3), e0150861 (2016).
Medeiros, C. A. et al. Effect of atorvastatin on 5-fluorouracil-induced experimental oral mucositis. Cancer Chemother. Pharmacol. 67(5), 1085–1100 (2011).
Boeing, T. et al. Luteolin prevents irinotecan-induced intestinal mucositis in mice through antioxidant and anti-inflammatory properties. Br. J. Pharmacol. 177(10), 2393–2408 (2020).
Cinausero, M. et al. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front. Pharmacol. 8, 354 (2017).
Oyedeji, K. O., Okeke, O. E., Talabi, Y. J. & Gboluwagwa, O. Effect of losartan (angiotensin II antagonist) on haematological and biochemical parameters in male Wistar rats. J. Pharm. Sci. Res. 10(4), 995–998 (2018).
Hashemzehi, M. et al. Angiotensin receptor blocker Losartan inhibits tumor growth of colorectal cancer. EXCLI J. 20, 506–521 (2021).
Silveira, K. D. et al. Mechanisms of the anti-inflammatory actions of the angiotensin type 1 receptor antagonist losartan in experimental models of arthritis. Peptides 46, 53–63 (2013).
Suganuma, E. et al. Losartan attenuates the coronary perivasculitis through its local and systemic anti-inflammatory properties in a murine model of Kawasaki disease. Pediatr. Res. 81(4), 593–600 (2017).
Karanovic, D. et al. Effects of single and combined losartan and tempol treatments on oxidative stress, kidney structure and function in spontaneously hypertensive rats with early course of proteinuric nephropathy. PLoS One. 11(8), e0161706 (2016).
Mendez-Barbero, N., Gutierrez-Munoz, C., Blazquez-Serra, R., Martin-Ventura, J. L. & Blanco-Colio, L. M. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK)/fibroblast growth factor-inducible 14 (Fn14) axis in cardiovascular diseases: Progress and challenges. Cells 9(2), 405 (2020).
Mustonen, E. et al. Tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor Fn14 during cardiac remodelling in rats. Acta Physiol. (Oxf.) 199(1), 11–22 (2010).
Dohi, T. et al. TWEAK/Fn14 pathway: A nonredundant role in intestinal damage in mice through a TWEAK/intestinal epithelial cell axis. Gastroenterology 136(3), 912–923 (2009).
Dohi, T. & Burkly, L. C. The TWEAK/Fn14 pathway as an aggravating and perpetuating factor in inflammatory diseases: Focus on inflammatory bowel diseases. J. Leukoc. Biol. 92(2), 265–279 (2012).
Di Martino, L., Osme, A., Kossak-Gupta, S., Pizarro, T. T. & Cominelli, F. TWEAK/Fn14 is overexpressed in Crohn’s disease and mediates experimental ileitis by regulating critical innate and adaptive immune pathways. Cell. Mol. Gastroenterol. Hepatol. 8(3), 427–446 (2019).
Leitao, R. F. et al. Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol. 11, 90 (2011).
Lima-Junior, R. C. et al. Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: role of cytokines on inducible nitric oxide synthase activation. Cancer Chemother. Pharmacol. 69(4), 931–942 (2012).
Costa, D. V. S. et al. The alpha-lipoic acid improves survival and prevents irinotecan-induced inflammation and intestinal dysmotility in mice. Pharmaceuticals (Basel) 13(11), 361 (2020).
Soares, P. M. et al. Role of platelet-activating factor in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Cancer Chemother. Pharmacol. 68(3), 713–720 (2011).
Araujo, A. A. et al. Effect of telmisartan on levels of IL-1, TNF-alpha, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J. Clin. Periodontol. 40(12), 1104–1111 (2013).
Sousa Gomes, M. et al. Anti-inflammatory and antioxidant activity of hydroethanolic extract of Spondias mombin leaf in an oral mucositis experimental model. Arch. Oral Biol. 111, 104664 (2020).
Sedlak, J. & Lindsay, R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 25, 192–205 (1968).
Rebrin, I., Kamzalov, S. & Sohal, R. S. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 35(6), 626–635 (2003).
Rao, X., Huang, X., Zhou, Z. & Lin, X. An improvement of the 2(−delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 3(3), 71–85 (2013).
Acknowledgements
We would like to thank to Neida da Mata, Flávio Maurílio dos Santos Lima, Cesar Augusto Trevisan Bordignon and Ramon Tadeu Galvão Alves Rodrigues for excellent technical support, and collaborators the Department of Morphology (UFC, Fortaleza, CE, Brazil). The graphic abstract presented in Fig. 7 was created with BioRender.com.
Funding
This work was financed by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001. C.A.C.X. d M. has Research Productivity Scholarship—PQ2 CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.M.B.O., C.d.S.M.R., R.F.d.C.L., C.A.C.X.d.M.; Methodology: M.M.B.O., S.B.R., P.C.M.d.S.M., V.B.M., C.d.S.M.R, G.A.d.C.B., R.F.d.C.L., G.C.B.G.; A.A.d.A., C.A.C.X.d.M.; Formal analysis: M.M.B.O., S.B.R., A.A.d.A., R.F.d.C.L., C.A.C.X.d.M.; Investigation: M.M.B.O., S.B.R., P.C.M.d.S.M., V.B.M., C.d.S.M.R., G.C.B.G., C.A.C.X.d.M.; Resources: M.M.B.O., A.A.d.A., R.F.d.C.L., G.A.d.C.B., C.A.C.X.d.M.; Writing-original draft preparation: M.M.B.O., R.F.d.C.L., C.A.C.X.d.M.; Writing-review and editing: M.M.B.O., R.F.d.C.L.; J.G.F., P.B.B., C.A.C.X.d.M.; Supervision: R.F.d.C.L., G.C.B.G.; A.A.d.A., C.A.C.X.d.M.; Project administration: C.A.C.X.d.M.; Funding acquisition: A.A.d.A., G.A.d.C.B.; R.F.d.C.L., C.A.C.X.d.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oliveira, M.M.B., de Araújo, A.A., Ribeiro, S.B. et al. Losartan improves intestinal mucositis induced by 5-fluorouracil in mice. Sci Rep 11, 23241 (2021). https://doi.org/10.1038/s41598-021-01969-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01969-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.