Abstract
With the aging of the population, an increasing number of elderly patients are opting for artificial joint replacement, leading to the exploration of various rapid rehabilitation programs in the perioperative period. In this study, we aimed to investigate the effectiveness of combining recombinant human erythropoietin and iron sucrose in altering the range and trend of postoperative hemoglobin in patients undergoing arthroplasty. Specifically, we will examine whether this combination can effectively alter the rise and fall of postoperative haemoglobin, identify the inflection point of haemoglobin change or recovery after arthroplasty, and assess the effect of treatment on serum iron in postoperative blood. We conducted a retrospective study of 138 patients who underwent unilateral total joint arthroplasty by the same surgeon in the same hospital before July 2022. The results of this study may provide valuable insights for the development of effective rehabilitation programs for patients undergoing arthroplasty.
Similar content being viewed by others
Introduction
For arthroplasty, perioperative blood management is an important sector that cannot be ignored. Relevant experiments show that in the initial TKA and THA, the average bleeding volume calculated in the visible and invisible aspects is approximately 1500 ml1,2, and then the haemoglobin values decrease by approximately 3 g/dl1,3. However, with advances in surgical techniques and increased emphasis on perioperative blood management, intraoperative bleeding in joint replacement surgery is kept low and hardly affects intraoperative hemoglobin levels4. Moreover, total perioperative bleeding was significantly lower at 290–860 ml5,6,7. The experiment of Park et al. deemed that in patients receiving THA, multivariate analysis showed that blood loss increased with age (3.44 ml increased every year) and body mass index (12.45 ml increased every 1 kg/m2)3. Sehat et al. concluded that perioperative recessive blood loss in TKA is often more than 50% of the total blood loss2. In addition, it was confirmed that there are a series of complications in blood transfusion, and there is a certain connection with joint infection8,9,10,11,12. Therefore, many institutions and hospitals are studying how to reduce the total blood loss, improve the hemoglobin value, and reduce the blood transfusion rate during the perioperative period. Maempel et al. reported that the preoperative haemoglobin levels of women and men undergoing TKA were 12.75 g/dl and 13.75 g/dl, respectively, which have been identified as the lowest threshold for reducing the need for blood transfusion, with maximum comprehensive sensitivity and specificity8. However, preoperative optimization often requires a long treatment time, which increases various economic costs. In addition, the self-management of nonhospital patients is poor. The outcome is that it is difficult to ensure that the haemoglobin value of patients on the day before surgery reaches the threshold. Therefore, intraoperative and postoperative blood management will receive more attention.
The use of tranexamic acid in joint replacement surgery has been proven to be able to effectively reduce the amount of bleeding and reduce the intraoperative and postoperative blood transfusion rates13,14,15,16,17,18,19,20,21,22,23,24,25. The usage scheme is from one dose to six doses. Although venous thrombosis is often a concern for the side effects of tranexamic acid, any combination of these dosages has been reported not to increase the incidence or risk of postoperative deep venous thrombosis in patients undergoing arthroplasty13,14,16,17,18,19,20,25. This is significantly lower than the increased costs associated with postoperative rehabilitation, such as blood transfusion, hospital inspection, length of stay, diet and so on26,27, so this measure has been routinely adopted by major hospitals.
Ferrimodulin is the main systemic iron regulatory hormone of iron metabolism, and its synthesis is controlled by a variety of signaling pathways (such as inflammation, hypoxia, and erythropoietin)28. The high level of ferrimodulin caused by inflammation related to surgical stress will inhibit the intestinal absorption of oral iron. In addition, adverse reactions of the gastrointestinal tract after surgery are of large probability. Therefore, postoperative anemia is more common (up to 80–90%). Sometimes, high-dose oral iron therapy is not better than placebo29,30,31. Intravenous iron is similar to or better than oral iron in improving hemoglobin levels and decreasing blood transfusion rates32. Therefore, in the case of intraoperative or postoperative anemia, intravenous iron is preferred to oral iron. If the reaction of intravenous iron is insufficient and there is no contraindication for patients, the combination of recombinant human erythropoietin (EPO) and intravenous iron should be considered29. Relevant studies have shown that the addition of a single dose of rh-EPO (40,000 IU) can enhance the erythropoiesis effect of intravenous iron and further reduce the demand for blood transfusion33.
For the use of rh-EPO, most studies tend to give preoperative medication to patients with preoperative anemia. Usually, 30,000–40,000 IU/w or 10,000 IU/day is given subcutaneously, and the program starts 2–4 weeks before surgery. On this basis, some experiments have also used various iron agents orally or intravenously34,35,36,37,38,39,40. There are also many experiments that have verified more convenient schemes: we can use rh-EPO and intravenous iron once before or on the day after surgery and then inject rh-EPO (3000–10,000 IU) subcutaneously every day or every other day after surgery, equipping intravenous iron (100–200 mg)41,42,43. In contrast, there are individual reports suggesting that this kind of practice is not worth taking because the high cost may make it too expensive for routine use, and the effectiveness of reducing the blood transfusion rate and accelerating postoperative rehabilitation after surgery is not largely obvious44,45. The main purpose of this study was to verify the short-term effect of the combined use of rh-EPO and sucrose iron on postoperative haemoglobin.
Methods
All methods were carried out in accordance with relevant guidelines and regulations. The study was performed in accordance with the Declaration of Helsinki. All experimental protocols were approved by the ethics committee of the First Hospital of Jilin University, and the need for informed consent was waived by the ethics committee of the First Hospital of Jilin University since only anonymized patient data were used for the study.
The study included adult patients (> 18 years old) who underwent total joint arthroplasty performed by the same team of orthopedic surgeons. Preoperative exclusion criteria consisted of preoperative autologous blood donation, iv iron or erythropoietin therapy; active infection; pregnancy or lactation; major comorbidities (previous history of stroke, transient ischemic attacks, or seizures); significant respiratory disease (forced expiratory volume in the first second < 50% predicted), renal disease (creatinine > 200 µmol/l], or liver disease (hepatitis, cirrhosis); uncontrolled hypertension (systolic > 180, diastolic > 100 mmHg); and any hematological diseases (e.g., thromboembolic events, hemoglobinopathy, coagulopathy, or hemolytic disease). Additionally, patients with ongoing hemorrhage or evidence of organ dysfunction on postoperative days were also excluded.
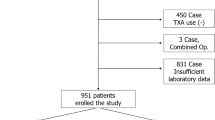
A total of 150 patients with unilateral total joint replacement performed by the same surgeon in the same hospital were selected consecutively, and the final sample size for the study was 138 (99 in the control group and 39 in the experimental group). The preoperative examination of all patients was in line with the surgical indications, and there was no surgical contraindication. In the experimental group, rh-EPO (10,000 iu) was injected subcutaneously every day; a dose of sucrose iron (200 mg) dissolved in 250 ml normal saline was administered intravenously every other day after the operation. The control group was only supported by symptomatic treatment after the operation. No drainage tube was used in any patients.
The demographic data of the two groups were similar (p > 0.05) and comparable (Tables 1, 2). We collected the haemoglobin and serum iron of patients who were included in the experimental study on the first, third and fifth days after the operation and calculated the difference values of haemoglobin (HB1-3, HB5-3) and serum iron (Fe3-1, Fe5-3) and then judged the normality of clinical data between the two groups. If the data were normally distributed, an independent sample t test was used. If the distribution was skewed, a rank sum test was used. We assigned 1 to the patients whose values were greater than or equal to 0 in HB1-3 and HB5-3 and 2 to the patients whose values were less than 0 to convert them into categorical variables. For categorical variables, the chi-square test was adopted. According to the trend and extent of the increase or decrease in hemoglobin value, we will evaluate the effect of the combined use of rh-EPO and sucrose iron on the rapid recovery of patients undergoing TKA or THA. At the same time, according to the change in serum iron content, we can analyse whether rh-EPO can effectively promote the optimal utilization of iron in the blood and then promote the formation of red blood cells.
Presurgery Hb levels and Hb measured on the day after surgery were used to calculate the Hb change due to surgery. The Nadler formula was used to calculate blood volume (BV): k1 × h3 + k2 × m + k3 (where h is height, unit: m, m is weight, unit: kg); k values were as follows: males—k1 = 0.366 9, k2 = 0.032 19, k3 = 0.604 1 and females—k1 = 0.3561, k2 = 0.03308, k3 = 0.183346.
Meunier’s formula was used to calculate and report total blood loss: BV × [(Hbi − Hbe)/Hbe] (where Hbi = haemoglobin concentration preoperatively; Hbe = haemoglobin concentration postoperatively)47.
All statistical analyses were performed using SPSS software (IBM SPSS Statistics, Version 25). Metric data are presented as the mean ± standard deviation (SD) (\(\overline{{\text{x}}}\) ± s), and t tests were performed. Nominal data are expressed as interquartile and percentages (M (IQR)), and the rank sum test was performed for comparison. Enumeration data were expressed as rates, and chi-square tests were performed. The Mann‒Whitney U test was used to compare nonparametric variables, whereas the Wilcoxon signed-rank test was used to compare related samples of nonparametric data. A p value < 0.05 was defined as statistically significant.
Result
In the study of hemoglobin, there were 99 patients in the control group, with average values of HB1-3 (14.07 ± 9.01) (g/l) and HB5-3 (2.99 ± 6.87) (g/l); there were 39 patients in the experimental group, with average values of HB1-3 (11.15 ± 7.27) (g/l) and HB5-3 (4.79 ± 6.63) (g/l). There was no significant difference between the two groups (THB3-1 = 1.802, pHB3-1 = 0.074; THB5-3 = − 1.404, pHB5-3 = 0.064) (Table 3).
When studying serum iron, there were 63 patients in the control group. The mean value of Fe3-1 was 0.816 ± 4.230 µmol/l, and the median value of Fe5-3 was 3.100 µmol/l (1.400, 4.900). There were 33 patients in the experimental group. The mean value of Fe3-1 was (− 14.724 ± 16.535) µmol/l, and the median value of Fe5-3 was 0.400 µmol/l (− 5.950, 2.900). There was a significant difference between the two groups (TFe3-1 = 5.309, pFe3-1 < 0.001; ZFe5-3 = − 3.183, pFe5-3 = 0.001) (Tables 4, 5).
For the change trend of hemoglobin, the data show that for HB1-3, the proportion of the control group assigned 1 is 93.9%, and the proportion of the experimental group assigned 1 is 94.9% (chi square value < 0.0001, p = 1). For HB5-3, 72.7% of the control group and 82.1% of the experimental group are assigned 1 (chi square = 1.310, p = 0.252). There was no significant difference between the two groups. It can be concluded from the data that from the first day to the third day after surgery, the haemoglobin of the experimental group and the control group generally showed a downwards trend, and from the third day to the fifth day after surgery, the haemoglobin of the patients in the two groups generally tended to be stable or upwards (Tables 6, 7). The results shown in the data plot of all patients are basically the same as above. The hemoglobin values of most patients on the first and fifth days after surgery were greater than those on the third day (Figs. 1, 2).
Overall comparison of postoperative hemoglobin of the control group. The vertical coordinate represents the hemoglobin value and the vertical coordinate represents the patient. And the blue line represents the first day, the orange line represents the third day, and the grey line represents the fifth day. This graph not only intuitively reflects the trend of hemoglobin change over time in the control group, but also shows the differences between individuals of different patients, suggesting the significance of individualized medical treatment.
Overall comparison of postoperative hemoglobin of the experimental group. The vertical coordinate represents the hemoglobin value and the vertical coordinate represents the patient. And the blue line represents the first day, the orange line represents the third day, and the grey line represents the fifth day. This graph not only intuitively reflects the trend of hemoglobin change over time in the experimental group, but also shows the differences between individuals of different patients, suggesting the significance of individualized medical treatment.
There was no statistically significant difference in the amount of perioperative blood loss over time between the control and experimental groups (p = 0.95 and 0.091, respectively), as depicted in Table 8.
The data on adverse events after the use of the drug are listed in Table 9. Upon assessing the correlation between the complications and drug utilization, there was no statistical significance for deep vein thrombosis (6.1% vs 7.7%, p = 0.727), gastrointestinal symptoms (12.1% vs 17.9%, p = 0.371) and fever (17.1% vs 20.5%, p = 0.528). None of the patients had allergic reactions, pulmonary embolisms, periprosthetic fractures, or wound infections postoperatively.
Discussion
In recent years, the perioperative blood transfusion rate of arthroplasty has decreased yearly, which benefits from gradual attention to perioperative blood management and the update and progress of knowledge48. There is a certain relationship between blood transfusion and joint infection, and joint infection can be said to be a catastrophic complication for such surgery8,9,10,11,12. Therefore, many institutions and hospitals are studying how to reduce the blood transfusion rate. Hemoglobin value is one of the most important indications of whether blood transfusion is needed.
Long-term preoperative intervention programs have been proven to be effective in reducing the blood transfusion rate and rapid recovery after surgery, but their controllability and compliance are poor and cannot be generally implemented34,35,36,37,38,39,40. Patients are now generally willing to accept intraoperative and postoperative blood management programs. The use of different doses of tranexamic acid on the day of operation has been proven to be able to effectively reduce the amount of bleeding caused by the operation to reduce the rate of blood transfusion13,14,15,16,17,18,19,20,21,22,23,24,25. Similarly, some experimental studies claim that the combined use of rh-EPO and iron agents can have an effective effect in a short period of time41,42,43, but such data do not seem to be completely consistent with our conclusions.
In our retrospective study, the mean values of HB1-3 in the two groups were positive, and the difference was approximately 2.9 g/l (within the 95% confidence interval), which means that the hemoglobin values of the two groups were declining, and the declined value of the patients in the experimental group was smaller than that of the control group. It can be roughly believed that the combined use of these two types of drugs has a certain effect on improving the haemoglobin value in the short term to some extent. However, such a small change has no significant impact on the overall change amplitude, and there is no significant difference (THB3-1 = 1.802, PHB3-1 = 0.074). The same is true for HB5-3. From the perspective of the change trend of the whole haemoglobin value, there was no significant difference between the two groups. The hemoglobin values of the two groups of patients were basically in the decline stage in the first 3 days after surgery and rose or basically remained the same 3 days later (chi square value < 0.0001, p = 1).
The reason why the results are different from those obtained in other articles may be as follows: First, some articles research the numerical changes on the day of operation and the first 3 days after operation. They believed that more than 80% of the total blood loss often occurs within 24 h after the operation41. Some experiments also showed that the decrease in haemoglobin within 1 day after the operation was the largest42. Our experiment was performed to study the numerical changes on the first, third and fifth days after surgery. At this stage, perhaps the hemoglobin values in the human body have become relatively stable without large fluctuations, so even if these two drugs are used, the values will not float significantly. Second, the method of using tranexamic acid during the operation of patients in this experiment is to apply a dose of tranexamic acid (20 mg/kg) half an hour before the beginning. If the surgery lasted more than three hours, another dose of tranexamic acid was applied; otherwise, it was not administered. However, the scheme in the related article is to inject two doses of tranexamic acid (every 3 h)42. Whether the difference in the use of tranexamic acid will affect the experimental results remains to be investigated. Third, many researchers started using rh-EPO and iron more than 2 weeks before surgery. They believed that although rh-EPO could promote the use of iron and the production of red blood cells, it could not rapidly and significantly increase hemoglobin in a short time29,33,34,35,36,37,38,39,40. Whether our experimental research is too short to observe the real effect also needs to be further explored. Since our research shows that hemoglobin begins to rise or stabilize three days after surgery, it is meaningless that these drugs have an effective impact even on the fourth day.
Reducing the blood transfusion rate is the ultimate objective of most experiments. In our study, we mainly discussed the impact on hemoglobin. Although the impact is small and not statistically significant, small changes here may have a greater influence on whether blood transfusion is needed. Strict adherence to appropriate transfusion standards often has a huge effect on the results of the study. In postoperative patients, when the standard for triggering blood transfusion was set as a hemoglobin value lower than 7–8 g/dl rather than a more relaxed threshold (9 or 10 g/dl), no difference was found49,50,51,52. A meta-analysis of relevant experiments showed that the use of restrictive transfusion strategies with hemoglobin values less than 7 g/dl could significantly reduce various complications, such as acute coronary syndrome, pulmonary edema, rebleeding, infection, and total mortality, compared with more liberal strategies. There may also be clinical conditions requiring blood transfusion when the hemoglobin value is higher than 7–8 g/dl or even higher. The main signs include hypotension, tachycardia, shortness of breath, dizziness, fatigue, etc. In the case of the above situations, a blood transfusion scheme is required when necessary52,53,54,55. Because our control of the blood transfusion standard is not strict enough and this study is aimed at the analysis of the range and trend of the value changes from the first day to the fifth day after surgery, we do not consider whether blood transfusion was implemented during surgery as one of the influencing factors. In contrast, we excluded all patients who received blood transfusion during surgery to avoid affecting the data analysis.
According to the collected serum iron data, during the five days of hospitalization after surgery, the rise and fall of serum iron in patients in the control group were significantly weaker than those in the experimental group, and there was a significant difference between the two groups (TFe3-1 = 5.309, PFe3-1 < 0.001; ZFe5-3 = − 3.183, PFe5-3 = 0.001). Theoretically, compared with the control group, the serum iron in the human body tends to increase significantly in a short period of time after the supplementation of iron, but in the case of our retrospective study, the serum iron of the patients in the experimental group decreased evidently. Based on the above research data and phenomena, we can conclude that the use of rhEPO will accelerate the redistribution and utilization of iron ions in the body, which will reduce the content of iron ions in the blood.
The Meunier formula is widely used to calculate postoperative blood loss and is particularly applicable for calculating transfusion risk47,56,57. The present study found that the combined use of rhEPO and iron source in the short term did not reduce perioperative blood loss, which is consistent with the results of other studies58. The potential causes are that up to 10% of patients exhibit EPO resistance or hyporesponsiveness59. In addition, factors may be more significant drivers of perioperative blood loss, such as extensive soft tissue release and bone cuts in joint arthroplasty60, use of anticoagulants61, and individual coagulation factors62.
The relatively mild adverse events associated with rhEPO administration include a local rash, fever, edema, and constipation63, which will resolve gradually over time. Meanwhile, the incidence of hypertension with erythropoietin use is estimated to be approximately 10% to 15% and is associated with NO, endothelins, and the sympathoadrenal and renin-angiotensin pathways64,65. Nevertheless, no abnormal hypertension was found in this study because rhEPO-induced hypertension may not occur in the short term66. In addition, several studies have shown that rhEPO can cause severe adverse effects, such as cerebral hemorrhage and thrombosis, suggesting that rhEPO has tumor-promoting ability67. However, the adverse events among the patients in this study were mainly DVT, gastrointestinal reactions and fever. The incidence of adverse events was not statistically significant compared to the control group, which is similar to the results of previous studies58. This may be due to the strict control of preoperative patients’ physiological indexes, strict control of the indications for drug use, and timely detection and management of patients’ preexisting symptoms. Therefore, it is important to formulate appropriate individualized therapeutic measures when treating patients with rhEPO combined with iron and to pay timely attention to patients’ postoperative status to prevent the occurrence of serious complications.
We learned from the relevant literature that the use of tranexamic acid on the day of operation with no more than six doses (6 g in total, intravenous once every three hours) had no effect on the probability of postoperative deep vein thrombosis13,14,16,17,18,19,20,25, and it was feasible to reduce the probability of blood transfusion in hospitals13,14,15,16,17,18,19,20,21,22,23,24,25. Based on this and the change trend of hemoglobin value obtained by our research, a scheme seems to achieve the maximum optimization effect: we can only use rh-EPO (10,000 iu) and iron sucrose (200 mg) together during the operation and the first 3 days after the operation (once a day). In addition to the two doses of tranexamic acid on the day of operation, we can take a daily intravenous drip (1 g/day) in the first 3 days after the operation. In addition, according to our research results, the average value of HB1-3 is 14.07 ± 9.01 g/l, and if the hemoglobin value on the first day after surgery is greater than 90 g/l, we can choose not to take any intervention measures.
Conclusion
In conclusion, the combined use of rh-EPO and iron sucrose after surgery can accelerate the redistribution and utilization of iron ions in the body in a short period of time, but there is no statistical significance for the change in hemoglobin value, and it cannot advance the rebound of hemoglobin. Therefore, it not only cannot play a greater role in the postoperative rehabilitation of patients undergoing arthroplasty but also incurs additional medical costs.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Change history
29 December 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-48937-1
References
Sehat, K. R., Evans, R. L. & Newman, J. H. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J. Bone Jt. Surg. Br. 86, 561–565 (2004).
Sehat, K. R., Evans, R. & Newman, J. H. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee 7, 151–155. https://doi.org/10.1016/s0968-0160(00)00047-8 (2000).
Park, J. H. et al. Predictors of perioperative blood loss in total joint arthroplasty. J. Bone Jt. Surg. Am. 95, 1777–1783. https://doi.org/10.2106/jbjs.L.01335 (2013).
Yang, T. et al. Comparison of the efficacy of low-molecular-weight heparin and fondaparinux sodium after total knee arthroplasty: A retrospective cohort study. BMC Musculoskelet. Disord. 24, 552. https://doi.org/10.1186/s12891-023-06674-6 (2023).
Magill, P. et al. Oral tranexamic acid for an additional 24 hours postoperatively versus a single preoperative intravenous dose for reducing blood loss in total knee arthroplasty: Results of a randomized controlled trial (TRAC-24). Bone Jt. J. 103(10), 1595–1603. https://doi.org/10.1302/0301-620X.103B10.BJJ-2020-2308.R1 (2021).
Song, K., Pan, P., Yao, Y., Jiang, T. & Jiang, Q. The incidence and risk factors for allogenic blood transfusion in total knee and hip arthroplasty. J. Orthop. Surg. Res. 14, 273. https://doi.org/10.1186/s13018-019-1329-0 (2019).
Fan, D., Ma, J., Liu, X. & Zhang, L. Peri-articular administration of tranexamic acid is an alternative route in total knee arthroplasty: A systematic review and meta-analysis. J. Orthop. Surg. Res. 17, 211. https://doi.org/10.1186/s13018-022-03095-4 (2022).
Maempel, J. F., Wickramasinghe, N. R., Clement, N. D., Brenkel, I. J. & Walmsley, P. J. The pre-operative levels of haemoglobin in the blood can be used to predict the risk of allogenic blood transfusion after total knee arthroplasty. Bone Jt. J. 98, 490–497. https://doi.org/10.1302/0301-620x.98b4.36245 (2016).
Friedman, R., Homering, M., Holberg, G. & Berkowitz, S. D. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J. Bone Jt. Surg. Am. 96, 272–278. https://doi.org/10.2106/jbjs.L.01268 (2014).
Klika, A. K. et al. Primary total knee arthroplasty allogenic transfusion trends, length of stay, and complications: Nationwide inpatient sample 2000–2009. J. Arthroplasty 29, 2070–2077. https://doi.org/10.1016/j.arth.2014.06.018 (2014).
Ponnusamy, K. E., Kim, T. J. & Khanuja, H. S. Perioperative blood transfusions in orthopaedic surgery. J. Bone Jt. Surg. Am. 96, 1836–1844. https://doi.org/10.2106/JBJS.N.00128 (2014).
Newman, E. T. et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J. Bone Jt. Surg. Am. 96, 279–284. https://doi.org/10.2106/jbjs.L.01041 (2014).
Xie, J., Ma, J., Yao, H., Yue, C. & Pei, F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: A randomized clinical trial. J. Arthroplasty 31, 2458–2464. https://doi.org/10.1016/j.arth.2016.04.034 (2016).
Jain, N. P., Nisthane, P. P. & Shah, N. A. Combined administration of systemic and topical tranexamic acid for total knee arthroplasty: Can it be a better regimen and yet safe? A randomized controlled trial. J. Arthroplasty 31, 542–547. https://doi.org/10.1016/j.arth.2015.09.029 (2016).
Kopanidis, P. et al. Perioperative blood management programme reduces the use of allogenic blood transfusion in patients undergoing total hip and knee arthroplasty. J. Orthop. Surg. Res. 11, 28. https://doi.org/10.1186/s13018-016-0358-1 (2016).
Watts, C. D., Houdek, M. T., Sems, S. A., Cross, W. W. & Pagnano, M. W. Tranexamic acid safely reduced blood loss in hemi- and total hip arthroplasty for acute femoral neck fracture: A randomized clinical trial. J. Orthop. Trauma 31, 345–351. https://doi.org/10.1097/bot.0000000000000837 (2017).
Huang, Z. et al. Intravenous and topical tranexamic acid alone are superior to tourniquet use for primary total knee arthroplasty: A prospective, randomized controlled trial. J. Bone Jt. Surg. Am. 99, 2053–2061. https://doi.org/10.2106/jbjs.16.01525 (2017).
Xie, J., Hu, Q., Ma, J., Huang, Q. & Pei, F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss and the inflammatory response following enhanced-recovery primary total hip arthroplasty: A randomised clinical trial. Bone Jt. J. 99, 1442–1449. https://doi.org/10.1302/0301-620X.99B11.BJJ-2017-0488.R1 (2017).
Lei, Y. et al. The efficacy and safety of multiple-dose intravenous tranexamic acid on blood loss following total knee arthroplasty: A randomized controlled trial. Int. Orthop. 41, 2053–2059. https://doi.org/10.1007/s00264-017-3519-x (2017).
Lei, Y. et al. Multiple-dose intravenous tranexamic acid further reduces hidden blood loss after total hip arthroplasty: A randomized controlled trial. J. Arthroplasty 33, 2940–2945. https://doi.org/10.1016/j.arth.2018.04.024 (2018).
Gianakos, A. L., Hurley, E. T., Haring, R. S., Yoon, R. S. & Liporace, F. A. Reduction of blood loss by tranexamic acid following total hip and knee arthroplasty: A meta-analysis. JBJS Rev. 6, e1. https://doi.org/10.2106/jbjs.Rvw.17.00103 (2018).
Abdel, M. P. et al. Intravenous versus topical tranexamic acid in total knee arthroplasty: Both effective in a randomized clinical trial of 640 patients. J. Bone Jt. Surg. Am. 100, 1023–1029. https://doi.org/10.2106/jbjs.17.00908 (2018).
Yazdi, H. et al. Tranexamic acid is associated with reduced periprosthetic joint infection after primary total joint arthroplasty. J. Arthroplasty 35, 840–844. https://doi.org/10.1016/j.arth.2019.10.029 (2020).
Changjun, C. et al. Tranexamic acid attenuates early post-operative systemic inflammatory response and nutritional loss and avoids reduction of fibrinogen in total hip arthroplasty within an enhanced recovery after surgery pathway. Int. Orthop. 45, 2811–2818. https://doi.org/10.1007/s00264-021-05182-3 (2021).
Zhang, S. et al. Six-dose intravenous tranexamic acid regimen further inhibits postoperative fibrinolysis and reduces hidden blood loss following total knee arthroplasty. J. Knee Surg. 34, 224–232. https://doi.org/10.1055/s-0039-1694768 (2021).
Gillette, B. P. et al. Economic impact of tranexamic acid in healthy patients undergoing primary total hip and knee arthroplasty. J. Arthroplasty 28, 137–139. https://doi.org/10.1016/j.arth.2013.04.054 (2013).
Tuttle, J. R. et al. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J. Arthroplasty 29, 1512–1515. https://doi.org/10.1016/j.arth.2014.01.031 (2014).
Muñoz, M., García-Erce, J. A. & Remacha, A. F. Disorders of iron metabolism. Part 1: Molecular basis of iron homoeostasis. J. Clin. Pathol. 64, 281–286. https://doi.org/10.1136/jcp.2010.079046 (2011).
Gómez-Ramírez, S., Bisbe, E., Shander, A., Spahn, D. R. & Muñoz, M. Management of perioperative iron deficiency anemia. Acta Haematol. 142, 21–29. https://doi.org/10.1159/000496965 (2019).
Muñoz, M. et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 73, 1418–1431. https://doi.org/10.1111/anae.14358 (2018).
Muñoz, M. & Gómez-Ramírez, S. Is there a role for iron supplementation in critically ill patients? Med. Intens. 43, 103–107. https://doi.org/10.1016/j.medin.2018.03.003 (2019).
Lee, S. H., Kim, J. I., Choi, W., Kim, T. W. & Lee, Y. S. Effectiveness of iron supplementation in the perioperative management of total knee arthroplasty: A systematic review. Knee Surg. Relat. Res. 32, 44. https://doi.org/10.1186/s43019-020-00064-1 (2020).
García-Erce, J. A. et al. Efficacy of preoperative recombinant human erythropoietin administration for reducing transfusion requirements in patients undergoing surgery for hip fracture repair. An observational cohort study. Vox Sang. 97, 260–267. https://doi.org/10.1111/j.1423-0410.2009.01200.x (2009).
So-Osman, C. et al. Patient blood management in elective total hip- and knee-replacement surgery (Part 1): A randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietin-eligible patients. Anesthesiology 120, 839–851. https://doi.org/10.1097/aln.0000000000000134 (2014).
Voorn, V. M. et al. Erythropoietin to reduce allogeneic red blood cell transfusion in patients undergoing total hip or knee arthroplasty. Vox Sang. 111, 219–225. https://doi.org/10.1111/vox.12412 (2016).
Zhao, Y. et al. The effectiveness and safety of preoperative use of erythropoietin in patients scheduled for total hip or knee arthroplasty: A systematic review and meta-analysis of randomized controlled trials. Medicine 95, e4122. https://doi.org/10.1097/md.0000000000004122 (2016).
Polanco-García, M. et al. Effectiveness of a patient blood management protocol on reduction of allogeneic red blood cell transfusions in orthopedic surgery. Med. Clin. 152, 90–97. https://doi.org/10.1016/j.medcli.2018.04.021 (2019).
Hourlier, H. & Fennema, P. Surgical prescription of epoetin alfa in contemporary total hip arthroplasty: A prospective comparative study. Int. Orthop. 44, 261–266. https://doi.org/10.1007/s00264-019-04399-7 (2020).
Biboulet, P. et al. Preoperative erythropoietin within a patient blood management program decreases both blood transfusion and postoperative anemia: A prospective observational study. Transfusion 60, 1732–1740. https://doi.org/10.1111/trf.15900 (2020).
Muñoz, M. et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: A pooled analysis of observational data from 2547 patients. Transfusion 54, 289–299. https://doi.org/10.1111/trf.12195 (2014).
Na, H. S. et al. Effects of intravenous iron combined with low-dose recombinant human erythropoietin on transfusion requirements in iron-deficient patients undergoing bilateral total knee replacement arthroplasty. Transfusion 51, 118–124. https://doi.org/10.1111/j.1537-2995.2010.02783.x (2011).
Ruan, R. X. et al. Does subcutaneous administration of recombinant human erythropoietin increase thrombotic events in total hip arthroplasty? A prospective thrombelastography analysis. J. Orthop. Surg. Res. 15, 546. https://doi.org/10.1186/s13018-020-02083-w (2020).
Suh, Y. S., Nho, J. H., Seo, J., Jang, B. W. & Park, J. S. Hip fracture surgery without transfusion in patients with hemoglobin less than 10 g/dl. Clin. Orthop. Surg. 13, 30–36. https://doi.org/10.4055/cios20070 (2021).
Bedair, H., Yang, J., Dwyer, M. K. & McCarthy, J. C. Preoperative erythropoietin alpha reduces postoperative transfusions in THA and TKA but may not be cost-effective. Clin. Orthop. Relat. Res. 473, 590–596. https://doi.org/10.1007/s11999-014-3819-z (2015).
Jeong, J. H. et al. Postoperative intravenous iron supplementation does not improve hemoglobin level and transfusion rate following staged bilateral total knee arthroplasty. J. Arthroplasty 35, 2444–2450. https://doi.org/10.1016/j.arth.2020.04.086 (2020).
Nadler, S. B., Hidalgo, J. H. & Bloch, T. Prediction of blood volume in normal human adults. Surgery 51, 224–232 (1962).
Donovan, R. L., Lostis, E., Jones, I. & Whitehouse, M. R. Estimation of blood volume and blood loss in primary total hip and knee replacement: An analysis of formulae for perioperative calculations and their ability to predict length of stay and blood transfusion requirements. J. Orthop. 24, 227–232. https://doi.org/10.1016/j.jor.2021.03.004 (2021).
Bedard, N. A. et al. Recent trends in blood utilization after primary hip and knee arthroplasty. J. Arthroplasty 32, 724–727. https://doi.org/10.1016/j.arth.2016.09.026 (2017).
Hébert, P. C. et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N. Engl. J. Med. 340, 409–417. https://doi.org/10.1056/nejm199902113400601 (1999).
Boral, L. I. et al. How do I implement a more restrictive transfusion trigger of hemoglobin level of 7 g/dL at my hospital? Transfusion 55, 937–945. https://doi.org/10.1111/trf.12982 (2015).
Doodeman, H. J. et al. The effect of a preoperative erythropoietin protocol as part of a multifaceted blood management program in daily clinical practice (CME). Transfusion 53, 1930–1939. https://doi.org/10.1111/trf.12016 (2013).
Carson, J. L. et al. Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann. Intern. Med. 157, 49–58. https://doi.org/10.7326/0003-4819-157-1-201206190-00429 (2012).
Liumbruno, G., Bennardello, F., Lattanzio, A., Piccoli, P. & Rossetti, G. Recommendations for the transfusion of red blood cells. Blood Transfus 7, 49–64. https://doi.org/10.2450/2008.0020-08 (2009).
Liumbruno, G. M., Bennardello, F., Lattanzio, A., Piccoli, P. & Rossetti, G. Recommendations for the transfusion management of patients in the peri-operative period. II. The intra-operative period. Blood Transfus 9, 189–217. https://doi.org/10.2450/2011.0075-10 (2011).
Liumbruno, G. M., Bennardello, F., Lattanzio, A., Piccoli, P. & Rossetti, G. Recommendations for the transfusion management of patients in the peri-operative period. III. The post-operative period. Blood Transfus 9, 320–335. https://doi.org/10.2450/2011.0076-10 (2011).
King, L., Randle, R., Dare, W. & Bernaitis, N. Comparison of oral vs combined topical/intravenous/oral tranexamic acid in the prevention of blood loss in total knee arthroplasty: A randomised clinical trial. Orthop. Traumatol. Surg. Res. 105, 1073–1077. https://doi.org/10.1016/j.otsr.2019.06.008 (2019).
Levent, A., Kose, O., Linke, P., Gehrke, T. & Citak, M. Does tourniquet use decrease blood loss following primary total knee arthroplasty in Jehovah’s Witness patients? Jt. Dis. Relat. Surg. 31, 419–425. https://doi.org/10.5606/ehc.2020.76244 (2020).
Kaufner, L. et al. Erythropoietin plus iron versus control treatment including placebo or iron for preoperative anaemic adults undergoing non-cardiac surgery. Cochrane Database Syst. Rev. 8, CD012451. https://doi.org/10.1002/14651858.CD012451.pub2 (2020).
Zou, H., Xu, P., Wong, R. S. M. & Yan, X. A novel combination therapy of erythropoietin and thrombopoietin to treat erythropoietin-resistance anemia. Pharm. Res. 39, 1249–1265. https://doi.org/10.1007/s11095-022-03304-z (2022).
Prasad, N., Padmanabhan, V. & Mullaji, A. Blood loss in total knee arthroplasty: An analysis of risk factors. Int. Orthop. 31, 39–44. https://doi.org/10.1007/s00264-006-0096-9 (2007).
Wang, T. F. et al. Risk of venous thromboembolism or hemorrhage among individuals with chronic kidney disease on prophylactic anticoagulant after hip or knee arthroplasty. Am. J. Hematol. https://doi.org/10.1002/ajh.26994 (2023).
Kleiboer, B. et al. Postoperative bleeding complications in patients with hemophilia undergoing major orthopedic surgery: A prospective multicenter observational study. J. Thromb. Haemost. 20, 857–865. https://doi.org/10.1111/jth.15654 (2022).
Kettelhack, C., Hones, C., Messinger, D. & Schlag, P. M. Randomized multicentre trial of the influence of recombinant human erythropoietin on intraoperative and postoperative transfusion need in anaemic patients undergoing right hemicolectomy for carcinoma. Br. J. Surg. 85, 63–67. https://doi.org/10.1046/j.1365-2168.1998.00564.x (1998).
Agarwal, R. Mechanisms and mediators of hypertension induced by erythropoietin and related molecules. Nephrol. Dial Transplant. 33, 1690–1698. https://doi.org/10.1093/ndt/gfx324 (2018).
Winearls, C. G. et al. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet 2, 1175–1178. https://doi.org/10.1016/s0140-6736(86)92192-6 (1986).
Frank, H. et al. Effect of erythropoietin on cardiovascular prognosis parameters in hemodialysis patients. Kidney Int. 66, 832–840. https://doi.org/10.1111/j.1523-1755.2004.00810.x (2004).
Liu, W. et al. Erythropoietin signaling in the microenvironment of tumors and healthy tissues. Adv. Exp. Med. Biol. 1223, 17–30. https://doi.org/10.1007/978-3-030-35582-1_2 (2020).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the Affiliations. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, W., Liu, C. & Bi, Z. Effect of recombinant human erythropoietin combined with iron sucrose on postoperative hemoglobin in patients undergoing artificial joint replacement. Sci Rep 13, 18919 (2023). https://doi.org/10.1038/s41598-023-41887-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41887-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.