Abstract
Perioperative anaemia increases postoperative morbidity and mortality, and iron deficiency is anaemia’s most common cause in surgical patients. Preoperative intravenous iron increases postoperative haemoglobin; however, data regarding intraoperative intravenous iron’s effectiveness are inadequate. This study examined intraoperative intravenous iron’s effects on postoperative haemoglobin levels in adults. Fifty-seven healthy subjects (aged 19–40 years) scheduled for bimaxillary orthognathic surgery were assigned randomly to the iron (n = 28) or control (n = 29) groups. The iron group received intravenous ferric derisomaltose (1,000 mg) after anaesthetic induction. The control group received an identical volume of intravenous normal saline. The primary outcome was postoperative haemoglobin level. Secondary outcomes included other postoperative haematologic and iron parameters. Laboratory data were obtained preoperatively and at 1 day, 2 weeks, and 4 weeks postoperatively. Haemoglobin was higher in the iron group 2 weeks postoperatively (12.9 g/dL vs. 12.2 g/dL), but the between-group difference was not significant after adjustment for multiple testing. However, the reticulocyte production index was significantly higher in the iron group 2 weeks postoperatively. Intraoperative intravenous iron maintains postoperative haemoglobin values in patients undergoing bimaxillary orthognathic surgery by increasing haematopoietic function and iron bioavailability and therefore appears to be a useful strategy for blood management.
Similar content being viewed by others
Introduction
Perioperative anaemia increases postoperative morbidity and mortality1. Up to 75% of patients present with anaemia before surgery2. Iron deficiency, secondary to intraoperative blood loss and increased iron requirements, is the leading cause of anaemia in surgical patients3, 4. Identification of preoperative anaemia and iron deficiency has been recommended for all those with anticipated moderate to high (> 500 mL) surgical blood loss5, 6.
Bimaxillary orthognathic surgery is performed for correction of facial abnormalities, such as prognathism, retrognathism, and facial asymmetry7. Because of high vascularity and poor visualisation of the surgical site, this surgery is accompanied by a substantial likelihood of excessive blood loss and the potential need for blood transfusion8, 9. Preoperative autologous blood donation and hypotensive anaesthesia are often used to reduce the need for allogenic transfusion with bimaxillary orthognathic surgery10, but preoperative autologous blood donation has several drawbacks. The donation process can be uncomfortable for the patient, it can lead to anaemia, and its cost is relatively high.
Preoperative intravenous (IV) iron has been demonstrated to reduce transfusion requirements and increase postoperative haemoglobin (Hb) levels11,12,13,14,15,16. Furthermore, there are a number of trials reporting on the improved erythropoietic function (increased reticulocyte count) using preoperative IV iron11, 17, 18. However, administration of preoperative IV iron is not always feasible, especially in emergency surgery. Infusing iron intraoperatively is more convenient, but data regarding the effectiveness of this approach are inadequate19. We thereby conducted a randomised controlled trial to evaluate the effects of intraoperative IV iron on postoperative Hb levels and transfusion requirements in patients undergoing bimaxillary orthognathic surgery.
Methods
Patients
The Severance Hospital Institutional Review Board (Yonsei University Health System, Seoul, Republic of Korea, Chairperson Professor Seung Min Kim) approved the study protocol on 17 February 2017 (No. 4-2016-1146), which is registered at ClinicalTrials.gov (No. NCT03094182, 29/03/17). This study was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent before randomisation. We enrolled 57 adults (aged 19–40 years) who underwent elective bimaxillary orthognathic surgery between September 2017 and March 2019 by one surgeon (Y-S.J.). The American Society of Anesthesiologists (ASA) physical status class of all subjects was I. The exclusion criteria were pregnancy, haematologic disease, kidney-related anaemia, hepatitis, severe atopy, or drug allergies. None received iron supplements. The preoperative autologous blood donation was performed 2 weeks prior to surgery.
Randomisation and interventions
Participants were assigned at random to one of two groups—iron group (n = 28) or control group (n = 29)—using a computer-generated randomisation table (available at https://www.random.org). An anaesthesiologist not involved in data collection conducted the randomisation and group assignments. The iron group received 1000 mg ferric derisomaltose (Monofer, 200 mg/2 mL, Solupharm, Melsungen, Germany), which was added to normal saline to generate a total volume of 100 mL. This solution was infused over 30 min after anaesthesia induction. The same volume of normal saline was administered in an identical manner to the control group. An anaesthesiologist not involved in data collection prepared the two solutions. The investigator, surgeons, and study participants were blinded to the group assignment.
Anaesthesia
Anaesthesia was induced with 2 mg kg−1 propofol (Fresofol MCT 1%, Fresenius Kabi Korea Ltd, Seoul, Korea) and 0.5–1 µg kg−1 remifentanil (Ultiva, GlaxoSmithKline Korea, Seoul, Korea). Tracheal intubation was facilitated with 1.0 mg kg−1 rocuronium (Esmeron, MSD Korea Ltd, Seoul, Korea). After intubation, a radial artery catheter and an additional peripheral venous line were inserted. Anaesthesia was maintained with sevoflurane in 40% O2 and remifentanil infused at 0.05–0.15 µg kg−1 min−1. Controlled hypotension, with a systolic blood pressure < 100 mmHg, was used during maxillary manipulation. Red blood cells were transfused when the Hb fell below 8.5 g/dL.
Data collection and outcome assessments
The primary outcome was the postoperative blood Hb level. Secondary outcomes included reticulocyte count, and reticulocyte production index, serum levels of iron, ferritin, transferrin, transferrin saturation, and total iron binding capacity (TIBC), and perioperative blood transfusion requirements. Reticulocyte production index was the reticulocyte percentage corrected for both the haematocrit and reticulocyte lifespan20. The reticulocyte count and reticulocyte percentage were measured automatically using the ADVIA 2120i analyser (Siemens Healthcare, Erlangen, Germany). All laboratory values were obtained before surgery and at 1 day, 2 weeks, and 4 weeks postoperatively.
Statistical analysis
The sample size was calculated for the primary outcome (postoperative Hb). An Hb difference of > 0.8 mg/dL between iron and control groups was considered clinically relevant11. Twenty-six subjects were required in each group for a power of 80% at a significance level of 5%. To account for 10% dropout, we enrolled 29 in each group.
Demographic and intraoperative data are presented as number of patients, mean ± standard deviation, or median (interquartile range). Haematologic and iron parameters are expressed as estimated mean ± standard error values obtained from a linear mixed model. For continuous variables, the independent t-test was used to compare parametric data, and the Mann–Whitney U test was used for nonparametric data. The χ2 or Fisher’s exact test was used to evaluate categorical variables. Linear mixed models of fixed and random effects between groups were used to examine repeated measurements of haematologic and iron parameters. Intergroup comparisons of parameter changes over time included group-by-time interactions. Correlations between repeated measures were examined using an unstructured covariance matrix. Post hoc analysis was performed with Bonferroni correction to adjust for multiple comparisons. P values < 0.05 were considered statistically significant. Statistical analyses were conducted using SPSS 25.0 (IBM Corp., Armonk, NY, USA), R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), and SAS 9.4 (SAS Inc., Cary, NC, USA).
Results
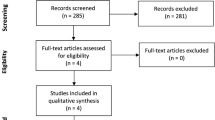
Of the 58 subjects evaluated for eligibility, 57 were enrolled and assigned either to the iron (n = 28) or control group (n = 29) (Fig. 1). One subject was excluded due to allergic reaction to antibiotics in pre-anaesthesia room after informed consent. The remaining participants completed the study. As shown in Table 1, patient characteristics and intraoperative variables were not significantly different between groups. Before surgery, 26 patients (male:female, 4:22) had iron deficiency (serum ferritin < 30 µg/L) and 10 (male:female, 1:9) had a transferrin saturation of < 20% and serum ferritin of < 100 µg/L. Requirements of blood transfusion perioperatively did not differ significantly between groups: one (4%) individual in the iron group and three (10%) in the control group (P = 0.630).
At 2 weeks postoperatively, the Hb was higher in the iron group than in the control group but there was no statistical difference after adjustment for multiple testing (12.9 g/dL vs. 12.2 g/dL, P = 0.108) (Table 2). The reticulocyte count was higher in the iron group than in the control group at 4 weeks (95.1 × 103/\(\mu\)L vs. 66.8 × 103/\(\mu\)L, P = 0.045) after surgery. Corroborating these observations, the reticulocyte production index at 2 weeks postoperatively was higher in the iron group than in the control group (1.51 vs. 1.10, P = 0.018) (Fig. 2).
Both serum iron and transferrin saturation were higher in the iron group than in the control group at 1 day and 2 weeks postoperatively but were similar in the two groups at 4 weeks after surgery (Table 3). Serum ferritin was higher in the iron group than in the control group at 2 weeks and 4 weeks after surgery. As expected, TIBC and transferrin levels were higher in the control group than in the iron group at 2 weeks and 4 weeks postoperatively.
The linear mixed model revealed that there were statistically significant between-group differences in changes in reticulocyte count, reticulocyte production index, iron, ferritin, transferrin saturation, TIBC, and transferrin during the 4 weeks following surgery (Table 4).
No surgical complication or adverse reaction related to IV iron was observed. Postoperative hospital length of stay did not differ between the groups (3 days vs. 3 days, P = 0.326).
Discussion
In this randomised clinical trial, intraoperative administration of IV iron maintained Hb in the postoperative period by increasing haematopoiesis and iron bioavailability.
Patient blood management (PBM) is a multidisciplinary concept that focuses on patient safety by minimising blood loss and optimising physiological tolerance of anaemia21. Iron is necessary for erythroid cell proliferation, in addition to Hb synthesis, as it functions as a cofactor for several enzymes, such as DNA replicases, DNA polymerases, and DNA helicases22. These enzymes are necessary for cell division of all cells, including haematopoietic stem cells necessary for erythropoiesis23. Because iron is an essential component of red blood cell production, management of iron reserves during the perioperative period is important. However, surgically induced inflammatory responses upregulate hepcidin synthesis, and hepcidin decreases iron bioavailability by inhibiting iron absorption from the gastrointestinal tract and preventing the release of stored iron24. These effects lead to hypoferremia and iron-restricted erythropoiesis, even when iron stores are normal25. IV iron provides a readily available source of iron25, creating a five-fold erythropoietic response to anaemia resulting from blood loss26. Besides preoperative anaemia management, there are many perioperative care and alternative PBM strategies, including antifibrinolytics (tranexamic acid), intraoperative autologous cell salvage, anaesthetic management (autologous normovolemic haemodilution, controlled hypotension, normothermia), reduced phlebotomy blood loss (smaller tubes, eliminate unnecessary testing), and point-of-care coagulation monitoring (thromboelastography)27, 28.
Low serum ferritin level before IV iron and transferrin saturation were the useful parameters of iron stores within the body. Serum ferritin < 30 µg/L alone, or < 100 µg/L in the presence of serum transferrin saturation < 20%, indicated iron deficiency5. Serum ferritin < 100 µg/L or transferrin saturation < 20% was recommended to detect the iron deficiency in patients with chronic heart failure, chronic kidney disease, and inflammatory bowel disease29. A preoperative ferritin < 100 μg/L may indicate insufficient iron stores to recover from a 3–4 g/dL decrease in Hb decrease5. Treating preoperative iron deficiency can decrease the need for perioperative blood transfusion and consequently improve outcomes12, 30. However, the presence of iron deficiency is often not evaluated before surgery in healthy patients. Unexpectedly, 91% of subjects in our study had inadequate iron stores for moderate to high blood loss surgery, as evidenced by a serum ferritin < 100 µg/L preoperatively. Females were especially affected: 73% had a serum ferritin < 30 µg/L, and 90% had a serum ferritin < 100 µg/L plus transferrin saturation < 20% preoperatively. Of note, these subjects were all healthy (ASA class 1), but they did not have adequate iron stores for moderate blood loss surgery.
In cases with inadequate iron stores or iron deficiency but not anaemia, iron supplementation before surgery with an anticipated blood loss of > 500 mL might be beneficial4. Preoperative assessment and treatment of iron deficiency may be particularly important in women. Because of their lower blood volume, women have higher transfusion rates than males, when undergoing the same type of surgery31. Females with a preoperative Hb of 12 g/dL are two times more likely to receive a blood transfusion than males with an Hb of 13 g/dL5.
A high reticulocyte count after surgery reflects an increased erythropoietic response to blood loss11. Reticulocyte production index is used to assess whether the bone marrow is responding appropriately to the presence of anaemia20. The reticulocyte count and reticulocyte production index were higher in the iron group than in the control group at 2 weeks and 4 weeks after surgery, indicating that the intraoperative IV iron increased haematopoietic activity.
During progressive iron depletion, laboratory results typically show reduced serum ferritin and iron levels and increased serum transferrin and TIBC values20. These findings were evident in the control group at 2 weeks postoperatively, as expected, but not in the iron group. IV iron has been previously shown to improve iron biochemical outcomes and haematopoietic response to severe anaemia and provide long-term normalisation of Hb levels32. Prior studies reported that IV iron administered 2 to 4 weeks before surgery decreased perioperative red blood cell transfusion rates and hospital length of stay14. Furthermore, IV iron administration less than 1 week preoperatively has been associated with improved outcomes11, 16. However, iron supplementation is not always administered before surgery, and unexpected bleeding may occur during the operation. In these situations, intraoperative iron supplementation appears to be a good alternative, as our results showed that, in the absence of iron supplementation before surgery, intraoperative IV iron promoted haematopoiesis and iron bioavailability.
This study has some limitations. We included only healthy subjects; thus, the effects of intraoperative IV iron in patients with chronic disease remain unknown. We excluded candidates with anaemia due to kidney disease because they had a decreased ability of erythropoietin production and the error between subjects could be large33. Healthy people have a good haematopoietic response to blood loss, which can underestimate the effect of intravenous iron. In patients whose iron availability is inhibited by chronic inflammation, intravenous iron facilitates the rapid replenishment of available iron and Hb levels, resulting in a greater haematopoietic effect than that in healthy individuals29. Although previous research suggested that supplemental iron improved markers of iron status and anaemia in patients with chronic disease and older persons15, 34,35,36, investigations of intraoperative iron use in this patient population should be done separately. Another potential limitation of our study was that the amount of bleeding during surgery was less than expected. However, because decreases in Hb are greater with larger volumes of blood loss, the postoperative difference in Hb levels between groups may have been even larger if blood loss was as high as anticipated. Lastly, we started administering intravenous iron immediately after induction to give it before the start of surgery. A certain amount of iron might be lost due to intraoperative bleeding. However, the results showed that iron parameters are not significantly affected by intraoperative blood loss. The preoperative anaemia or iron deficiency assessment has not been performed in some patients with a high risk of developing postoperative anaemia undergoing major surgery. In this case, even after anaesthesia, administration of iron before starting surgery would be effective for haematopoiesis based on our study.
In conclusion, intraoperative administration of IV iron maintains postoperative Hb values in patients undergoing bimaxillary orthognathic surgery by increasing iron bioavailability and haematopoiesis. Thus, intraoperative IV iron appears to be a useful strategy for blood management in patients undergoing operations with anticipated moderate to high blood loss.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Munoz, M. et al. “Fit to fly”: Overcoming barriers to preoperative haemoglobin optimization in surgical patients. Br. J. Anaesth. 115, 15–24 (2015).
Shander, A., Knight, K., Thurer, R., Adamson, J. & Spence, R. Prevalence and outcomes of anemia in surgery: A systematic review of the literature. Am. J. Med. 116(Suppl 7A), 58s–69s (2004).
Munoz, M. et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 73, 1418–1431 (2018).
Munoz, M. et al. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 72, 826–834 (2017).
Munoz, M. et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 72, 233–247 (2017).
Koo, B.-N. et al. Korean clinical practice guideline for perioperative red blood cell transfusion from Korean Society of Anesthesiologists. Korean J. Anesthesiol. 72, 91–118 (2019).
Choi, B. K., Yang, E. J., Oh, K. S. & Lo, L. J. Assessment of blood loss and need for transfusion during bimaxillary surgery with or without maxillary setback. J. Oral Maxillofac. Surg. 71, 358–365 (2013).
Varol, A., Basa, S. & Ozturk, S. The role of controlled hypotension upon transfusion requirement during maxillary downfracture in double-jaw surgery. J. Cranio-Maxillo-Facial Surg. 38, 345–349 (2010).
Lanigan, D. T., Hey, J. H. & West, R. A. Major vascular complications of orthognathic surgery: Hemorrhage associated with Le Fort I osteotomies. J. Oral Maxillofac. Surg. 48, 561–573 (1990).
Oh, A. Y., Seo, K. S., Lee, G. E. & Kim, H. J. Effect of preoperative autologous blood donation on patients undergoing bimaxillary orthognathic surgery: A retrospective analysis. Int. J. Oral Maxillofac. Surg. 45, 486–489 (2016).
Johansson, P. I., Rasmussen, A. S. & Thomsen, L. L. Intravenous iron isomaltoside 1000 (Monofer(R)) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: A randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang. 109, 257–266 (2015).
Quinn, E. M., Meland, E., McGinn, S. & Anderson, J. H. Correction of iron-deficiency anaemia in colorectal surgery reduces perioperative transfusion rates: A before and after study. Int. J. Surg. (Lond., Engl.) 38, 1–8 (2017).
Gonzalez-Porras, J. R. et al. An individualized pre-operative blood saving protocol can increase pre-operative haemoglobin levels and reduce the need for transfusion in elective total hip or knee arthroplasty. Transfus. Med. 19, 35–42 (2009).
Froessler, B. et al. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: A randomized controlled trial. Ann. Surg. 264, 41–46 (2016).
Calleja, J. L. et al. Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Int. J. Colorectal Dis. 31, 543–551 (2016).
Munoz, M. et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: A pooled analysis of observational data from 2547 patients. Transfusion 54, 289–299 (2014).
Spahn, D. R. et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: A prospective randomised trial. Lancet (Lond., Engl.) 393, 2201–2212 (2019).
Karkouti, K. et al. Intravenous iron and recombinant erythropoietin for the treatment of postoperative anemia. Can. J. Anaesth. 53, 11–19 (2006).
Park, H. S. et al. The effect of intraoperative ferric carboxymaltose in joint arthroplasty patients: A randomized trial. J. Clin. Med. 8, 2 (2019).
Harrison's Principles of internal medicine 19th edition edn eds. Dennis, L. K., Stephen, L. H., J, L. J., Anthony, S. F., Dan, L. L., Joseph, L.) 393–399 (Mc Graw Hill Education, 2015).
Zacharowski, K. & Spahn, D. R. Patient blood management equals patient safety. Best Pract. Res. Clin. Anaesthesiol. 30, 159–169 (2016).
Rishi, G. & Subramaniam, V. N. The relationship between systemic iron homeostasis and erythropoiesis. Biosci. Rep. 37, 2 (2017).
Zhang, C. Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell 5, 750–760 (2014).
Munoz, M., Garcia-Erce, J. A. & Remacha, A. F. Disorders of iron metabolism. Part 1: molecular basis of iron homoeostasis. J. Clin. Pathol. 64, 281–286 (2011).
Munoz, M., Garcia-Erce, J. A. & Remacha, A. F. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J. Clin. Pathol. 64, 287–296 (2011).
Goodnough, L. T., Skikne, B. & Brugnara, C. Erythropoietin, iron, and erythropoiesis. Blood 96, 823–833 (2000).
Frank, S. M. et al. Implementing a health system-wide patient blood management program with a clinical community approach. Anesthesiology 127, 754–764 (2017).
Baron, D. M. et al. Evaluation of clinical practice in perioperative patient blood management. BJA Br. J. Anaesth. 117, 610–616 (2016).
Cappellini, M. D. et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 92, 1068–1078 (2017).
Gybel-Brask, M., Seeberg, J., Thomsen, L. L. & Johansson, P. I. Intravenous iron isomaltoside improves hemoglobin concentration and iron stores in female iron-deficient blood donors: A randomized double-blind placebo-controlled clinical trial. Transfusion 58, 974–981 (2018).
Rosencher, N. et al. Orthopedic surgery transfusion hemoglobin European overview (OSTHEO) study: Blood management in elective knee and hip arthroplasty in Europe. Transfusion 43, 459–469 (2003).
Holm, C., Thomsen, L. L., Norgaard, A. & Langhoff-Roos, J. Single-dose intravenous iron infusion versus red blood cell transfusion for the treatment of severe postpartum anaemia: A randomized controlled pilot study. Vox Sang. 112, 122–131 (2017).
Shih, H. M., Wu, C. J. & Lin, S. L. Physiology and pathophysiology of renal erythropoietin-producing cells. J. Formosan Med. Assoc. 117, 955–963 (2018).
Kalra, P. A. & Bhandari, S. Efficacy and safety of iron isomaltoside (Monofer((R))) in the management of patients with iron deficiency anemia. Int. J. Nephrol. Renovasc. Dis. 9, 53–64 (2016).
Petis, S. M. et al. Is there a role for preoperative iron supplementation in patients preparing for a total hip or total knee arthroplasty?. J. Arthroplasty 32, 2688–2693 (2017).
Macdougall, I. C. et al. Intravenous iron in patients undergoing maintenance hemodialysis. New Engl. J. Med. 380, 447–458 (2019).
Acknowledgements
The authors thank Pharmbio Korea, Seoul, Korea, for supplying ferric derisomaltose (Monofer, 200 mg/2 mL, Solupharm, Melsungen, Germany).
Funding
This study was funded by a National Research Foundation of Korea grant from the Korean government (MSIP) (NRF-2017R1A2B4009478).
Author information
Authors and Affiliations
Contributions
B.L.: Recruitment of patients, collection and analysis of the data, and preparation of the first draft of this manuscript. E.J.K.: Collection of data and revision of the manuscript. J.S.: Collection of data and revision of the manuscript. J.-Y.S., B.-N.K.: Design of the study, randomisation of the patients, collection of data, and preparation of the final version of this manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, B., Kim, E.J., Song, J. et al. A randomised trial evaluating the effect of intraoperative iron administration. Sci Rep 10, 15853 (2020). https://doi.org/10.1038/s41598-020-72827-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72827-5
This article is cited by
-
What we should consider to facilitate recovery of the hematological profile in all patients after pancreaticoduodenectomy: the role of preoperative intravenous iron treatment
BMC Surgery (2023)
-
Preemptive intravenous iron therapy versus autologous whole blood therapy for early postoperative hemoglobin level in patients undergoing bimaxillary orthognathic surgery: a prospective randomized noninferiority trial
BMC Oral Health (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.