Abstract
We examined whether the extent of initial peritoneal dissemination affected the prognosis of patients with advanced ovarian, fallopian tube, and peritoneal carcinoma when initially disseminated lesions > 1 cm in diameter were removed, regardless of the timing of aggressive cytoreductive surgery. The extent of peritoneal dissemination was assessed by the peritoneal cancer index (PCI) at initial laparotomy in 186 consecutive patients with stage IIIC/IV. Sixty patients underwent primary debulking surgery and 109 patients underwent neoadjuvant chemotherapy followed by interval debulking surgery. Seventeen patients could not undergo debulking surgery because of disease progression during neoadjuvant chemotherapy. The median initial PCI were 17. Upper abdominal surgery and bowel resection were performed in 149 (80%) and 171 patients (92%), respectively. Residual disease ≤ 1 cm after surgery was achieved in 164 patients (89%). The initial PCI was not significantly associated with progression-free survival (PFS; p = 0.13) and overall survival (OS; p = 0.09). No residual disease and a high-complexity surgery significantly prolonged PFS (p < 0.01 and p = 0.02, respectively) and OS (p < 0.01 and p ≤ 0.01, respectively). The extent of initial peritoneal dissemination did not affect the prognosis when initially disseminated lesions > 1 cm were resected.
Similar content being viewed by others
Introduction
Majority of ovarian, fallopian tube, and peritoneal carcinoma have already spread to the peritoneal cavity beyond the pelvis upon diagnosis. Among the several assessment tools for the extent of peritoneal dissemination1,2,3,4,5,6, peritoneal cancer index (PCI) is precise and reproducible for the assessment of the location and size of lesions in 13 abdominopelvic regions1,2. It has been universally used for the assessment of the prognosis or surgical resectability of gastrointestinal carcinomas. Some reports have shown that PCI is also one of the prognostic factors for ovarian cancer4,6,7,8,9. A negative correlation has been reported between PCI and complete resection rate10, which is the most significant prognostic factor for ovarian cancer11,12.
Whether aggressive cytoreductive surgery overcome the extent of peritoneal dissemination remains debatable3,13,14,15. Aggressive surgery is necessary to achieve complete resection in patients with a high PCI score. However, in practice, aggressive surgery is not often performed for patients with a high PCI score at primary debulking surgery (PDS). Only 22% patients with high disease burden received high-complexity surgery in the Gynecologic Oncology Group 182 study3. In addition, complete cytoreduction rate in patients treated with PDS was approximately 20–50% even in highly experienced centers in which aggressive surgery is performed16,17,18, except for a few7,19. Perioperative death or severe perioperative complications in patients with a high PCI score could occur when aggressive surgery with PDS was performed18.
The treatment option for patients who could not achieve complete resection with PDS is neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS). It is unclear whether aggressive surgery overcomes the extent of dissemination for the patients treated with IDS. Usually, aggressive surgery is not performed during IDS in patients with a high initial PCI score before initiation of NACT18 because many initially disseminated tumors become invisible after NACT, and only visible tumors can be resected during IDS. Therefore, initially disseminated tumors were not resected in such cases. However, our previous study showed that lesions > 1 cm in diameter before NACT administration may harbor microscopic disease even though the initially disseminated tumor becomes invisible after NACT20. The previous study observed that the median progression-free survival (PFS) was longer in patients who underwent aggressive surgery with resection of lesions measuring > 1 cm before NACT than in those who underwent resection of only visible lesions during IDS20. Therefore, we concluded that initially disseminated tumors that could not be resected during IDS contributed to poor prognosis in patients with a high PCI score.
We hypothesized that aggressive surgery with resection of the initial > 1 cm dissemination would overcome the high PCI score by selecting PDS or NACT followed by IDS depending on whether cytoreduction to no residual disease is achievable at initial laparotomy before starting treatment. In this study, we examined whether PCI affected the prognosis of patients with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC/IV ovarian, fallopian tube, and peritoneal carcinoma when initially disseminated lesions > 1 cm were resected, regardless of the timing of cytoreductive surgery.
Results
Patients' characteristics and correlation with the initial PCI (Table 1)
The median initial PCI was 17 (minimum 2, maximum 31, first to third quartile range 10 to 22). The initial PCI was significantly associated with primary site, histology, timing of cytoreductive surgery, residual disease, and surgical complexity score. The initial PCI was not associated with performance status and FIGO stage.
Sixty patients (32%) underwent PDS. One hundred and nine patients underwent IDS after NACT. Seventeen patients did not undergo IDS because of disease progression during NACT (no debulking surgery). The median initial PCI of the patients who underwent PDS, IDS, and no debulking surgery were 8, 19, and 22, respectively. The initial PCI of the patients who underwent PDS was significantly lower than that of the patients who underwent IDS (p < 0.01).
Surgical procedure and outcome
Of the 186 patients, 149 patients (80%) underwent upper abdominal procedure, including right diaphragm resection (n = 145) and splenectomy with or without distal pancreatectomy (n = 98), and 171 patients (92%) underwent bowel resection, including rectosigmoid colon (n = 163), large bowel other than the rectosigmoid colon (n = 90), and small bowel (n = 20). The surgical complexity score had a median value of 12 and was significantly higher in the patients who underwent IDS than in those who underwent PDS (13 vs 10, p < 0.001).
Overall, cytoreductive surgery achieved no residual disease in 148 patients (80%), residual disease measuring 0.1–1 cm in 16 (9%), and residual disease > 1 cm or no debulking surgery in 22 (12%). Among the 60 patients who underwent PDS, cytoreductive surgery was associated with no residual disease in 47 (78%), residual disease measuring 0.1–1 cm in 11 (18%), and residual disease > 1 cm in 2 patients (3%). Among the 109 patients who underwent IDS, cytoreductive surgery was associated with no residual disease in 101 (93%), residual disease measuring 0.1–1 cm in 5 (5%), and residual disease > 1 cm or no debulking surgery in 3 patients (3%).
The 30-day and 3-month mortality rates after cytoreductive surgery were 0% and 0.005%, respectively.
Residual disease and surgical complexity score in each of the 3 groups of PCI (Fig. 1)
Residual disease and surgical complexity scores in subgroups of patients with PCI scores 1–10, 11–20, and 21–39. (a) Residual disease in patients who underwent PDS. (b) Residual disease in patients who underwent IDS. (c) Surgical complexity score in patients who underwent PDS. (d) Surgical complexity score in patients who underwent IDS. PDS: primary debulking surgery, IDS: interval debulking surgery, PCI: peritoneal cancer index, SCS: surgical complexity score.
Distribution of residual disease in 60 patients who underwent PDS was as follows: among 36 patients with PCI scores 1–10, cytoreductive surgery was associated with no residual disease in 31 (86%), residual disease measuring 0.1–1 cm in 3 (8%), and residual disease > 1 cm in 2 patients (6%). Among 20 patients with PCI scores 11–20, cytoreductive surgery was associated with no residual disease in 15 (75%), residual disease measuring 0.1–1 cm in 5 (25%), and residual disease > 1 cm in 0 patients (0%). Among 4 patients with PCI scores 21–39, cytoreductive surgery was associated with no residual disease in 1 (25%), residual disease measuring 0.1–1 cm in 3 (75%), and residual disease > 1 cm in 0 patients (0%).
Distribution of residual disease in 109 patients who underwent IDS was as follows: cytoreductive surgery was associated with no residual disease in all 13 patients (100%) with PCI scores 1–10. Among 54 patients with PCI scores 11–20, cytoreductive surgery was associated with no residual disease in 52 (96%), residual disease measuring 0.1–1 cm in 1 (2%), and residual disease > 1 cm in 1 patient (2%). Among 42 patients with PCI scores 21–39, cytoreductive surgery was associated with no residual disease in 36 (86%), residual disease measuring 0.1–1 cm in 4 (10%), and residual disease > 1 cm in 2 patients (5%).
The surgical complexity score in 60 patients who underwent PDS was as follows: among 36 patients with PCI scores 1–10, high-complexity surgery (score of 8–18) was performed in 14 (39%), moderate-complexity surgery (score of 4–7) in 15 (42%), and low-complexity surgery (score of 1–3) in 7 patients (19%). Among 20 patients with PCI scores 11–20, high-complexity surgery was performed in 17 (85%) and moderate-complexity surgery in 3 patients (15%). All 4 patients (100%) with PCI scores 21–39 underwent high-complexity surgery.
The surgical complexity score in 109 patients who underwent IDS was as follows: among 13 patients with PCI scores 1–10, high-complexity surgery was performed in 9 (69%) and moderate-complexity surgery in 4 patients (31%). Among 54 patients with PCI scores 11–20, high-complexity surgery was performed in 50 (93%), moderate-complexity surgery in 3 (6%), and low-complexity surgery in 1 patient (2%). Among 42 patients with PCI scores 21–39, high-complexity surgery was performed in 38 (90%), moderate-complexity surgery in 2 (5%), and low-complexity surgery in 2 patients (5%).
Survival analysis
After a median follow-up duration of 40 months (range, 0.8–127 months), the median PFS for the entire cohort was 28 months, and the median overall survival (OS) was not reached.
In the Kaplan–Meier analysis (Fig. 2), PFS and OS did not significantly differ among the initial PCI groups, which were classified into three groups based on the PCI scores: 1–10 (n = 49), 11–20 (n = 81), 21–39 (n = 56) (p = 0.18 and p = 0.39, respectively). Kaplan–Meier curves were drawn for subgroup analyses of patients who underwent PDS, for those who underwent IDS, and for those who received chemotherapy without IDS (no debulking surgery). We observed that the PCI was not associated with PFS and OS in the subgroups of patients who underwent PDS and IDS (Fig. 3a,b,d,e). In contrast, the PCI was associated with PFS and OS in the subgroup of patients who received chemotherapy without IDS (Fig. 3c,f). The PDS, IDS, and no debulking surgery groups had a median PFS of 33, 28, and 6 months, respectively, and a median OS of not reached, 72, and 8 months, respectively.
The Kaplan–Meier curves classified the patients into three groups, according to the initial PCI scores of 1–10 (n = 49), 11–20 (n = 81), 21–39 (n = 56). These PCI groups did not significantly differ in terms of progression-free survival (p = 0.18) and overall survival (p = 0.39). PCI: peritoneal cancer index.
The Kaplan–Meier curves for subgroup analyses of patients who underwent PDS, for those who underwent IDS, and for those who received chemotherapy without debulking surgery. (a, b, c) progression-free survival. (c, d, e) overall survival. (a, c) patients who underwent PDS. (b, d) patients who underwent IDS. (c, e) patients who received chemotherapy without debulking surgery (no debulking surgery). Kaplan–Meier curves showed that PCI was not associated with progression-free survival and overall survival in both subgroups of patients who underwent PDS and those who underwent IDS. In contrast, PCI was associated with progression-free survival and overall survival in subgroup of patients who received chemotherapy without debulking surgery. PDS: primary debulking surgery, IDS: interval debulking surgery, PCI: peritoneal cancer index.
Kaplan–Meier curves were drawn for subgroup analyses of patients without and with residual disease (those with lesions measuring 0.1–1 cm and also those with lesions measuring > 1 cm). We observed that the PCI was not associated with PFS and OS in patients without residual disease (Fig. 4a–d). In contrast, the PCI was associated with OS in patients with residual disease (Fig. 4e,f).
The Kaplan–Meier curves in each in each subgroup of patients with no residual disease, patients with residual disease 0.1–1 cm, and patients with residual disease > 1 cm. (a, b, c) progression-free survival. (c, d, e) overall survival. (a, c) patients with no residual disease. (b, d) patients with residual disease 0.1–1 cm. (c, e) patients with residual disease > 1 cm. Kaplan–Meier curves showed that PCI was not associated with progression-free survival and overall survival in subgroups of patients without residual disease. In contrast, PCI was associated with overall survival in patients with residual disease. PCI: peritoneal cancer index.
Univariate Cox regression analysis showed that the initial PCI was not significantly associated with the PFS (hazard ratio [HR] 1.83, 95% confidence interval [CI] 0.83–4.09, p = 0.13) and with the OS (HR 2.43, 95% CI 0.87–6.86, p = 0.09) (Table 2). Univariate analysis showed that absence of residual disease and high surgical complexity scores significantly prolonged the PFS (HR 0.36, 95% CI 0.24–0.57, p < 0.01 and HR 0.62, 95% CI 0.42–0.94, p = 0.02, respectively) and the OS (HR 0.23, 95% CI 0.14–0.39, p < 0.01, and HR 0.42, 95% CI 0.26–0.70, p < 0.01, respectively).
Multivariate Cox regression analysis (Table 2) confirmed that the initial PCI was not significantly associated with the PFS (HR 1.83, 95% CI 0.74–4.52, p = 0.19) and with the OS (HR 3.24, 95% CI 0.98–10.6, p = 0.05). Multivariate analysis showed that absence of residual disease significantly prolonged the PFS (HR 0.44, 95% CI 0.28–0.73, p < 0.01), and absence of residual disease and a high surgical complexity score significantly prolonged the OS (HR 0.34, 95% CI 0.19–0.63, p < 0.01, and HR 0.43, 95% CI 0.23–0.79, p < 0.01, respectively).
Discussion
This study showed that PCI did not affect the prognosis of patients with FIGO stage IIIC/IV ovarian, fallopian tube, and peritoneal carcinoma when initially disseminated lesions > 1 cm in diameter were resected, regardless of the timing of cytoreductive surgery. No residual disease after surgery and high-surgical complexity score affected the prognosis. In other words, aggressive surgery could overcome the extent of peritoneal dissemination when patients with a high PCI score undergo high-complexity surgery by selecting PDS or IDS, depending on whether cytoreduction to no residual disease is achievable at initial laparotomy before starting treatment.
In this study, the timing of cytoreductive surgery was determined at initial laparotomy on the based on the feasibility of achieving no residual disease. As a result, the number of the patients who did not underwent PDS was more than twice as large as the number of the patients underwent PDS. Contrary to our study, the timing of cytoreductive surgery was usually primary at many institutions where aggressive cytoreductive surgery was performed16,17,18,19. However, studies have shown that the PCI score was negatively associated with the completeness of cytoreduction and residual disease after PDS10. Moreover, the rate of complications with PDS was higher than that with IDS18,24. Conversely, the use of NACT/IDS enabled the surgeons to accomplish a safe and high-complexity surgery in patients with high extent of peritoneal dissemination because NACT reduces the massive ascites and the tumor volume, improves the general condition, and decreases the difficulty of complex surgery. In this study, high-complexity surgery was performed on 71% of the entire cohort; this led to high rate (80%) of complete cytoreduction rate. Our results showed that not only PDS but also selective use of NACT/IDS allowed many patients to receive complete cytoreduction safely, regardless of the initial PCI.
In this study, the surgical complexity score of the patients who underwent IDS was higher than those of the patients who underwent PDS because initial PCI of the patients who underwent IDS was higher than those of the patients who underwent PDS. On the contrary, several studies showed that the surgical complexity and the rate of extra-gynecologic surgery were lower with IDS than with PDS18,25,26. The difference between this study and others lies in the surgical policy for IDS. In the present study, all disseminated tumors > 1 cm in diameter identified at initial laparotomy was removed even if the initially disseminated tumor was no longer visible at the time of IDS after NACT. In other words, at IDS, we performed the surgical procedures that would be performed to achieve residual tumor < 1 cm if PDS were to be performed. A drawback of the conventional IDS is that the tumors > 1 cm diameter identified at initial laparotomy would not be resected during IDS if they become invisible due to a good response to NACT. This may lead to a risk of early recurrence or relapse, similar to residual disease > 1 cm diameter after PDS. Our previous study showed that microscopic disease remains present especially in the rectosigmoid colon, transverse mesentery, greater omentum, right diaphragm, paracolic gutters, and vesicouterine pouch even if the tumors become invisible during IDS20. Similar to ours, Lim et al. reported that detached scars had residual cancer cells that assumedly included cancer stem cells27,28. The surgical removal of all the tumors > 1 cm identified at initial laparotomy could overcome tumor biology and tumor burden.
Unlike gastrointestinal carcinoma, another reason that PCI did not affect prognosis in this study is the high response rate to chemotherapy for ovarian cancer. In this study, 87% patients did not have disease progression during NACT and could undergo IDS. In other reports, the response rates to chemotherapy in FIGO stage III/IV ovarian carcinoma is higher in colorectal carcinoma (73–90 vs 15–62%)18,29,30,31,32. For colorectal cancer, patients with high score of PCI were reported to have poor prognosis29,33,34,35,36 because PCI directly affect the rate of no residual disease after surgery, frequency of postoperative complications, and prognosis29,33,34,35,36. Similar results have been reported for gastric and appendix cancers.
This study had some limitations such as the single-institution analysis, the small number of patients, and a higher percentage of IDS cases compared with that of PDS cases. The selection criteria for performing PDS or IDS varies among institutions. Notably, 42 of 56 patients with a high PCI score (21–39) were included in the NACT/IDS group; therefore, it is reasonable to conclude that in addition to aggressive surgery, chemotherapy may have affected the prognosis. In this study, 73% of patients with a high PCI score who could not undergo PDS underwent high-complexity surgery following the administration of NACT. Therefore, optimal patient selection for PDS or IDS can enable high-complexity surgery with favorable prognosis even in patients with a high PCI score.
In conclusion, the extent of initial peritoneal dissemination did not affect the prognosis for patients with FIGO stage IIIC/IV ovarian, fallopian, and peritoneal carcinoma when initially disseminated lesions > 1 cm was resected, regardless of the timing of cytoreductive surgery. No residual disease after surgery and a high-surgical complexity led to favorable prognosis. Aggressive cytoreductive surgery with selective use of IDS could overcome the extent of peritoneal dissemination.
Methods
Patient selection
This study was approved by the Institutional Review Board of Chiba University Graduate School of Medicine. All methods were carried out in accordance with relevant guidelines and regulations. Patients with FIGO 2014 stage IIIC/IV ovarian, fallopian tube, and peritoneal carcinoma who were consecutively treated at Chiba University Hospital from January 2008 to December 2017 were included. Written informed consent was obtained from all patients before surgery. Of the 208 patients during the study period, 22 patients who did not undergo exploratory laparotomy before NACT because of poor general condition (performance status ≥ 3 or ileus) and/or those aged ≥ 80 years, or who did not be evaluated in whole abdominal cavity due to adhesion were excluded. Therefore, this study included 186 patients in whom the extent of peritoneal dissemination upon initial laparotomy was assessed.
Selection of Primary or Interval debulking surgery
The treatment protocol used in this study is described in our previous reports20,21. In brief, the selection of primary or interval debulking surgery was decided during the initial laparotomy which was performed as early as possible after a patient's first visit. The patients was selected for IDS when disseminated tumor burden explored at initial laparotomy was too high to achieve no residual disease; gastrectomy, resection of the hepatic hilum or head of the pancreas, massive intestinal resection, or total colectomy was required; and/or massive ascites caused coagulopathy. At that time, patients underwent ovarian, fallopian tube, or omental biopsies, followed by NACT. Patients other than those mentioned above were selected for primary debulking surgery including upper abdominal surgery and bowel resection, to achieve no residual disease after surgery.
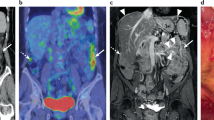
The exploration of initial peritoneal dissemination
The extent of peritoneal dissemination was assessed during initial laparotomy according to the peritoneal cancer index (PCI)1,2. The initial PCI groups were classified into three groups based on the PCI scores: 1–10, 11–20, 21–39. When the patients were selected for IDS, the margins of the disseminated tumor (> 1 cm) were marked with a non-absorbable, 3–0 black silk suture after diagnostic biopsy of ovary, fallopian tube or omentum was performed20,21.
Interval debulking surgery
The timing of IDS was described at our previous reports20,21. An implantable port system placed in the abdominal cavity at initial laparotomy was used for collection of peritoneal washing cytology, which was performed every 3 to 4 weeks during NACT. IDS was performed when the peritoneal washing cytology was negative and/or the serum CA-125 had decreased to 15 IU/mL or the serum CA-125 level stopped decreasing when the peritoneal washing cytology remained positive. If the disease progressed during NACT, we did not perform IDS. During IDS, the regions in which the disseminated tumors > 1 cm at the initial laparotomy was removed even when initial tumors were invisible, using the non-absorbable suture marked at initial laparotomy as landmarks.
The complexity of surgery performed
The complexity of surgery performed were scored according to the surgical complexity score22,23. The surgical complexity score was classified into three groups: the score of 1–3 was low, 4–7 was moderate, and 8–18 was high22,23.
Chemotherapy
For the adjuvant chemotherapy after PDS, six cycles of weekly paclitaxel (60–80 mg/m2) and carboplatin (AUC 2–3) for 3 weeks were administered. For the NACT, weekly paclitaxel and carboplatin were administered until the conditions mentioned above20. The median number of NACT cycles was 5. For adjuvant chemotherapy after IDS, six cycles of gemcitabine (500 mg/m2) and irinotecan (50 mg/m2) on days 1 and 8 every 3 weeks were administered. After December 2013, upon its approval for use by the Japanese public insurance, bevacizumab (15 mg/kg) every 21 days for 21 cycles was administered as the first-line therapy.
Statistical analysis
The association between the initial PCI and the clinical factors were analyzed using the Mann–Whitney U-test and Kruskal–Wallis H-test. Age and initial PCI score were entered as continuous variables. The association between the initial PCI and survival was analyzed by the Kaplan–Meier. Kaplan–Meier curves were drawn for the entire cohort and for subgroups of patients who underwent PDS, for those who underwent IDS, and for those who received chemotherapy without IDS. Kaplan–Meier curves were also drawn for subgroups of patients without and with residual disease (those with lesions measuring 0.1–1 cm and also those with lesions measuring > 1 cm). Univariate and multivariate Cox proportional hazards regression analyses were performed to analyze the prognostic factors. All statistical analyses were performed using JMP ver. 11(SAS, Cary, NC, USA). The Kaplan–Meier survival curves were drawn using IBM SPSS Statistical ver. 22 (IBM Japan Services Co., Ltd., Tokyo, Japan). For all statistical tests, the differences were considered significant at p < 0.05.
References
Prat, J. FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 124, 1–5 (2014).
Griffiths, C. T. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl. Cancer Inst. Monogr. 42, 101–104 (1975).
Bristow, R. E., Tomacruz, R. S., Armstrong, D. K., Trimble, E. L. & Montz, F. J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 20, 1248–1259 (2002).
Wimberger, P. et al. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol. Oncol. 106, 69–74 (2007).
Eisenhauer, E. L. et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol. Oncol. 103, 1083–1090 (2006).
Aletti, G. D. et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 107, 77–85 (2006).
Chi, D. S. et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol. Oncol. 114, 26–31 (2009).
Tentes, A. A. K. et al. Peritoneal cancer index: a prognostic indicator of survival in advanced ovarian cancer. Eur. J. Surg. Oncol. 29, 69–73 (2003).
Crawford, S. C. et al. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J. Clin. Oncol. 23, 8802–8811 (2005).
Sehouli, J. et al. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J. Surg. Oncol. 99, 424–427 (2009).
Chereau, E. et al. Comparison of peritoneal carcinomatosis scoring methods in predicting resectability and prognosis in advanced ovarian cancer. Am. J. Obstet. Gynecol. 202(178), e1–e10 (2010).
Hamilton, C. A. et al. The impact of disease distribution on survival in patients with stage III epithelial ovarian cancer cytoreduced to microscopic residual: a Gynecologic Oncology Group study. Gynecol. Oncol. 122, 521–526 (2011).
Aletti, G. D. et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol. Oncol. 120, 23–28 (2011).
Horowitz, N. S. et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J. Clin. Oncol. 33, 937–943 (2015).
Llueca, A. & Escrig, J. Prognostic value of peritoneal cancer index in primary advanced ovarian cancer. Eur. J. Surg. Oncol. 44, 163–169 (2018).
Fagotti, A. et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 59, 22–33 (2016).
Eisenkop, S. M., Friedman, R. L. & Wang, H. J. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol. Oncol. 69, 103–108 (1998).
Sugarbaker, P. H. & Jablonski, K. A. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann. Surg. 221, 124–132 (1995).
Jacquet, P. & Sugarbaker, P. H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In Peritoneal Carcinomatosis: Principles of Management (ed. Sugarbaker, P. H.) 359–374 (Springer, Berlin, 1996).
Tate, S., Kato, K., Nishikimi, K., Matsuoka, A. & Shozu, M. Survival and safety associated with aggressive surgery for stage III/IV epithelial ovarian cancer: a single institution observation study. Gynecol. Oncol. 147, 73–80 (2017).
Aletti, G. D. et al. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol. Oncol. 107, 99–106 (2007).
Kehoe, S. et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 386, 249–257 (2015).
Vergote, I. et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 363, 943–953 (2010).
Kang, S. et al. Is neo-adjuvant chemotherapy a “waiver” of extensive upper abdominal surgery in advanced epithelial ovarian cancer?. Ann. Surg. 18, 3824–3827 (2011).
Lim, M. C. et al. Residual cancer stem cells after interval cytoreductive surgery following neoadjuvant chemotherapy could result in poor treatment outcomes for ovarian cancer. Onkologie. 33, 324–330 (2010).
Lim, M. C. et al. Survival outcomes after extensive cytoreductive surgery and selective neoadjuvant chemotherapy according to institutional criteria in bulky stage IIIC and IV epithelial ovarian cancer. J. Gynecol. Oncol. 28, e48 (2017).
Eisenkop, S. M. et al. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol. Oncol. 90, 390–396 (2003).
Fagotti, A. et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann. Surg. Oncol. 13, 1156–1161 (2006).
da Silva, R. G. & Sugarbaker, P. H. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J. Am. Coll. Surg. 203, 878–886 (2006).
Elias, D. et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a Multicentric French Study. J. Clin. Oncol. 28, 63–68 (2010).
Yonemura, Y., Canbay, E. & Ishibashi, H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. Sci. World J. 2013, 978394 (2013).
Huang, Y., Alzahrani, N. A., Chua, T. C., Liauw, W. & Morris, D. L. Impacts of peritoneal cancer index on the survival outcomes of patients with colorectal peritoneal carcinomatosis. Int. J. Surg. 32, 65–70 (2016).
Kabbinavar, F. F. et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J. Clin. Oncol. 23, 3697–3705 (2005).
Ceelen, W. Peritoneal Carcinomatosis: A Multidisciplinary Approach 426–435 (Springer, Berlin, 2007).
Köhne, C.-H. et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J. Clin. Oncol. 23, 4856–4865 (2005).
McGuire, W. P. et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 334, 1–6 (1996).
Author information
Authors and Affiliations
Contributions
K.N. and S.T. designed the research; K.N., S.T., and A.M. collected the data; K.N. and S.T. analyzed and interpreted the data; K.N. wrote the main manuscript; S.T., A.M., and M.S. edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishikimi, K., Tate, S., Matsuoka, A. et al. Aggressive surgery could overcome the extent of initial peritoneal dissemination for advanced ovarian, fallopian tube, and peritoneal carcinoma. Sci Rep 10, 21307 (2020). https://doi.org/10.1038/s41598-020-78296-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78296-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.