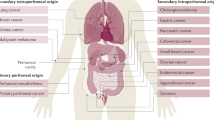
Abstract
Peritoneal surface malignancies (PSMs) are usually associated with a poor prognosis. Nonetheless, in line with advances in the management of most abdominopelvic metastatic diseases, considerable progress has been made over the past decade. An improved understanding of disease biology has led to the more accurate prediction of neoplasia aggressiveness and the treatment response and has been reflected in the proposal of new classification systems. Achieving complete cytoreductive surgery remains the cornerstone of curative-intent treatment of PSMs. Alongside centralization in expert centres, enabling the delivery of multimodal and multidisciplinary strategies, preoperative management is a crucial step in order to select patients who are most likely to benefit from surgery. Depending on the specific PSM, the role of intraperitoneal chemotherapy and of perioperative systemic chemotherapy, in particular, in the neoadjuvant setting, is established in certain scenarios but questioned in several others, although more prospective data are required. In this Review, we describe advances in all aspects of the management of PSMs including disease biology, assessment and improvement of disease resectability, perioperative management, systemic therapy and pre-emptive management, and we speculate on future research directions.
Key points
-
Pathological, molecular and genetic biomarkers should be integrated into the selection processes for appropriate multidisciplinary, palliative or curative management of peritoneal surface malignancies (PSMs).
-
Centralization of curative treatment in PSM-specific expert centres is needed to decrease the incidence of postoperative complications and improve survival outcomes owing to better patient selection, surgical expertise and perioperative care.
-
The combination of functional imaging (MRI, PET–CT) with appropriate laparoscopy improves the preoperative assessment of resectability of patients with peritoneal metastases, which is the most important determinant of eligibility for curative treatment.
-
Perioperative prehabilitation and enhanced postoperative recovery (ERAS) pathways should be integrated into curative management, especially in patients >70 years of age who require specific comprehensive geriatric assessments.
-
Intraperitoneal chemotherapy (HIPEC, PIPAC, NIPEC or EPIC) has an important role in the management of PSM and requires further evaluation in the neoadjuvant, consolidative therapy and pre-emptive settings.
-
The role of perioperative systemic chemotherapy, targeted therapy and immunotherapy should be evaluated in specific studies involving patients with PSMs, considering the common chemoresistance to systemic treatments in these patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cortés-Guiral, D. et al. Primary and metastatic peritoneal surface malignancies. Nat. Rev. Dis. Prim. 7, 91 (2021).
Sadeghi, B. et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88, 358–363 (2000).
Chu, D. Z., Lang, N. P., Thompson, C., Osteen, P. K. & Westbrook, K. C. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 63, 364–367 (1989).
Jayne, D. G., Fook, S., Loi, C. & Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 89, 1545–1550 (2002).
Sugarbaker, P. H. Peritonectomy procedures. Ann. Surg. 221, 29–42 (1995).
Arquillière, J., Glehen, O. & Passot, G. Cytoreductive surgery in peritoneal carcinomatosis. J. Visc. Surg. 158, 258–264 (2021).
Chi, D. S. et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol. Oncol. 94, 650–654 (2004).
Glehen, O., Mohamed, F. & Gilly, F. N. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 5, 219–228 (2004).
Glehen, O. et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1290 patients. Cancer 116, 5608–5618 (2010).
Speeten, K. V., der, Lemoine, L. & Sugarbaker, P. Overview of the optimal perioperative intraperitoneal chemotherapy regimens used in current clinical practice. Pleura Peritoneum 2, 63–72 (2017).
Bristow, R. E., Tomacruz, R. S., Armstrong, D. K., Trimble, E. L. & Montz, F. J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 20, 1248–1259 (2002).
Elias, D. et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 28, 63–68 (2010).
Kusamura, S. et al. The role of hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei after cytoreductive surgery. JAMA Surg. 156, e206363 (2021).
Jacquet, P. & Sugarbaker, P. H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 82, 359–374 (1996).
Goere, D. et al. Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: results from a worldwide analysis issue of the peritoneal surface oncology group international (PSOGI). Int. J. Hyperthermia 33, 520–527 (2017).
Chirurgie, A. Fde et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 17, 2370–2377 (2010).
Bakrin, N. et al. Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. 39, 1435–1443 (2013).
Villeneuve, L. et al. A new internet tool to report peritoneal malignancy extent. PeRitOneal malignancy stage evaluation (PROMISE) application. Eur. J. Surg. Oncol. 42, 877–882 (2016).
Chapel, D. B. et al. Malignant peritoneal mesothelioma: prognostic significance of clinical and pathologic parameters and validation of a nuclear-grading system in a multi-institutional series of 225 cases. Mod. Pathol. 34, 380–395 (2020).
Benzerdjeb, N. et al. Combined grade and nuclear grade are prognosis predictors of epithelioid malignant peritoneal mesothelioma: a multi-institutional retrospective study. Virchows Arch. 479, 927–936 (2021).
Panou, V. et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J. Clin. Oncol. 36, 2863–2871 (2018).
Chirac, P. et al. Genomic copy number alterations in 33 malignant peritoneal mesothelioma analyzed by comparative genomic hybridization array. Hum. Pathol. 55, 72–82 (2016).
Alakus, H. et al. BAP1 mutation is a frequent somatic event in peritoneal malignant mesothelioma. J. Transl. Med. 13, 122 (2015).
Joseph, N. M. et al. Genomic profiling of malignant peritoneal mesothelioma reveals recurrent alterations in epigenetic regulatory genes BAP1, SETD2, and DDX3X. Mod. Pathol. 30, 246–254 (2017).
Leblay, N. et al. BAP1 is altered by copy number loss, mutation, and/or loss of protein expression in more than 70% of malignant peritoneal mesotheliomas. J. Thorac. Oncol. 12, 724–733 (2017).
Shrestha, R. et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med. 11, 8 (2019).
Deraco, M. et al. In Pathology of Peritoneal Metastases (eds Glehen, O. & Bhatt, A.) 117–129 (Springer, 2020) https://doi.org/10.1007/978-981-15-3773-8_6.
Carbone, M. et al. BAP1 and cancer. Nat. Rev. Cancer 13, 153–159 (2013).
Sugarbaker, P. H. et al. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann. Surg. 219, 109–111 (1994).
Carr, N. J. et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) mModified Delphi process. Am. J. Surg. Pathol. 40, 14–26 (2016).
Lin, Y. L. et al. Consensuses and controversies on pseudomyxoma peritonei: a review of the published consensus statements and guidelines. Orphanet J. Rare Dis. 16, 85 (2021).
Valasek, M. A. & Pai, R. K. An update on the diagnosis, grading, and staging of appendiceal mucinous neoplasms. Adv. Anat. Pathol. 25, 38–60 (2018).
Levine, E. A. et al. Prognostic molecular subtypes of low-grade cancer of the appendix. J. Am. Coll. Surg. 222, 493–503 (2016).
Vaira, M. et al. In Pathology of Peritoneal Metastases (eds Glehen, O. & Bhatt, A.) 163–173 (Springer, 2020).
Su, J. et al. Prognostic molecular classification of appendiceal mucinous neoplasms treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 27, 1439–1447 (2020).
Moaven, O. et al. Clinical implications of genetic signatures in appendiceal cancer patients with incomplete cytoreduction/HIPEC. Ann. Surg. Oncol. 27, 5016–5023 (2020).
Ajani, J. A. et al. Gastric adenocarcinoma. Nat. Rev. Dis. Prim. 3, 17036 (2017).
Gao, D. et al. Cdh1 regulates cell cycle through modulating the Claspin/Chk1 and the Rb/E2F1 pathways. Mol. Biol. Cell 20, 3305–3316 (2009).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Cristescu, R. et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 21, 449–456 (2015).
Wang, K. et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 46, 573–582 (2014).
Oh, S. C. et al. Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat. Commun. 9, 1777 (2018).
Tanaka, Y. et al. Multi-omic profiling of peritoneal metastases in gastric cancer identifies molecular subtypes and therapeutic vulnerabilities. Nat. Cancer 2, 962–977 (2021).
Wang, R. et al. Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut 69, 18–31 (2020).
Bettington, M. et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology 62, 367–386 (2013).
Zajac, O. et al. Tumour spheres with inverted polarity drive the formation of peritoneal metastases in patients with hypermethylated colorectal carcinomas. Nat. Cell Biol. 20, 296–306 (2018).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Ubink, I. et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br. J. Surg. 105, e204–e211 (2018).
Heinemann, V., Stintzing, S., Kirchner, T., Boeck, S. & Jung, A. Clinical relevance of EGFR- and KRAS-status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat. Rev. 35, 262–271 (2009).
Deng, G. et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin. Cancer Res. 10, 191–195 (2004).
Arjona-Sanchez, A. et al. RAS mutation decreases overall survival after optimal cytoreductive surgery and hyperthermic intraperitoneal chemotherapy of colorectal peritoneal metastasis: a modification proposal of the peritoneal surface disease severity score. Ann. Surg. Oncol. 26, 2595–2604 (2019).
Schneider, M. A. et al. Mutations of RAS/RAF proto-oncogenes impair survival after cytoreductive surgery and HIPEC for peritoneal metastasis of colorectal origin. Ann. Surg. 268, 845–853 (2018).
Cohen, R., Pudlarz, T., Delattre, J.-F., Colle, R. & Andre, T. Molecular targets for the treatment of metastatic colorectal cancer. Cancers 12, 2350 (2020).
Pietrantonio, F. et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J. Natl Cancer Inst. 109, djx089 (2017).
André, T. et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Sallum, L. F. et al. WT1, p53 and p16 expression in the diagnosis of low- and high-grade serous ovarian carcinomas and their relation to prognosis. Oncotarget 9, 15818–15827 (2018).
Moore, K. et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 379, 2495–2505 (2018).
Miller, R. E. et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann. Oncol. 31, 1606–1622 (2020).
Chiang, Y.-C., Lin, P.-H. & Cheng, W.-F. Homologous recombination deficiency assays in epithelial ovarian cancer: current status and future direction. Front. Oncol. 11, 675972 (2021).
Ubink, I. et al. Organoids from colorectal peritoneal metastases as a platform for improving hyperthermic intraperitoneal chemotherapy. Br. J. Surg. 106, 1404–1414 (2019).
Narasimhan, V. et al. Medium-throughput drug screening of patient-derived organoids from colorectal peritoneal metastases to direct personalized therapy. Clin. Cancer Res. 26, 3662–3670 (2020).
Votanopoulos, K. I. et al. Appendiceal cancer patient-specific tumor organoid model for predicting chemotherapy efficacy prior to initiation of treatment: a feasibility study. Ann. Surg. Oncol. 26, 139–147 (2019).
Letai, A. Functional precision cancer medicine — moving beyond pure genomics. Nat. Med. 23, 1028–1035 (2017).
Roy, P. et al. Organoids as preclinical models to improve intraperitoneal chemotherapy effectiveness for colorectal cancer patients with peritoneal metastases: preclinical models to improve HIPEC. Int. J. Pharm. 531, 143–152 (2017).
Mazzocchi, A. R., Rajan, S. A. P., Votanopoulos, K. I., Hall, A. R. & Skardal, A. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci. Rep. 8, 2886 (2018).
Votanopoulos, K. I. et al. Model of patient-specific immune-enhanced organoids for immunotherapy screening: feasibility study. Ann. Surg. Oncol. 27, 1956–1967 (2020).
Dhanisha, S. S., Guruvayoorappan, C., Drishya, S. & Abeesh, P. Mucins: structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 122, 98–122 (2018).
Rhodes, J. M. Usefulness of novel tumour markers. Ann. Oncol. 10, S118–S121 (1999).
Young, R. H. Pseudomyxoma peritonei and selected other aspects of the spread of appendiceal neoplasms. Semin. Diagn. Pathol. 21, 134–150 (2004).
O’Connell, J. T., Tomlinson, J. S., Roberts, A. A., McGonigle, K. F. & Barsky, S. H. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am. J. Pathol. 161, 551–564 (2002).
Lohani, K. et al. Pseudomyxoma peritonei: inflammatory responses in the peritoneal microenvironment. Ann. Surg. Oncol. 21, 1441–1447 (2014).
O’Connell, J. T., Hacker, C. M. & Barsky, S. H. MUC2 is a molecular marker for pseudomyxoma peritonei. Mod. Pathol. 15, 958–972 (2002).
Amini, A., Masoumi-Moghaddam, S., Ehteda, A. & Morris, D. L. Secreted mucins in pseudomyxoma peritonei: pathophysiological significance and potential therapeutic prospects. Orphanet J. Rare Dis. 9, 71 (2014).
Huang, Y. et al. Intraoperative macroscopic tumour consistency is associated with overall survival after cytoreductive surgery and intraperitoneal chemotherapy for appendiceal adenocarcinoma with peritoneal metastases: a retrospective observational study. Am. J. Surg. 217, 704–712 (2019).
Nagtegaal, I. D. et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 76, 182–188 (2020).
Benesch, M. G. K. & Mathieson, A. Epidemiology of signet ring cell adenocarcinomas. Cancers 12, 1544 (2020).
Berger, Y. et al. Correlation between intraoperative and pathological findings for patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 26, 1103–1109 (2019).
Bhatt, A. et al. The pathologic peritoneal cancer index (PCI) strongly differs from the surgical PCI in peritoneal metastases arising from various primary tumors. Ann. Surg. Oncol. 10, 3–12 (2020).
Sessa, C. et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 30, 672–705 (2019).
Bois, Adu et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115, 1234–1244 (2009).
Harter, P. et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N. Engl. J. Med. 385, 2123–2131 (2021).
Quenet, F. et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 22, 256–266 (2021).
Franko, J. et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 17, 1709–1719 (2016).
Elias, D. et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 27, 681–685 (2009).
Chua, T. C. et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 30, 2449–2456 (2012).
Yan, T. D. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J. Clin. Oncol. 27, 6237–6242 (2009).
Govaerts, K. et al. Appendiceal tumours and pseudomyxoma peritonei: literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 47, 11–35 (2021).
Kusamura, S. et al. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. 47, 36–59 (2021).
Ansari, N. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur. J. Surg. Oncol. 42, 1035–1041 (2016).
Chia, C. S. et al. Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann. Surg. Oncol. 23, 1971–1979 (2016).
Brandl, A., Yonemura, Y., Glehen, O., Sugarbaker, P. & Rau, B. Long term survival in patients with peritoneal metastasised gastric cancer treated with cytoreductive surgery and HIPEC: a multi-institutional cohort from PSOGI. Eur. J. Surg. Oncol. 47, 172–180 (2021).
Bonnot, P.-E. et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J. Clin. Oncol. 37, 2028–2040 (2019).
Bonnot, P. E. et al. Prognosis of poorly cohesive gastric cancer after complete cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (CYTO-CHIP study). Br. J. Surg. 108, 1225–1235 (2021).
Kusamura, S. et al. Multicentre study of the learning curve and surgical performance of cytoreductive surgery with intraperitoneal chemotherapy for pseudomyxoma peritonei. Br. J. Surg. 101, 1758–1765 (2014).
Passot, G. et al. A perioperative clinical pathway can dramatically reduce failure-to-rescue rates after cytoreductive surgery for peritoneal carcinomatosis: a retrospective study of 666 consecutive cytoreductions. Ann. Surg. 265, 806–813 (2017).
Noiret, B. et al. Centralization and oncologic training reduce postoperative morbidity and failure-to-rescue rates after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: study on a 10-year national french practice. Ann. Surg. 272, 847–854 (2020).
Stang, N. L. et al. Incidence and survival of peritoneal malignant mesothelioma between 1989 and 2015: a population-based study. Cancer Epidemiol. 60, 106–111 (2019).
Villeneuve, L. et al. The RENAPE observational registry: rationale and framework of the rare peritoneal tumors French patient registry. Orphanet J. Rare Dis. 12, 37–39 (2017).
Cavaliere, F. et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur. J. Surg. Oncol. 37, 148–154 (2011).
Manzanedo, I. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer with peritoneal carcinomatosis: multicenter study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann. Surg. Oncol. 26, 2615–2621 (2019).
Arjona-Sánchez, Á. et al. A minimally invasive approach for peritonectomy procedures and hyperthermic intraperitoneal chemotherapy (HIPEC) in limited peritoneal carcinomatosis: the American society of peritoneal surface malignancies (ASPSM) multi-institution analysis. Surg. Endosc. 33, 854–860 (2018).
Kusamura, S. et al. Learning curve, training program, and monitorization of surgical performance of peritoneal surface malignancies centers. Surg. Oncol. Clin. N. Am. 27, 507–517 (2018).
Reuss, A. et al. TRUST: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int. J. Gynecol. Cancer 29, 1327 (2019).
Mariani, A. et al. Strategies for managing intraoperative discovery of limited colorectal peritoneal metastases. Ann. Surg. Oncol. 26, 1437–1444 (2019).
Faron, M. et al. Linear relationship of peritoneal cancer index and survival in patients with peritoneal metastases from colorectal cancer. Ann. Surg. Oncol. 23, 114–119 (2016).
Goéré, D. et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann. Surg. Oncol. 22, 2958–2964 (2015).
van’t Sant, I. et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur. Radiol. 30, 3101–3112 (2020).
Mohkam, K. et al. Resectability of peritoneal carcinomatosis: learnings from a prospective cohort of 533 consecutive patients selected for cytoreductive surgery. Ann. Surg. Oncol. 23, 1261–1270 (2016).
Sugarbaker, P. H. & Low, R. N. (eds) Pictorial Essays On Peritoneal Metastases Imaging: CT, MRI and PET-CT (Nova Science Publishers, 2020).
Low, R. N., Barone, R. M. & Rousset, P. Peritoneal MRI in patients undergoing cytoreductive surgery and HIPEC: history, clinical applications, and implementation. Eur. J. Surg. Oncol. 47, 65–74 (2021).
Dohan, A. et al. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br. J. Surg. 104, 1244–1249 (2017).
Sant, I. V. T. et al. Diffusion-weighted MRI assessment of the peritoneal cancer index before cytoreductive surgery. Br. J. Surg. 106, 491–498 (2019).
Delhorme, J.-B. et al. Appendiceal tumors and pseudomyxoma peritonei: French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (RENAPE, RENAPATH, SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, SFR). Dig. Liver Dis. 54, 30–39 (2022).
Menassel, B. et al. Preoperative CT and MRI prediction of non-resectability in patients treated for pseudomyxoma peritonei from mucinous appendiceal neoplasms. Eur. J. Surg. Oncol. 42, 558–566 (2016).
Lehmann, K. et al. 18FDG-PET-CT improves specificity of preoperative lymph-node staging in patients with intestinal but not diffuse-type esophagogastric adenocarcinoma. Eur. J. Surg. Oncol. 43, 196–202 (2017).
Kim, S.-J. & Lee, S.-W. Diagnostic accuracy of 18F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis. Br. J. Radiol. 91, 20170519 (2018).
Sugarbaker, P. H. et al. Concerning CT features used to select patients for treatment of peritoneal metastases, a pictoral essay. Int. J. Hyperthermia 33, 497–504 (2017).
Chandramohan, A. et al. Communicating imaging findings in peritoneal mesothelioma: the impact of ‘PAUSE’ on surgical decision-making. Insights Imaging 12, 174 (2021).
Chandramohan, A., Thrower, A., Smith, S. A., Shah, N. & Moran, B. “PAUSE”: a method for communicating radiological extent of peritoneal malignancy. Clin. Radiol. 72, 972–980 (2017).
Lennartz, S. et al. Iodine overlays to improve differentiation between peritoneal carcinomatosis and benign peritoneal lesions. Eur. Radiol. 30, 3968–3976 (2020).
Darras, K. E. et al. Virtual monoenergetic reconstruction of contrast-enhanced CT scans of the abdomen and pelvis at 40 keV improves the detection of peritoneal metastatic deposits. Abdom. Radiol. 44, 422–428 (2019).
Thivolet, A. et al. Spectral photon-counting CT imaging of colorectal peritoneal metastases: initial experience in rats. Sci. Rep. 10, 13394 (2020).
Zhao, L. et al. Role of [68Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [18F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. I 48, 1944–1955 (2021).
Kuten, J. et al. Head-to-head comparison of [68Ga]Ga-FAPI-04 and [18F]-FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 49, 743–750 (2022).
van’t Sant, I. et al. Seeing the whole picture: added value of MRI for extraperitoneal findings in CRS-HIPEC candidates. Eur. J. Surg. Oncol. 48, 462–469 (2022).
Wang, W. et al. Are positron emission tomography-computed tomography (PET-CT) scans useful in preoperative assessment of patients with peritoneal disease before cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC)? Int. J. Hyperthermia 34, 524–531 (2018).
Vuysere, S. D. et al. Accuracy of whole-body diffusion-weighted MRI (WB-DWI/MRI) in diagnosis, staging and follow-up of gastric cancer, in comparison to CT: a pilot study. BMC Med. Imaging 21, 18 (2021).
Dresen, R. C. et al. Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases. Cancer Imaging 19, 1 (2019).
Brendle, C. et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur. J. Nucl. Med. Mol. I 43, 123–132 (2016).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Bhatt, A. et al. Patterns of peritoneal dissemination and response to systemic chemotherapy in common and rare peritoneal tumours treated by cytoreductive surgery: study protocol of a prospective, multicentre, observational study. BMJ Open 11, e046819 (2021).
Bhatt, A. et al. Clinical and radiologic predictors of a pathologic complete response to neoadjuvant chemotherapy (NACT) in patients undergoing cytoreductive surgery for colorectal peritoneal metastases: results of a prospective multi-center study. Ann. Surg. Oncol. 28, 3840–3849 (2021).
Liberale, G. et al. Accuracy of FDG-PET/CT in colorectal peritoneal carcinomatosis: potential tool for evaluation of chemotherapeutic response. Anticancer Res. 37, 929–934 (2017).
Park, S. J. et al. Reduction of cycles of neoadjuvant chemotherapy for advanced epithelial ovarian, fallopian or primary peritoneal cancer (ROCOCO): study protocol for a phase III randomized controlled trial. BMC Cancer 20, 385 (2020).
Sala, E. et al. Advanced ovarian cancer: multiparametric MR imaging demonstrates response- and metastasis-specific effects. Radiology 263, 149–159 (2012).
Himoto, Y. et al. Computed tomography–derived radiomic metrics can identify responders to immunotherapy in ovarian cancer. JCO Precis. Oncol. 3, 1–13 (2019).
Nougaret, S. et al. Radiomics and radiogenomics in ovarian cancer: a literature review. Abdom. Radiol. 46, 2308–2322 (2021).
Mikkelsen, M. S. et al. Assessment of peritoneal metastases with DW-MRI, CT, and FDG PET/CT before cytoreductive surgery for advanced stage epithelial ovarian cancer. Eur. J. Surg. Oncol. 47, 2134–2141 (2021).
Engbersen, M. P. et al. Dedicated MRI staging versus surgical staging of peritoneal metastases in colorectal cancer patients considered for CRS-HIPEC; the DISCO randomized multicenter trial. BMC Cancer 21, 464 (2021).
Passot, G. et al. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br. J. Surg. 105, 663–667 (2018).
Iversen, L. H., Rasmussen, P. C. & Laurberg, S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br. J. Surg. 100, 285–292 (2013).
Sell, N. M. et al. Staging laparoscopy not only saves patients an incision, but may also help them live longer. Ann. Surg. Oncol. 25, 1009–1016 (2018).
Allen, C. J. et al. Yield of peritoneal cytology in staging patients with gastric and gastroesophageal cancer. J. Surg. Oncol. 120, 1350–1357 (2019).
Blackshaw, G. R. J. C. et al. Laparoscopy significantly improves the perceived preoperative stage of gastric cancer. Gastric Cancer 6, 225–229 (2003).
Sarela, A. I., Lefkowitz, R., Brennan, M. F. & Karpeh, M. S. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am. J. Surg. 191, 134–138 (2006).
Passot, G. et al. Postoperative outcomes of laparoscopic vs open cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for treatment of peritoneal surface malignancies. Eur. J. Surg. Oncol. 40, 957–962 (2014).
Dong, D. et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann. Oncol. 30, 431–438 (2019).
Jiang, Y. et al. Predicting peritoneal recurrence and disease-free survival from CT images in gastric cancer with multitask deep learning: a retrospective study. Lancet Digital Heal. 4, e340–e350 (2022).
Passot, G. et al. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann. Surg. Oncol. 21, 2608–2614 (2014).
Goere, D. et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 21, 1147–1154 (2020).
Bhatt, A. et al. Prospective correlation of the radiological, surgical and pathological findings in patients undergoing cytoreductive surgery for colorectal peritoneal metastases: implications for the preoperative estimation of the peritoneal cancer index. Colorectal Dis. 22, 2123–2132 (2020).
Bhatt, A. & Glehen, O. Extent of peritoneal resection for peritoneal metastases: looking beyond a complete cytoreduction. Ann. Surg. Oncol. 27, 1458–1470 (2020).
Veys, I. et al. ICG-fluorescence imaging for detection of peritoneal metastases and residual tumoral scars in locally advanced ovarian cancer: a pilot study. J. Surg. Oncol. 117, 228–235 (2018).
Zapardiel, I. et al. Utility of intraoperative fluorescence imaging in gynecologic surgery: systematic review and consensus statement. Ann. Surg. Oncol. 28, 3266–3278 (2021).
Bhatt, A. & Glehen, O. ASO author reflections: tailoring the extent of peritoneal resection for peritoneal metastases according to the primary tumor site. Ann. Surg. Oncol. 27, 1471–1472 (2020).
Baratti, D., Kusamura, S., Cabras, A. D. & Deraco, M. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Ann. Surg. Oncol. 19, 1416–1424 (2012).
Bhatt, A. et al. Total parietal peritonectomy can be performed with acceptable morbidity for patients with advanced ovarian cancer after neoadjuvant chemotherapy: results from a prospective multi-centric study. Ann. Surg. Oncol. 28, 1118–1129 (2021).
Sinukumar, S. et al. A comparison of outcomes following total and selective peritonectomy performed at the time of interval cytoreductive surgery for advanced serous epithelial ovarian, fallopian tube and primary peritoneal cancer–a study by INDEPSO. Eur. J. Surg. Oncol. 47, 75–81 (2021).
Lawrence, V. A. et al. Functional independence after major abdominal surgery in the elderly. J. Am. Coll. Surg. 199, 762–772 (2004).
Christensen, T. & Kehlet, H. Postoperative fatigue. World J. Surg. 17, 220–225 (1993).
Baratti, D. et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis. Colon Rectum 57, 858–868 (2014).
Ditmyer, M. M., Topp, R. & Pifer, M. Prehabilitation in preparation for orthopaedic surgery. Orthop. Nurs. 21, 43–54 (2002).
Hübner, M. et al. Guidelines for perioperative care in cytoreductive surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): enhanced recovery after surgery (ERAS®) Society Recommendations — Part I: preoperative and intraoperative management. Eur. J. Surg. Oncol. 46, 2292–2310 (2020).
Hübner, M. et al. Guidelines for perioperative care in cytoreductive surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): enhanced recovery after surgery (ERAS®) society recommendations–Part II: postoperative management and special considerations. Eur. J. Surg. Oncol. 46, 2311–2323 (2020).
Dhiman, A. et al. Guide to enhanced recovery for cancer patients undergoing surgery: ERAS for patients undergoing cytoreductive surgery with or without HIPEC. Ann. Surg. Oncol. 28, 6955–6964 (2021).
Vashi, P. G. et al. The relationship between baseline nutritional status with subsequent parenteral nutrition and clinical outcomes in cancer patients undergoing hyperthermic intraperitoneal chemotherapy. Nutr. J. 12, 118–118 (2013).
Mills, E. et al. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am. J. Med. 124, 144–154.e8 (2011).
Thomsen, T., Villebro, N. & Møller, A. M. Interventions for preoperative smoking cessation. Cochrane Database Syst. Rev. 3, CD002294 (2014).
Iqbal, U. et al. Preoperative patient preparation in enhanced recovery pathways. J. Anaesthesiol. Clin. Pharmacol. 35, S14–S23 (2019).
Pouwels, S. et al. Preoperative exercise therapy for elective major abdominal surgery: a systematic review. Int. J. Surg. 12, 134–140 (2014).
Boukili, I. E. et al. Prehabilitation before major abdominal surgery: evaluation of the impact of a perioperative clinical pathway, a pilot study. Scand. J. Surg. 111, 145749692210833 (2022).
Alyami, M. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis in the elderly: a case-controlled, multicenter study. Ann. Surg. Oncol. 23, 737–745 (2016).
Dion, L. et al. Ovarian cancer in the elderly: time to move towards a more logical approach to improve prognosis — a study from the FRANCOGYN Group. J. Clin. Med. 9, 1339 (2020).
Gagniere, J. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the elderly: is it reasonable? a meta-analysis. Ann. Surg. Oncol. 25, 709–719 (2018).
Rubenstein, L. Z., Stuck, A. E., Siu, A. L. & Wieland, D. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J. Am. Geriatr. Soc. 39, 8S–16S (1991).
Soubeyran, P. et al. Screening for vulnerability in older cancer patients: the ONCODAGE prospective multicenter cohort study. PLoS ONE 9, e115060 (2014).
Hamaker, M. E. et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 13, e437–e444 (2012).
Wildiers, H. et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 32, 2595–2603 (2014).
Mohanty, S. et al. Optimal perioperative management of the geriatric patient: a best practices guideline from the american college of surgeons NSQIP and the American Geriatrics Society. J. Am. Coll. Surg. 222, 930–947 (2016).
Minnella, E. M. et al. Patients with poor baseline walking capacity are most likely to improve their functional status with multimodal prehabilitation. Surgery 160, 1070–1079 (2016).
Roche, M. et al. Feasibility of a prehabilitation programme dedicated to older patients with cancer before complex medical–surgical procedures: the PROADAPT pilot study protocol. BMJ Open 11, e042960 (2021).
Falandry, C. et al. Interventions to improve physical performances of older people with cancer before complex medico-surgical procedures. Medicine 99, e21780 (2020).
Cederholm, T. et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin. Nutr. 38, 1–9 (2019).
Boereboom, C., Doleman, B., Lund, J. N. & Williams, J. P. Systematic review of pre-operative exercise in colorectal cancer patients. Tech. Coloproctol. 20, 81–89 (2016).
Launay-Savary, M.-V. et al. Are enhanced recovery programs in colorectal surgery feasible and useful in the elderly? A systematic review of the literature. J. Visc. Surg. 154, 29–35 (2017).
Dedrick, R. L. Theoretical and experimental bases of intraperitoneal chemotherapy. Semin. Oncol. 12 (3 Suppl. 4), 1–6 (1985).
Dedrick, R. L., Myers, C. E., Bungay, P. M. & DeVita, V. T. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat. Rep. 62, 1–11 (1978).
Ceelen, W. P. & Flessner, M. F. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat. Rev. Clin. Oncol. 7, 108–115 (2010).
Nagy, J. A., Chang, S.-H., Shih, S.-C., Dvorak, A. M. & Dvorak, H. F. Heterogeneity of the tumor vasculature. Semin. Thromb. Hemost. 36, 321–331 (2010).
Heldin, C.-H., Rubin, K., Pietras, K. & Östman, A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 (2004).
Spratt, J. S., Adcock, R. A., Muskovin, M., Sherrill, W. & McKeown, J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 40, 256–260 (1980).
Issels, R. D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 44, 2546–2554 (2008).
Reymond, M. A. et al. Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg. Endosc. 14, 51–55 (2000).
Reis, A. C. V. et al. Hemodynamic and respiratory implications of high intra-abdominal pressure during HIPEC. Eur. J. surg. Oncol. 46, 1896–1901 (2020).
Sugarbaker, P. H. & Jablonski, K. A. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann. Surg. 221, 124–132 (1995).
der Speeten, K. V. & Lemoine, L. In Management of Peritoneal Metastases: Cytoreductive Surgery, HIPEC and Beyond (ed. Bhatt A.) 79–102 (Springer, 2017).
Urano, M., Kuroda, M. & Nishimura, Y. For the clinical application of thermochemotherapy given at mild temperatures. Int. J. Hyperthermia 15, 79–107 (1999).
Leunig, M. et al. Interstitial fluid pressure in solid tumors following hyperthermia: possible correlation with therapeutic response. Cancer Res. 52, 487–490 (1992).
Hettinga, J. et al. Mechanism of hyperthermic potentiation of cisplatin action in cisplatin-sensitive and -resistant tumour cells. Br. J. Cancer 75, 1735–1743 (1997).
Hettinga, J. V., Konings, A. W. & Kampinga, H. H. Reduction of cellular cisplatin resistance by hyperthermia-a review. Int. J. Hyperthermia 13, 439–457 (1997).
Wallner, K. E. & Li, G. C. Effect of drug exposure duration and sequencing on hyperthermic potentiation of mitomycin-C and cisplatin. Cancer Res. 47, 493–495 (1987).
Yurttas, C. et al. Systematic review of variations in hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastasis from colorectal cancer. J. Clin. Med. 7, 567 (2018).
Bhatt, A. et al. HIPEC methodology and regimens: the need for an expert consensus. Ann. Surg. Oncol. 28, 9098–9113 (2021).
Driel, W. Jvan et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 378, 230–240 (2018).
Yang, X.-J. et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase iii randomized clinical trial. Ann. Surg. Oncol. 18, 1575–1581 (2011).
Park, E. J. et al. Pharmacologic properties of the carrier solutions for hyperthermic intraperitoneal chemotherapy: comparative analyses between water and lipid carrier solutions in the rat model. Ann. Surg. Oncol. 25, 3185–3192 (2018).
Piché, N. et al. Rationale for heating oxaliplatin for the intraperitoneal treatment of peritoneal carcinomatosis: a study of the effect of heat on intraperitoneal oxaliplatin using a murine model. Ann. Surg. 254, 138–144 (2011).
Bespalov, V. G. et al. Comparative efficacy evaluation of catheter intraperitoneal chemotherapy, normothermic and hyperthermic chemoperfusion in a rat model of ascitic ovarian cancer. Int. J. Hyperthermia 34, 545–550 (2017).
Raue, W. et al. Multimodal approach for treatment of peritoneal surface malignancies in a tumour-bearing rat model. Int. J. Colorectal Dis. 25, 245–250 (2010).
Ortega-Deballon, P. et al. Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin? An experimental comparison of open and closed techniques. Ann. Surg. Oncol. 17, 1957–1963 (2010).
Helderman, R. F. C. P. A. et al. Preclinical in vivo-models to investigate HIPEC; current methodologies and challenges. Cancers 13, 3430 (2021).
Lemoine, L. et al. Body surface area-based versus concentration-based intraperitoneal perioperative chemotherapy in a rat model of colorectal peritoneal surface malignancy: pharmacologic guidance towards standardization. Oncotarget 10, 1407–1424 (2019).
Löffler, M. W. et al. Pharmacodynamics of oxaliplatin-derived platinum compounds during hyperthermic intraperitoneal chemotherapy (HIPEC): an emerging aspect supporting the rational design of treatment protocols. Ann. Surg. Oncol. 24, 1650–1657 (2017).
Elekonawo, F. M. K. et al. Effect of intraperitoneal chemotherapy concentration on morbidity and survival. BJS Open 4, 293–300 (2020).
Löke, D. R. et al. Simulating drug penetration during hyperthermic intraperitoneal chemotherapy. Drug Deliv. 28, 145–161 (2021).
Prabhu, A. et al. Effect of oxaliplatin-based chemotherapy on chemosensitivity in patients with peritoneal metastasis from colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: proof-of-concept study. BJS Open 5, zraa075 (2021).
Hübner, M. et al. Pressurized IntraPeritoneal aerosol chemotherapy - practical aspects. Eur. J. Surg. Oncol. 43, 1102–1109 (2017).
Alyami, M. et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 20, e368–e377 (2019).
Davigo, A. et al. PIPAC versus HIPEC: cisplatin spatial distribution and diffusion in a swine model. Int. J. Hyperthermia 37, 144–150 (2020).
Solass, W. et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology 74, 1014–1024 (2019).
Solaß, W., Hetzel, A., Nadiradze, G., Sagynaliev, E. and Reymond, M.A. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg. Endosc. 26, 1849–1855 (2012).
Kurtz, F. et al. Feasibility, safety, and efficacy of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis: a registry study. Gastroent Res. Pract. 2018, 2743985 (2018).
Rovers, K. P. et al. Pressurized intraperitoneal aerosol chemotherapy (oxaliplatin) for unresectable colorectal peritoneal metastases: a multicenter, single-arm, phase II trial (CRC-PIPAC). Ann. Surg. Oncol. 28, 5311–5326 (2021).
Tempfer, C. B. et al. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol. Oncol. 150, 23–30 (2018).
Kepenekian, V. et al. Non-resectable malignant peritoneal mesothelioma treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) plus systemic chemotherapy could lead to secondary complete cytoreductive surgery: a cohort study. Ann. Surg. Oncol. 29, 2104–2113 (2022).
Ellebaek, S. B. et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC)-directed treatment of peritoneal metastasis in end-stage colo-rectal cancer patients. Pleura Peritoneum 5, 20200109 (2020).
Alyami, M. et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur. J. Surg. Oncol. 47, 123–127 (2020).
Ndaw, S. et al. Occupational exposure to platinum drugs during intraperitoneal chemotherapy. Biomonitoring and surface contamination. Toxicol. Lett. 298, 171–176 (2018).
Clerc, D. et al. Current practice and perceptions of safety protocols for the use of intraperitoneal chemotherapy in the operating room: results of the IP-OR international survey. Pleura Peritoneum 6, 39–45 (2021).
Tempfer, C., Giger-Pabst, U., Hilal, Z., Dogan, A. & Rezniczek, G. A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch. Gynecol. Obstet. 298, 243–257 (2018).
Hübner, M. et al. Consensus guidelines for pressurized intraperitoneal aerosol chemotherapy: technical aspects and treatment protocols. Eur. J. Surg. Oncol. 48, 789–794 (2022).
Dumont, F. et al. A phase I dose-escalation study of oxaliplatin delivered via a laparoscopic approach using pressurised intraperitoneal aerosol chemotherapy for advanced peritoneal metastases of gastrointestinal tract cancers. Eur. J. Cancer 140, 37–44 (2020).
Taibi, A. et al. Feasibility and safety of oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy with or without intraoperative intravenous 5-fluorouracil and leucovorin for colorectal peritoneal metastases: a multicenter comparative cohort study. Ann. Surg. Oncol. 29, 5243–5251 (2022).
Sande, L. V. D. et al. Intraperitoneal aerosolization of albumin-stabilized paclitaxel nanoparticles (AbraxaneTM) for peritoneal carcinomatosis–a phase I first-in-human study. Pleura Peritoneum 3, 20180112 (2018).
Sgarbura, O. et al. MESOTIP: phase II multicenter randomized trial evaluating the association of PIPAC and systemic chemotherapy vs. systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma. Pleura Peritoneum 4, 20190010 (2019).
Eveno, C., Jouvin, I. & Pocard, M. PIPAC EstoK 01: pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: a randomized and multicenter phase II study. Pleura Peritoneum 3, 20180116 (2018).
Alyami, M. et al. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 47, 128–133 (2021).
Tate, S. J., Sande, L. V., de, Ceelen, W. P., Torkington, J. & Parker, A. L. The feasibility of pressurised intraperitoneal aerosolised virotherapy (PIPAV) to administer oncolytic adenoviruses. Pharm 13, 2043 (2021).
Sugarbaker, P. H., Stuart, O. A., Vidal-Jove, J., Pessagno, A. M. & DeBruijn, E. A. Peritoneal Carcinomatosis: Principles Of Management (Springer, 1996).
Flessner, M. F. The transport barrier in intraperitoneal therapy. Am. J. Physiol. Ren. Physiol. 288, F433–F342 (2005).
Wilson, R. B. Hypoxia, cytokines and stromal recruitment: parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Peritoneum 3, 20180103 (2018).
Seebauer, C. T. et al. Peritoneal carcinomatosis of colorectal cancer is characterized by structural and functional reorganization of the tumor microenvironment inducing senescence and proliferation arrest in cancer cells. Oncoimmunology 5, e1242543 (2016).
Wang, E. et al. Abundant intratumoral fibrosis prevents lymphocyte infiltration into peritoneal metastases of colorectal cancer. PLoS ONE 16, e0255049 (2021).
Morgan, R. D. et al. Objective responses to first-line neoadjuvant carboplatin–paclitaxel regimens for ovarian, fallopian tube, or primary peritoneal carcinoma (ICON8): post-hoc exploratory analysis of a randomised, phase 3 trial. Lancet Oncol. 22, 277–288 (2021).
Ray-Coquard, I. et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N. Engl. J. Med. 381, 2416–2428 (2019).
Coleman, R. L. et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 381, 2403–2415 (2019).
Ledermann, J. A. et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24, vi24–vi32 (2013).
Winter-Roach, B. A., Kitchener, H. C. & Dickinson, H. O. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst. Rev. 1, CD004706 (2009).
ICON1. International collaborative ovarian neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. J. Natl Cancer Inst. 95, 125–132 (2003).
McGuire, W. P. et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 334, 1–6 (1996).
Piccart, M. J. et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J. Natl Cancer Inst. 92, 699–708 (2000).
Aghajanian, C. et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 30, 2039–2045 (2012).
Pujade-Lauraine, E. et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol. 32, 1302–1308 (2014).
Perren, T. J. et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 365, 2484–2496 (2011).
Burger, R. A. et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 365, 2473–2483 (2011).
Martín, A. G. et al. Exploratory outcome analyses according to stage and/or residual disease in the ICON7 trial of carboplatin and paclitaxel with or without bevacizumab for newly diagnosed ovarian cancer. Gynecol. Oncol. 152, 53–60 (2019).
Monk, B. J. et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol. 22, 1275–1289 (2021).
Pujade-Lauraine, E. et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 22, 1034–1046 (2021).
Konstantinopoulos, P. A. et al. TOPACIO/Keynote-162 (NCT02657889): a phase 1/2 study of niraparib + pembrolizumab in patients (pts) with advanced triple-negative breast cancer or recurrent ovarian cancer (ROC) — results from ROC cohort. J. Clin. Oncol. 36 (suppl. 15), Abstr. 106 (2018).
Drew, Y. et al. 1190PD Phase II study of olaparib + durvalumab (MEDIOLA): updated results in germline BRCA-mutated platinum-sensitive relapsed (PSR) ovarian cancer (OC). Ann. Oncol. 30, v485–v486 (2019).
Zsiros, E. et al. Efficacy and safety of pembrolizumab in combination with Bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer. JAMA Oncol. 7, 78–85 (2021).
Breuer, E. et al. Site of recurrence and survival after surgery for colorectal peritoneal metastasis. J. Natl Cancer Inst. 113, djab001 (2021).
Franko, J. Therapeutic efficacy of systemic therapy for colorectal peritoneal carcinomatosis: surgeon’s perspective. Pleura Peritoneum 3, 20180102 (2018).
Cutsem, E. V., Cervantes, A., Nordlinger, B., Arnold, D. & Group, E. G. W. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25, iii1–iii9 (2014).
Nordlinger, B. et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 14, 1208–1215 (2013).
Loupakis, F. et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 371, 1609–1618 (2014).
Cremolini, C. et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 21, 497–507 (2020).
Ychou, M. et al. Induction chemotherapy (CT) with FOLFIRINOX or FOLFOX/FOLFIRI, plus cetuximab (CET) or bevacizumab (BEV) (by RAS status), in patients (pts) with primarily unresectable colorectal liver metastases (CRLM): results of the randomized UNICANCER PRODIGE 14-ACCORD 21 (METHEP-2) trial. J. Clin. Oncol. 36 (suppl. 15), Abstr. 3535 (2018).
Rovers, K. et al. LBA-6 Safety, feasibility, tolerability, and preliminary efficacy of perioperative systemic therapy for resectable colorectal peritoneal metastases: pilot phase of a randomised trial (CAIRO6). Ann. Oncol. 31, S243 (2020).
Rovers, K. P. et al. Perioperative systemic therapy vs cytoreductive surgery and hyperthermic intraperitoneal chemotherapy alone for resectable colorectal peritoneal metastases. JAMA Surg. 156, 710–720 (2021).
Rovers, K. P. et al. Adjuvant systemic chemotherapy vs active surveillance following up-front resection of isolated synchronous colorectal peritoneal metastases. JAMA Oncol. 6, e202701 (2020).
Beal, E. W. et al. Impact of neoadjuvant chemotherapy on the outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: a multi-institutional retrospective review. J. Clin. Med. 9, 748 (2020).
Waite, K. & Youssef, H. The role of neoadjuvant and adjuvant systemic chemotherapy with cytoreductive surgery and heated intraperitoneal chemotherapy for colorectal peritoneal metastases: a systematic review. Ann. Surg. Oncol. 24, 705–720 (2017).
Chau, I. et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer-pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J. Clin. Oncol. 22, 2395–2403 (2004).
Kim, J. G. et al. Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemoth Pharm. 61, 301–307 (2008).
Thomassen, I. et al. Chemotherapy as palliative treatment for peritoneal carcinomatosis of gastric origin. Acta Oncol. 53, 429–432 (2013).
Shitara, K. et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2, 277–287 (2017).
KINOSHITA, J. et al. Comparative study of the antitumor activity of Nab-paclitaxel and intraperitoneal solvent-based paclitaxel regarding peritoneal metastasis in gastric cancer. Oncol. Rep. 32, 89–96 (2014).
Kang, Y.-K. et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 2461–2471 (2017).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Shitara, K. et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 6, 1571–1580 (2020).
Takahashi, Y. et al. Real-world effectiveness of nivolumab in advanced gastric cancer: the DELIVER trial (JACCRO GC-08). Gastric Cancer 25, 235–244 (2022).
Goere, D., Glehen, O., Mariette, C., Auperin, A. & Elias, D. Results of a phase II randomized study evaluating the potential benefit of a postoperative intraperitoneal immunotherapy after resection of peritoneal metastases from gastric carcinoma metastases (IIPOP-NCT01784900). J. Clin. Oncol. 35 (suppl. 15), Abstr. 4064 (2017).
Thadi, A. et al. Early investigations and recent advances in intraperitoneal immunotherapy for peritoneal metastasis. Vaccines 6, 54 (2018).
Vogelzang, N. J. et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 21, 2636–2644 (2003).
Jänne, P. A. et al. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: outcomes of an expanded access program. Clin. Lung Cancer 7, 40–46 (2005).
Carteni, G. et al. Malignant peritoneal mesothelioma — results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 64, 211–218 (2009).
Obasaju, C. K. et al. Single-arm, open label study of pemetrexed plus cisplatin in chemotherapy naïve patients with malignant pleural mesothelioma: outcomes of an expanded access program. Lung Cancer 55, 187–194 (2007).
Kepenekian, V. et al. Diffuse malignant peritoneal mesothelioma: evaluation of systemic chemotherapy with comprehensive treatment through the RENAPE database: multi-institutional retrospective study. Eur. J. Cancer 65, 69–79 (2016).
Deraco, M., Baratti, D., Hutanu, I., Bertuli, R. & Kusamura, S. The role of perioperative systemic chemotherapy in diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 20, 1093–1100 (2013).
Naffouje, S. A., Tulla, K. A. & Salti, G. I. The impact of chemotherapy and its timing on survival in malignant peritoneal mesothelioma treated with complete debulking. Med. Oncol. 35, 69 (2018).
Disselhorst, M. J. et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir. Med. 7, 260–270 (2019).
Baas, P. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397, 375–386 (2021).
Raghav, K. et al. Clinical efficacy of immune checkpoint inhibitors in patients with advanced malignant peritoneal mesothelioma. JAMA Netw. Open 4, e2119934 (2021).
Maio, M. et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 18, 1261–1273 (2017).
Desai, A. et al. OA08.03 Phase II trial of pembrolizumab (NCT02399371) in previously-treated malignant mesothelioma (MM): final analysis. J. Thorac. Oncol. 13, S339 (2018).
Fennell, D. A. et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 22, 1530–1540 (2021).
Blackham, A. U. et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 109, 740–745 (2014).
Lu, P. et al. Systemic chemotherapy and survival in patients with metastatic low-grade appendiceal mucinous adenocarcinoma. J. Surg. Oncol. 120, 446–451 (2019).
Turner, K. M. et al. Assessment of neoadjuvant chemotherapy on operative parameters and outcome in patients with peritoneal dissemination from high-grade appendiceal cancer. Ann. Surg. Oncol. 20, 1068–1073 (2013).
Lieu, C. H. et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann. Oncol. 23, 652–658 (2012).
Bijelic, L., Kumar, A. S., Stuart, O. A. & Sugarbaker, P. H. Systemic chemotherapy prior to cytoreductive surgery and hipec for carcinomatosis from appendix cancer: impact on perioperative outcomes and short-term survival. Gastroenterol. Res. Pract. 2012, 163284 (2012).
Shapiro, J. F. et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: a single-institution experience. Cancer 116, 316–322 (2010).
Munoz-Zuluaga, C. A. et al. Systemic chemotherapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) in patients with high-grade mucinous carcinoma peritonei of appendiceal origin. Eur. J. Surg. Oncol. 45, 1598–1606 (2019).
Baratti, D. et al. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann. Surg. Oncol. 15, 526–534 (2007).
Milovanov, V. et al. Systemic chemotherapy (SC) before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) in patients with peritoneal mucinous carcinomatosis of appendiceal origin (PMCA). Eur. J. Surg. Oncol. 41, 707–712 (2015).
Choe, J. H. et al. Improved survival with anti-VEGF therapy in the treatment of unresectable appendiceal epithelial neoplasms. Ann. Surg. Oncol. 22, 2578–2584 (2015).
Kus, T. et al. Prediction of peritoneal recurrence in patients with gastric cancer: a multicenter study. J. Gastrointest. Cancer 52, 634–642 (2021).
Willett, C. G., Tepper, J. E., Cohen, A. M., Orlow, E. & Welch, C. E. Failure patterns following curative resection of colonic carcinoma. Ann. Surg. 200, 685–690 (1984).
Minton, J. P. et al. Results of a 400-patient carcinoembryonic antigen second-look colorectal cancer study. Cancer 55, 1284–1290 (1985).
Elias, D. et al. Results of systematic second-look surgery in patients at high risk of developing colorectal peritoneal carcinomatosis. Ann. Surg. 247, 445–450 (2008).
Sugarbaker, P. H. Revised guidelines for second-look surgery in patients with colon and rectal cancer. Clin. Transl. Oncol. 12, 621–628 (2010).
Honoré, C., Goere, D., Souadka, A., Dumont, F. & Elias, D. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann. Surg. Oncol. 20, 183–192 (2013).
Sugarbaker, P. H. Second-look surgery for colorectal cancer: revised selection factors and new treatment options for greater success. Int. J. Surg. Oncol. 2011, 915078 (2011).
Sammartino, P. et al. Proactive management for gastric, colorectal and appendiceal malignancies: preventing peritoneal metastases with hyperthermic intraperitoneal chemotherapy (HIPEC). Indian J. Surg. Oncol. 7, 215–224 (2016).
Rekhraj, S. et al. Can intra-operative intraperitoneal free cancer cell detection techniques identify patients at higher recurrence risk following curative colorectal cancer resection: a meta-analysis. Ann. Surg. Oncol. 15, 60–68 (2008).
Katoh, H. et al. Prognostic significance of peritoneal tumour cells identified at surgery for colorectal cancer. Br. J. Surg. 96, 769–777 (2009).
Tan, K.-L., Tan, W.-S., Lim, J.-F. & Eu, K.-W. Krukenberg tumors of colorectal origin: a dismal outcome — experience of a tertiary center. Int. J. Colorectal Dis. 25, 233–238 (2009).
Cheynel, N. et al. Incidence, patterns of failure, and prognosis of perforated colorectal cancers in a well-defined population. Dis. Colon Rectum 52, 406–411 (2009).
van Santvoort, H. C. et al. Peritoneal carcinomatosis in t4 colorectal cancer: occurrence and risk factors. Ann. Surg. Oncol. 21, 1686–1691 (2014).
Trilling, B. et al. Intraperitoneal-free cancer cells represent a major prognostic factor in colorectal peritoneal carcinomatosis. Dis. Colon Rectum 59, 615–622 (2016).
Bhatt, A. et al. Patients with extensive regional lymph node involvement (pN2) following potentially curative surgery for colorectal cancer are at increased risk for developing peritoneal metastases: a retrospective single-institution study. Colorectal Dis. 21, 287–296 (2019).
Klaver, C. E. L. et al. Locally advanced colorectal cancer: true peritoneal tumor penetration is associated with peritoneal metastases. Ann. Surg. Oncol. 25, 212–220 (2018).
Arrizabalaga, N. B. et al. Prophylactic HIPEC in pT4 colon tumors: proactive approach or overtreatment? Ann. Surg. Oncol. 27, 1094–1100 (2020).
Veld, J. V. et al. Synchronous and metachronous peritoneal metastases in patients with left-sided obstructive colon cancer. Ann. Surg. Oncol. 27, 2762–2773 (2020).
Elias, D. et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann. Surg. 254, 289–293 (2011).
Stewart, C. J. R., Hillery, S. & Plattell, C. Protocol for the examination of specimens from patients with primary carcinomas of the colon and rectum. Arch. Pathol. Lab. Med. 133, 1182–1193 (2009). 1359-60-author reply 1360-1.
Panarelli, N. C., Schreiner, A. M., Brandt, S. M., Shepherd, N. A. & Yantiss, R. K. Histologic features and cytologic techniques that aid pathologic stage assessment of colonic adenocarcinoma. Am. J. Surg. Pathol. 37, 1252–1258 (2013).
Frankel, W. L. & Jin, M. Serosal surfaces, mucin pools, and deposits, oh my: challenges in staging colorectal carcinoma. Mod. Pathol. 28 (Suppl. 1), S95–S108 (2015).
Klaver, C. E. L. et al. Interobserver, intraobserver, and interlaboratory variability in reporting pT4a colon cancer. Virchows Arch. 476, 219–230 (2019).
Sun, Z. et al. A novel subclassification of pT2 gastric cancers according to the depth of muscularis propria invasion. Ann. Surg. 249, 768–775 (2009).
Park, D. J. et al. Subclassification of pT2 gastric adenocarcinoma according to depth of invasion (pT2a vs pT2b) and lymph node status (pN). Surgery 141, 757–763 (2007).
Klaver, C. E. L. et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 4, 761–770 (2019).
Baratti, D. et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) at the time of primary curative surgery in patients with colorectal cancer at high risk for metachronous peritoneal metastases. Ann. Surg. Oncol. 24, 167–175 (2017).
Koh, J.-L., Yan, T. D., Glenn, D. & Morris, D. L. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 16, 327–333 (2009).
Nordlinger, B. et al. Adjuvant regional chemotherapy and systemic chemotherapy versus systemic chemotherapy alone in patients with stage II-III colorectal cancer: a multicentre randomised controlled phase III trial. Lancet Oncol. 6, 459–468 (2005).
Sloothaak, D. A. M. et al. Intraperitoneal chemotherapy as adjuvant treatment to prevent peritoneal carcinomatosis of colorectal cancer origin: a systematic review. Br. J. Cancer 111, 1112–1121 (2014).
Bennouna, J. et al. Rationale and design of the IROCAS study: multicenter, international, randomized phase 3 trial comparing adjuvant modified (m) FOLFIRINOX to mFOLFOX6 in patients with high-risk stage III (pT4 and/or N2) colon cancer — A UNICANCER GI-PRODIGE Trial. Clin. Colorectal Cancer 18, e69–e73 (2019).
Al-Batran, S.-E. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393, 1948–1957 (2019).
Arjona-Sánchez, Á. et al. HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of hyperthermic intra-peritoneal chemotherapy (HIPEC) with mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC cancer 18, 183–188 (2018).
Koga, S. et al. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 61, 232–237 (1988).
Zhu, L. et al. Prophylactic chemotherapeutic hyperthermic intraperitoneal perfusion reduces peritoneal metastasis in gastric cancer: a retrospective clinical study. BMC Cancer 20, 827 (2020).
Yonemura, Y. et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterol 48, 1776–1782 (2001).
Brenkman, H. J. F., Päeva, M., Hillegersberg, R., van, Ruurda, J. P. & Mohammad, N. H. Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer — a systematic review. J. Clin. Med. 8, 1685 (2019).
Glehen, O. et al. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer 14, 183 (2014).
Argani, P. et al. Pediatric mesothelioma with ALK fusions. Am. J. Surg. Pathol. 45, 653–661 (2021).
Desmeules, P. et al. A subset of malignant mesotheliomas in young adults are associated with recurrent EWSR1/FUS-ATF1 fusions. Am. J. Surg. Pathol. 41, 980–988 (2017).
Argani, P. et al. EWSR1/FUS-CREB fusions define a distinctive malignant epithelioid neoplasm with predilection for mesothelial-lined cavities. Mod. Pathol. 33, 2233–2243 (2020).
Raghav, K. et al. Efficacy, safety and biomarker analysis of combined PD-L1 (atezolizumab) and VEGF (bevacizumab) blockade in advanced malignant peritoneal mesothelioma. Cancer Discov. 11, 2738–2747 (2021).
Khanna, S. et al. Malignant mesothelioma effusions are infiltrated by CD3+ T cells highly expressing PD-L1 and the PD-L1+ tumor cells within these effusions are susceptible to ADCC by the anti–PD-L1 antibody avelumab. J. Thorac. Oncol. 11, 1993–2005 (2016).
Valmary-Degano, S. et al. Immunohistochemical evaluation of two antibodies against PD-L1 and prognostic significance of PD-L1 expression in epithelioid peritoneal malignant mesothelioma: a RENAPE study. Eur. J. Surg. Oncol. 43, 1915–1923 (2017).
Chapel, D. B. et al. Tumor PD-L1 expression in malignant pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and Dako PD-L1 28-8 pharmDx assays. Hum. Pathol. 87, 11–17 (2019).
Arjona-Sánchez, Á. et al. A proposal for modification of the PSOGI classification according to the Ki-67 proliferation index in pseudomyxoma peritonei. Ann. Surg. Oncol. 29, 126–136 (2022).
Tokunaga, R. et al. Molecular profiling of appendiceal adenocarcinoma and comparison with right-sided and left-sided colorectal cancer. Clin. Cancer Res. 25, 3096–3103 (2019).
Koopman, M. et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 100, 266–273 (2009).
Liu, X. et al. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol. Res. Pract. 216, 152881 (2020).
Fuchs, C. S. et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 Trial. JAMA Oncol. 4, e180013 (2018).
Wang, F. et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 30, 1479–1486 (2019).
Drakes, M. L. et al. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J. Ovarian Res. 11, 43 (2018).
Hamanishi, J. et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl Acad. Sci. USA 104, 3360–3365 (2007).
Verwaal, V. J. et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 21, 3737–3743 (2003).
Verwaal, V. J., Bruin, S., Boot, H., van Slooten, G. & van Tinteren, H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 15, 2426–2432 (2008).
Cashin, P. H. et al. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: a randomised trial. Eur. J. Cancer 53, 155–162 (2016).
Lemoine, L. et al. Body surface area-based vs concentration-based perioperative intraperitoneal chemotherapy after optimal cytoreductive surgery in colorectal peritoneal surface malignancy treatment: COBOX trial. J. Surg. Oncol. 119, 999–1010 (2019).
Zivanovic, O. et al. Secondary cytoreduction and carboplatin hyperthermic intraperitoneal chemotherapy for platinum-sensitive recurrent ovarian cancer: an MSK team ovary phase II study. J. Clin. Oncol. 39, 2594–2604 (2021).
Fagotti, A. et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 30, 1657–1664 (2020).
Lim, M. C. et al. Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer. JAMA Surg. 157, 374–383 (2022).
Levine, E. A. et al. A multicenter randomized trial to evaluate hematologic toxicities after hyperthermic intraperitoneal chemotherapy with oxaliplatin or mitomycin in patients with appendiceal tumors. J. Am. Coll. Surg. 226, 434–443 (2018).
Wallner, K. E., Banda, M. & Li, G. C. Hyperthermic enhancement of cell killing by mitomycin C in mitomycin C-resistant Chinese hamster ovary cells. Cancer Res. 47, 1308–1312 (1987).
Harrison, L. E., Bryan, M., Pliner, L. & Saunders, T. Phase I trial of pegylated liposomal doxorubicin with hyperthermic intraperitoneal chemotherapy in patients undergoing cytoreduction for advanced intra-abdominal malignancy. Ann. Surg. Oncol. 15, 1407–1413 (2008).
Huang, Y., Alzahrani, N. A., Liauw, W., Traiki, T. B. & Morris, D. L. Early postoperative intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasms with pseudomyxoma peritonei: is it beneficial? Ann. Surg. Oncol. 24, 176–183 (2017).
Sugarbaker, P. H. & Chang, D. Cytoreductive surgery plus HIPEC with and without NIPEC for malignant peritoneal mesothelioma: a propensity-matched analysis. Ann. Surg. Oncol. 28, 7109–7117 (2021).
Acknowledgements
The authors thank L. Villeneuve (of the Service de Recherche et d’Epidémiologie Cliniques, Hôpital Lyon Sud, Hospices Civils de Lyon, Université Claude Bernard Lyon-1) for assistance with obtaining relevant articles for this Review.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
O.G. has acted as a consultant for Gamida. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks C. Chan, I. de Hingh and D. L. Morris for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
PROMISE: http://www.e-promise.org
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kepenekian, V., Bhatt, A., Péron, J. et al. Advances in the management of peritoneal malignancies. Nat Rev Clin Oncol 19, 698–718 (2022). https://doi.org/10.1038/s41571-022-00675-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-022-00675-5
This article is cited by
-
Hyperthermic pressurized intraperitoneal aerosol drug delivery system in a large animal model: a feasibility and safety study
Surgical Endoscopy (2024)
-
Phase II clinical trial of sequential treatment with systemic chemotherapy and intraperitoneal paclitaxel for gastric and gastroesophageal junction peritoneal carcinomatosis - STOPGAP trial
BMC Cancer (2023)
-
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with peritoneal surface malignancies (PSM): a prospective single-center registry study
Journal of Cancer Research and Clinical Oncology (2023)
-
Primary Peritoneal Hepatoid Adenocarcinoma Patients Treated by Complete Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
Indian Journal of Surgical Oncology (2023)
-
Long-Term Survival in Patients Treated by Cytoreductive Surgery with or Without HIPEC for Peritoneal Surface Malignancies—A report from the Indian HIPEC Registry
Indian Journal of Surgical Oncology (2023)