Abstract
Small cell neuroendocrine carcinoma of the cervix (SCNECC) is a highly invasive cervical cancer. The immunohistochemical criteria is an important aspect for assistant diagnosis of SCNECC. However, which markers can be appropriate selection for diagnosing SCNECC were not determined. The aim was to systematically evaluate expression levels of four neuroendocrine markers (containing synaptophysin (Syn), neural cell adhesion molecules (CD56), neuron-specific enolase (NSE) and chromograninA (CgA)) and to find out the appropriate selection for diagnosing SCNECC. Four English and three Chinese libraries were retrieved between 1984 and 2020. 23 studies about NSE, 36 studies about Syn, 23 studies about CD56 and 36 studies about CgA (all studies containing 581 patients) were eligible for meta-analyses. The pooled positive expression percentages (95% CI; I2) were as follows: 84.84% (79.41–90.27%; 76.7%) for Syn, 84.53% (79.43–89.96%; 37.5%) for CD56, 77.94% (69.13–86.76%; 83.5%) for NSE, and 72.90% (67.40–78.86%; 59.7%) for CgA. The positive proportions (95% CI; I2) ranked top three of simultaneous expressions of two markers were 87.75% (82.03–93.87%, 33.3%) for Syn and CD56, 70.92% (50.50–87.68%, 82.7%) for Syn and NSE, 65.65% (53.33–76.98%, 73.5%) for Syn and CgA. This confirms that Syn and CD56 are reliable indicators for diagnosing SCNECC.
Similar content being viewed by others
Introduction
Neuroendocrine carcinoma of the cervix (NECC) is an aggressive histological variant of cervical malignancy. Small cell NECC (SCNECC) is the most common and high–grade poorly differentiated histological subtype of NECC1. SCNECC is associated with adverse outcome in spite of even a small component in mixed carcinomas of the uterine cervix2. Therefore, accurate initial diagnosis of SCNECC is paramount. SCNECC has its unique growth characteristic that the cancer cells have the capacity to invade the stroma extensively even in the early stage1,3. This may result in negative cytology and increase the difficulty of clinical diagnosis. Pathomorphological diagnosis is the basis for SCNECC, the immunohistochemical (IHC) criteria is an important aspect for the diagnosis too3.
To establish the SCNECC diagnosis, at least one or two positive staining neuroendocrine markers is recommended1,4,5,6. IHC staining for neuroendocrine markers include synaptophysin (Syn), neural cell adhesion molecules (CD56), neuron-specific enolase (NSE) and chromograninA (CgA), positive expression of which indicates the neoplasms arising from cells of the neuroendocrine system7,8. The above four neuroendocrine markers have been widely used for the assistant diagnosis of SCNECC. However, due to the low incidence of SCNECC, the relative literatures were clinical case reports or case series1,3. In addition, there are differences in values of positive expression rate among individual studies. Accordingly, the true levels of the four neuroendocrine markers may not be accurate by direct quantitative assessment of each study. It is also difficult to select the appropriate neuroendocrine markers to assist to diagnose SCNECC. Hence we performed a meta–analysis to evaluate the IHC expression of Syn, CD56, NSE and CgA, and aimed to provide an appropriate selection of neuroendocrine markers for assistant diagnosis of SCNECC.
Methods
Literature search
The study was approved by the Ethics Committee of Second Xiangya hospital. We only retrospectively extracted the clinical and pathological data of patients, which had no impact on the outcome of patients. The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Patients and public were not involved in this study. We interrogated four English libraries (the PubMed, Cochrane library, Web of science, and EMbase databases) and three Chinese libraries (the China National Knowledge Infrastructure (CNKI), VIP, and Wanfang databases) to perform a comprehensive search from inception to 20 January 2020. The broad search strategy used combinations of the following key terms: “Cervical tumor”, “small cell carcinoma”, “neuroendocrine carcinoma”, “neuroendocrine marker”. In order to interpret the results more objectively, the titles and abstracts were carefully screened according to the screening flow chart showed in the Fig. 1.
The screening flow chart of literature about small cell neuroendocrine carcinoma of the cervix (SCNECC). n1, n2, n3 and n4 respectively represent the number of the literature about Syn, CD56, NSE and CgA in each screening step. CNKI China National Knowledge Infrastructure; SCNECC small cell neuroendocrine carcinoma of the cervix; Syn synaptophysin; CD56 neural cell adhesion molecules; NSE neuron-specific enolase; CgA chromograninA; IHC immunohistochemical staining.
Eligibility criteria
The inclusion criteria were as follows: (i) English or Chinese published clinical studies; (ii) SCNECC patients confirmed by pathological diagnosis without age or racial restrictions; (iii) detailed IHC information with the positive expression rate of at least one of four neuroendocrine markers (Syn, CD56, NSE and CgA). The exclusion criteria were as follows: (i) case report of individual patient; (ii) systematic review or duplicate data; (iii) no detailed messages of IHC staining; (iv) non-SCNECC patients; (v) literature on basic research and animal studies. The research was still included if the study group was SCNECC patients and the control group was non-SCNECC patients.
All potentially relevant abstracts or full articles were reviewed independently by two researchers. When discrepancies between researchers occurred for inclusion or exclusion, discussion was conducted and disagreements were resolved by consensus. Quality assessment of all qualified literature was then done.
Data extraction
Information including types of studies, name of first author, institution, publication year and clinical data of patients was analyzed in each study.The clinical data were consisted of age, tumor stage, histopathological type, IHC results, treatment and prognosis.
We searched the definition of IHC positive staining in each study and found three methods of description. The first method only mentioned the positive expression without specific description. The second defined positive staining by positive staining percentage of tumor cells. When the staining rate of neuroendocrine markers was more than 5% or 10%, the expression of neuroendocrine markers was positive. The third was definition of positive staining by a four-point scale. Staining was graded as 0, 1 + (less than 5% or 10% tumor cells), 2 + (5% or 10–50% tumor cells), 3 + (more than 50% tumor cells) respectively. The positive expression of neuroendocrine markers by any of the above three methods was regarded as positive expression.
The positive expression rate of individual neuroendocrine marker in one study was defined as follows: number of positive expression SCNECC cases/number of tested SCNECC cases (percentage). In addition, there are 6 combinations of the two markers, which are Syn and CD56, Syn and NSE, Syn and CgA, CD56 and NSE, CD56 and CgA, NSE and CgA. Similarly, the positive expression rate of two markers can be calculated as followed: number of simultaneous positive expression cases/number of tested cases in one study (percentage). If the study included patients with non-SCNECC, we tried to calculate the sensitivity and specificity of neuroendocrine markers expression.
Statistical analysis
All raw data extracted from the predetermined studies were managed using MetaProp function in statistical software R 3.5.0. The detailed approach of meta-analysis was shown in Supplementary Fig. 1. The first step was to determine if a transformation of raw rate is needed. The converted rate was calculated with optimum one of the four proportion transform methods (log, logit, arcsine, and dsrsine) if the raw rate does not satisfy the normal distribution. Then the pooled expression proportions with 95% confidence intervals (CIs) of one marker or two markers could be determined using the appropriate transformation to give the effective value. A random effect model was selected when p ≤ 0.05 and a fixed effect model was used when p > 0.05.
Additionally, we also measured the effect of heterogeneity between the included studies using I2 = 100% × (Q − df)/Q. I2 value of 25%, 50%, and 75% were considered as low, moderate, and high degrees of heterogeneity respectively. The results of meta-analysis were presented in forest maps. Funnel plot asymmetry was assessed by the Egger's linear regression test. The results provided publication bias results for this meta-analysis. p < 0.05 was considered significant bias.
Results
Study searches and characteristics
A total of 369 literatures about Syn, 282 literatures about CD56, 300 literatures about NSE, and 278 literatures about CgA were identified as potentially eligible for inclusion. A flow diagram of the study selection is shown in Fig. 1. Of the remaining 42 studies warranting furthering review, 41 studies were case series and 1 study was a case report. 42 studies were harmonized for inclusion criteria, comprising 29 from Asia (China, Japan, Indian, and Thailand), 4 from Europe (England, Germany, Poland), 8 from the United States of America and 1 from Canada. All included studies were retrospective and a total of 581 patients were enrolled in the stratified meta-analyses. Characteristics of studies used in the research are enumerated in the Table 1.
For the 42 studies in the meta-analysis, 36 SCNECC studies involved Syn1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,30,31,32,33,34,35,36,37,38,39,40,41,42, 23 studies involved CD561,2,6,7,9,10,12,13,15,17,18,19,30,32,33,34,35,36,37,38,40,41,42, 23 studies involved NSE1,2,3,6,7,12,13,15,17,21,22,24,25,26,27,28,29,30,32,33,34,38,42and 36 studies involved CgA1,2,3,4,5,6,7,9,10,12,13,15,16,17,18,19,20,21,22,23,25,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. 26 studies provided information about positive expression rates for two markers. There were 15 studies about Syn and CD561,6,7,9,12,13,15,17,18,19,32,33,34,36,42, 15 studies about Syn and NSE1,3,6,7,12,13,17,18,22,25,32,33,34,38,42, 23 studies about Syn and CgA1,3,4,6,7,9,12,13,15,17,18,19,20,22,23,25,31,32,33,34,36,39,42, 9 studies about CD56 and NSE1,6,7,12,17,18,33,38,42, 12 studies about CD56 and CgA1,6,7,9,12,13,15,17,18,19,33,42and 15 studies about NSE and CgA1,3,6,7,12,13,17,18,21,22,25,29,33,38,42. The positive expressions of one marker and two markers are illustrated in the Table 2. For the description of IHC positive staining, 30 studies took the first method, 2 studies the second method, and 10 studies the third method respectively.
Meta–analyses of expression percentage of neuroendocrine markers
Results of the pooled positive expression percentages (95% CI) were as follows: 84.84% (79.41% to 90.27%) for Syn, 84.53% (79.43% to 89.96%) for CD56, 77.94% (69.13% to 86.76%) for NSE, and 72.90% (67.40% to 78.86%) for CgA (Fig. 2a–d). The transform methods of six combination expression levels were showed separately in Table 3. The positive proportions with 95% CIs expressed by the two markers from high to low were 87.75% (82.03% to 93.87%) for Syn and CD56, 70.92% (50.50% to 87.68%) for Syn and NSE, 65.65% (53.33% to 76.98%) for Syn and CgA, 64.09% (43.38% to 84.79%) for CD56 and NSE, 59.55% (45.53% to 72.81%) for NSE and CgA, 50.98% (40.52% to 61.39%) for CD56 and CgA (Fig. 3a–f).
Forest plots of expression proportion (95% CI) for single neuroendocrine markers in small cell neuroendocrine carcinoma of the cervix (SCNECC). (a) (Syn), (b) (CD56), (c) (NSE) and (d) (CgA).The weight represents the percentage of the results of a single study in the overall results. Generally, the larger the number of cases in a single study, the greater its weight in the overall population. CI confidence interval; Syn synaptophysin; CD56 neural cell adhesion molecules; NSE neuron-specific enolase; CgA chromograninA.
Forest plots of expression proportion (95% CI) for combination of two neuroendocrine markers in small cell neuroendocrine carcinoma of the cervix (SCNECC). (a) (Syn + CD56), (b) (Syn + NSE), (c) (Syn + CgA), (d) (CD56 + NSE), (e) (NSE + CgA), (f) (CD56 + CgA). The weight represents the percentage of the results of a single study in the overall results. Generally, the larger the number of cases in a single study, the greater its weight in the overall population. CI confidence interval; Syn synaptophysin; CD56 neural cell adhesion molecules; NSE neuron-specific enolase; CgA chromograninA.
Test of heterogeneity
The heterogeneity among studies included for the meta-analysis of Syn was evaluated firstly. As seen in Table 3, a random effect model was used because I2 was 76.7% with p value < 0.05. Similarly, random effect models were selected for other three single markers and four combinations (Syn and NSE, Syn and CgA, CD56 and NSE, NSE and CgA). While, fixed effect models were selected for two combinations (Syn and CD56, CD56 and CgA) since p value > 0.05.
The sensitivity and specificity of neuroendocrine markers expression
Only 6 studies described IHC staining of neuroendocrinenon markers in non-SCNECC patients8,9,10,24,28,29. In three of these studies, non-SCNECC patients were larger cell NECC (LCNECC) patients8,9,10. So the sensitivity and specificity of neuroendocrine markers can be calculated simultaneously in only 3 studies. The results were shown in Supplementary Table 1. This part of results was not further meta-analyzed because of the small sample size.
Quality assessment
42 studies included 39 full articles and 3 abstracts. Only the full articles were performed for quality assessment by appraisal checklists43, which included two different assessment forms, separately used for case series (38 studies) and case reports (1 study). The quality assessment of case series was shown in Supplementary Table 2. 80% enrolled studies gave positive response to 7 questions, and all studies satisfied 3 questions among them. The quality assessment of one case report was shown in Supplementary Table 3. The results showed that only the adverse events or unanticipated events were not identified in the study (question 7).
Publication bias
Publication bias was evaluated via Egger’s test. The Egger’s test (p > 0.05) suggested no significant publication bias. The results showed that the most of the literatures related to combined groups had no significant publication bias (Table 3, Fig. 4e–i). While, literatures about single markers had significant publication bias (Table 3, Fig. 4a-d).
Funnel plots estimating possible publication bias. The sample size of the combination of CD56 + NSE is too small to evaluate the publication bias. One requirement of asymmetric analysis is that the case number of included studies is greater than nine. So we eliminated the analysis of studies of CD56 and NSE for whose sample size did not meet analytical criteria.
Discussion
Precise diagnosis is very crucial for SCNECC treatment. This disease requires specialized management recommendations depending on its unique biological behavior. Our results confirmed that the positive expression percentage of Syn was the highest among four classic neuroendocrine markers, and the positive rates of combination (Syn and CD56) were the highest among six combinations (Fig. 5). This is the first meta-analysis of the expression levels of neuroendocrine markers in SCNECC studies with the largest sample size. Therefore, the results of quantitative evaluation will help us select suitable markers for assisting diagnosing SCNECC.
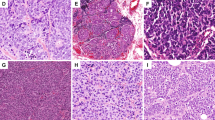
Brief overview of meta-analysis results. (a) (positive expression percentages per marker); (b) (a SCNECC cell), three proteins (containing Syn, CgA and NSE) locate in cell cytoplasm and only CD56 protein locates in the cell membrane; (c) (positive expression percentages per two markers); (d) (HE staining and Immunohistochemical staining of SCNECC), d1 (HE, × 100, d2 (Syn, × 100), d3 (CD56, × 400), d4 (NSE, × 100), d5 (CgA, × 100). All the pathological images come from our experiments. Syn synaptophysin; CD56 neural cell adhesion molecules; NSE neuron-specific enolase; CgA chromograninA; HE hematoxylin–eosin; × 100, magnification 100 times; × 400, magnification 400 times.
The cancer cells of SCNECC have the similarity of neuroendocrine characteristics44. This is also the basis for distinguishing other morphologically similar tumors from SCNECC. Our study showed Syn had the highest expression rate, followed by CD56 in single marker expression. The combination Syn and CD56 have the highest positive expression rate in double marker expression simultaneously. The differences in the expression levels of four neuroendocrine markers are related to their molecular characteristics. Syn is a calcium binding protein located on the membrane of synapse vesicles, which diffusely expresses in the cytoplasm of neuroendocrine cells4. The molecular biological characteristic may explain why it is expressed with high degree. The expression level of CD56 is second only to Syn. And the heterogeneity of CD56 expression is smaller than those of other three markers. CD56 is a glycoprotein on the surface of cell membrane and also a member of cell adhesion molecule, which plays an important role in infiltration and metastasis of tumor cells45. The high expression level of CD56 corresponds with the aggressive properties of SCNECC. Moreover, CD56 has its own unique advantages in terms of stable expression detected by IHC method.
The positive expression rates of NSE and CgA markers are relatively low in our study, especially the expression level of CgA is the lowest. CgA and NSE are valuable markers for diagnosing neuroendocrine cancer, and their expressions are relevant to the patient's prognosis6,12.Our study did not reach a similar conclusion. The potential possibilities are as follows. Firstly, SCNECC may have a decline in the expression of some neuroendocrine cell characteristics for high degree of malignancy and poor differentiation. Secondly, expression rates of neuroendocrine markers may be affected by detection technology. The expressions of CgA and NSE can be detected by a serological assay, which were not included in our studies.
The 5-year overall survival rates of SCNECC range from 20 to 46.6%, and the prognosis of patients with advanced stage was very poor regardless of therapy46. However, SCNECC patients with early stage have the potential to receive multimodality therapy and have long term survival47. This difference emphasises the importance of early accurate diagnosis of SCNECC. There are some controversies in diagnostic criteria of SCNECC focusing on the necessity of neuroendocrine markers in the diagnosis. Some researchers pointed out that SCNECC was a morphologic diagnosis and the IHC evidence of neuroendocrine differentiation was not a requirement for diagnosis25. But actually, many studies have confirmed that accurate diagnosis of SNCECC require IHC staining of neuroendocrine markers which have been performed in clinical work too1,3,12,19,22,29,48,49. These findings highlighted the expression of two or more markers was a necessary criteria for diagnosing SCNECC.
The evidences reveal that differential diagnosis by neuroendocrine markers is particularly important in two situations. One is to differentiate SCNECC from other tumors with small cell morphological characteristics, and the other is to determine whether cervical adenocarcinoma or squamous carcinoma coexist with SCNECC1,3,18. With the development of diagnostic technology, it is found that SCNECC frequently occur mixed with other pathological types. Of the 42 studies enrolled in our study, 16 were patients with mixed SCNECC. To obtain more valuable results, we also analyzed the combined expression of two markers. We found that the combination (Syn and CD56) had the highest expression rate, which was consistent with the level of positive expression rate for single markers. Syn and CD56 are sensitive indicators for diagnosing SCNECC. However, the expression levels of both markers are highly variable.
Our study managed to collect almost all the related studies. However, the quantity, quality, and type of these studies still limited the level of evidence of this meta-analysis. All the included studies were retrospective types with small sample sizes. Heterogeneity of some studies existed in this meta-analysis. There were not adequate data and studies for the meta-analysis of prognosis. Since the data including IHC expressions of the four neuroendocrine markers in non-SCNECC were too small, it was not possible to compare the diagnostic specificity of these four markers. Thus, more studies including patients with non-small cell neuroendocrine cancer or clinical trials with a larger sample size are expected in the future.
Conclusion
The positive expression percentage of Syn was the highest among four neuroendocrine markers, and the positive rates of combination (Syn and CD56) were the highest among six combinations. It is confirmed that Syn and CD56 are reliable indicators for diagnosing SCNECC.
Data availability
All data generated and analysed during the study are included in this published article.
References
Deng, G. H., Zhang, X. & Wu, L. Y. Clinicopathological analysis of nine cases of small cell carcinoma of the uterine cervix. Chin. J. Oncol. 32, 199–202. https://doi.org/10.3760/cma.j.issn.0253-3766.2010.03.009 (2010) (in Chinese).
Horn, L. C. et al. Mixed small cell carcinomas of the uterine cervix:prognostic impact of focal neuroendocrine differentiation but not of Ki-67 labeling index. Ann. Diagn. Pathol. 10, 140–143. https://doi.org/10.1016/j.anndiagpath.2005.07.019 (2006).
Cheng, M., Wu, L. Y., Bai, P., Zhang, R. & Zheng, S. Clinicopathologic characteristics of eight patients with small cell carcinoma of the cervix. Chin. J. Obstet. Gynecol. 43, 189–192. https://doi.org/10.3321/j.issn:0529-567X.2008.03.008 (2008) (in Chinese).
Ishida, G. M. et al. Small cell neuroendocrine carcinomas of the uterine cervix: a histological, immunohistochemical, and molecular genetic study. Int. J. Gynecol. Pathol. 23, 366–372. https://doi.org/10.1097/01.pgp.0000139637.01977.61 (2004).
Conner, M. G., Richter, H., Moran, C. A., Hameed, A. & Albores-Saavedra, J. Small cell carcinoma of the cervix: a clinicopathologic and immunohistochemical study of 23 cases. Ann. Diagn. Pathol. 6, 345–348 (2002).
Tsunoda, S. et al. Small-cell carcinoma of the uterine cervix: a clinicopathologic study of 11 cases. Int. J. Gynecol. Cancer 15, 295–300. https://doi.org/10.1111/j.1525-1438.2005.15219.x (2005).
Giorgadze, T. et al. Small cell carcinoma of the cervix in liquid-based Pap test: utilization of split-sample immunocytochemical and molecular analysis. Diagn. Cytopathol. 40, 214–219 (2012).
Ganesan, R. et al. Neuroendocrine carcinoma of the cervix: review of a series of cases and correlation with outcome. Int. J. Surg. Pathol. 24, 490–496. https://doi.org/10.1177/1066896916643385 (2016).
Kajiwara, H. et al. Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine carcinoma of uterine cervix. Arch. Gynecol. Obstet. 279, 521–525. https://doi.org/10.1007/s00404-008-0760-y (2009).
Kuji, S. et al. A new marker, insulinoma-associated protein 1 (INSM1), for high-grade neuroendocrine carcinoma of the uterine cervix: analysis of 37 cases. Gynecol. Oncol. 144, 384–390. https://doi.org/10.1016/j.ygyno.2016.11.020 (2017).
Lenczewski, A. et al. Small cell carcinoma of the uterine cervix-an uncommon variant of cervical cancer with neuroendocrine features. Folia Histochem. Cytobiol. 39(Suppl 2), 89–90 (2001).
Qin, J. C. et al. Clinicopathologic analysis of small cell neuroendocrine carcinoma of the cervix. Chin. J. Obstet. Gynecol. 46, 360–363 (2011) (in Chinese).
Rekhi, B. et al. Spectrum of neuroendocrine carcinomas of the uterine cervix, including histopathologic features, terminology, immunohistochemical immunohistochemical profile, and clinical outcomes in a series of 50 cases from a single institution in India. Ann. Diagn. Pathol. 17, 1–9. https://doi.org/10.1016/j.anndiagpath.2012.01.009 (2013).
Sato, Y., Shimamoto, T., Amada, S., Asada, Y. & Hayashi, T. Large cell neuroendocrine carcinoma of the uterine cervix a clinicopathological study of six cases. Int. J. Gynecol. Pathol. 22, 226–230. https://doi.org/10.1097/01.PGP.0000071046.12278.D1 (2003).
Viswanathan, A. N. et al. Small cell neuroendocrine carcinoma of the cervix outcome and patterns of recurrence. Gynecol. Oncol. 93, 27–33. https://doi.org/10.1016/j.ygyno.2003.12.027 (2004).
Wang, Y., Mei, K., Xiang, M. F., Li, J. M. & Xie, R. M. Clinicopathological characteristics and outcome of patients with small cell neuroendocrine carcinoma of the uterine cervix: case series and literature review. Eur. J. Gynaecol. Oncol. 34, 307–310 (2013).
Li, J. D. et al. A clinicopathological aspect of primary small-cell carcinoma of the uterine cervix a single-centre study of 25 cases. J. Clin. Pathol. 64, 1102–1107. https://doi.org/10.1136/jclinpath-2011-200274 (2011).
Sitthinamsuwan, P., Angkathunyakul, N., Chuangsuwanich, T. & Inthasorn, P. Neuroendocrine carcinomas of the uterine cervix a clinicopathological study. J. Med. Assoc. Thai 96, 83–90 (2013).
Li, S. & Zhu, H. Twelve cases of neuroendocrine carcinomas of the uterine cervix cytology, histopathology and discussion of their histogenesis. Acta Cytol. 57, 54–60. https://doi.org/10.1159/000342516 (2013).
Emerson, R. E. et al. Cervical carcinomas with neuroendocrine differentiation: a report of 28 cases with immunohistochemical analysis and molecular genetic evidence of common clonal origin with coexisting squamous and adenocarcinomas. Int. J. Gynecol. Pathol. 35, 372–384. https://doi.org/10.1097/PGP.0000000000000254 (2016).
Stoler, M. H., Mills, S. E., Gersell, D. J. & Walker, A. N. Small-cell neuroendocrine carcinoma of the cervix. A human papillomavirus type 18-associated cancer. Am. J. Surg. Pathol. 15, 28–32. https://doi.org/10.1097/00000478-199101000-00003 (1991).
Straughn, J. M., Richter, H. E., Conner, M. G., Meleth, S. & Barnes, M. N. Predictors of outcome in small cell carcinoma of the cervix-a case series. Gynecol. Oncol. 83, 216–220. https://doi.org/10.1006/gyno.2001.6385 (2001).
Xing, D. et al. Next-generation sequencing reveals recurrent somatic mutations in small cell neuroendocrine carcinoma of the uterine cervix. Am. J. Surg. Pathol. 42, 750–760. https://doi.org/10.1097/PAS.0000000000001042 (2018).
Inoue, M., Ueda, G. & Nakajima, T. Immunohistochemical demonstration of neuron-specific enolase in gynecologic malignant tumors. Cancer 55, 1686–1690 (1985).
Sheridan, E., Lorigan, P. C., Goepel, J., Radstone, D. J. & Coleman, R. E. Small cell carcinoma of the cervix. Clin. Oncol. 8, 102–105 (1996).
Fujii, S. et al. Small cell undifferentiated carcinoma of the uterine cervix: histology, ultrastructure, and immunohistochemistry of two cases. Ultrastruct. Pathol. 10, 337–346. https://doi.org/10.3109/01913128609064198 (1986).
Chen, C. A. et al. Serum neuronspecific enolase levels in patients with small cell carcinoma of the uterine cervix. J. Formos. Med. Assoc. 93, 81–83 (1994).
van Nagell, J. R. et al. Small cell carcinoma of the uterine cervix. Cancer 62, 1586–1593 (1988).
Ueda, G. et al. An immunohistochemical study of small-cell and poorly differentiated carcinomas of the cervix using neuroendocrine markers. Gynecol. Oncol. 34, 164–169. https://doi.org/10.1016/0090-8258(89)90134-0 (1989).
Hu, G. M. et al. Cervical small cell carcinoma: a clinicopathological study of 35 cases. Chin. J. Clin. Exp. Pathol. 34(5), 517–521. https://doi.org/10.13315/j.cnki.cjcep.2018.05.010 (2018) (in Chinese).
Han, C. & Kong, W. M. Small-cell neuroendocrine carcinoma of the cervix: a clinical study of 18 cases. Chin. J. Clin. 46(7), 850–853. https://doi.org/10.3969/j.issn.2095-8552.2018.07.032 (2018) (in Chinese).
Yang, D. P., Huang, L. F., Song, J. H. & Liang, J. Y. Small cell carcinoma of the cervix: a clinicopathological study of 18 cases. Beijing Med. J. 40(12), 1171–1174. https://doi.org/10.15932/j.0253-9713.2018.12.019 (2018) (in Chinese).
Zeng, S. et al. Small cell carcinoma of the uterine cervix: a clinical study of 8 cases and literature review. Guizhou Med. J. 42(9), 1121–1123 (2018) (in Chinese).
Zhi, L. R. et al. Small-cell carcinoma of the cervix: a clinicopathological study of 10 cases. Shaanxi Med. J. 47(02), 259–261. https://doi.org/10.3969/j.issn.1000-7377.2018.02.039 (2018) (in Chinese).
Wang, G. Y., Li, Q., Qian, L. X., Du, M. Y. & Jia, X. M. Clinicopathological characteristics and prognosis of 26 cases with cervical small cell carcinoma. J. Chin. Oncol. 25(6), 541–546. https://doi.org/10.11735/j.issn.1671-170X.2019.06.B010 (2019) (in Chinese).
Tong, Y., Xie, Q. M., Wu, Q. & Long, Z. L. Clinicopathological analysis of small-cell carcinoma of the cervix. Guizhou Med. J. 42(3), 342–343 (2018) (in Chinese).
Wang, R. R. Clinicopathological analysis of small-cell carcinoma of the cervix. Health Guide 18, 251 (2018) ((in Chinese)).
Li, R. G. et al. Analysis on clinicopathological characteristics and prognosis of small cell carcinoma of the cervix in 26 cases. China Mod. Med. 9, 107–110 (2015) (in Chinese).
Morgan, S., Slodkowska, E., Parra-Herran, C. & Mirkovic, J. PD-L1, RB1 and mismatch repair protein immunohistochemical expression in neuroendocrine carcinoma, small cell type, of the uterine cervix. Histopathology 74(7), 997–1004. https://doi.org/10.1111/his.13825 (2019).
Liu, H. et al. Differential expression of neuroendocrine markers, TTF-1, p53, and Ki-67 in cervicaland pulmonary small cell carcinoma. Medicine (Baltimore) 97(30), e11604. https://doi.org/10.1097/MD.0000000000011604 (2018).
Ning, L. et al. Prognostic factors of FIGO stage I-IIA small-cell neuroendocrine carcinoma of the uterine cervix. Int. J. Gynaecol. Obstet. 142(3), 374–375. https://doi.org/10.1002/ijgo.12544 (2018).
Jain, E., Sharma, S., Kini, L., Dewan, A. & Dhakal, H. P. Small cell carcinoma of the cervix: a retrospective clinicopathologic analysis over a period of 3 years in a referral laboratory in India. Indian J. Gynecol. Oncol. 17, 90. https://doi.org/10.1007/s40944-019-0339-5 (2019).
The Joanna Briggs Institute. Joanna Briggs institute reviewers manual: 2016 edition[M].
Wagenaar, S. S. New WHO-classification of lung and pleural tumors. Ned Tijdschr Geneeskd 143, 984–990 (1999).
Farinola, M. A., Weir, E. G. & Ali, S. Z. CD56 expression of neuroendocrine neoplasms on immunophenotyping by flow cytometry: a novel diagnostic approach to fine-needle aspiration biopsy. Cancer 99, 240–246. https://doi.org/10.1002/cncr.11458 (2003).
Xu, F. et al. Clinicopathological aspects of small cell neuroendocrine carcinoma of the uterine cervix: a multicenter retrospective study and meta-analysis. Cell Physiol. Biochem. 50, 1113–1122. https://doi.org/10.1159/000494538 (2018).
McCann, G. A. et al. Neuroendocrine carcinoma of the uterine cervix: the role of multimodality therapy in early-stage disease. Gynecol. Oncol. 129, 135–139. https://doi.org/10.1016/j.ygyno.2013.01.014 (2013).
Wang, P. H. et al. Small cell carcinoma of the cervix: analysis of clinical and pathologic findings. Eur. J. Gynaecol. Oncol. 19(2), 189–192 (1998).
Gersell, D. J. et al. Small-cell undifferentiated carcinoma of the cervix. Am. J. Surg. Pathol. 12(9), 684–698 (1988).
Acknowledgements
This work was supported by the National Natural Science Foundation Project of China (81771546), Hunan Provincial Department of Finance and Education Project (2018) No. 33: (2050205-30299-50502) and Hunan Science and Technology Innovation Project (2018SK52508) at the Second Xiangya Hospital of Central South University, Changsha, Hunan, China.
Author information
Authors and Affiliations
Contributions
R.H. and C.F. developed the project. R.H., L.Y. and Q.L. collected the data of included studies. R.H. and H.Y. analysed the data using related software. R.H., C.Z. and C.F. wrote the main manuscript text. C.Z., X.Y., S.S., Z.R., L.S., Z.Z., H.C. reviewed and editored the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, R., Yu, L., Zheng, C. et al. Diagnostic value of four neuroendocrine markers in small cell neuroendocrine carcinomas of the cervix: a meta-analysis. Sci Rep 10, 14975 (2020). https://doi.org/10.1038/s41598-020-72055-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72055-x
This article is cited by
-
Clinical value of combined serum CA125, NSE and 24-hour urine VMA for the prediction of recurrence in children with neuroblastoma
Italian Journal of Pediatrics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.