Abstract
Breast cancers with neuroendocrine (NE) differentiation are very heterogeneous, comprising broadly cancers that are morphologically similar to NE tumors (NET) of other anatomic sites, infiltrating breast carcinomas, no special type (IBC-NST) and other special subtypes with NE morphology and/or NE markers expression. Depending on the classification schemes, they are variably included into “NE breast cancers”. The latest WHO classification harmonized NE breast cancers with NE neoplasms (NEN) of other organ systems, defined NEN into well-differentiated NET (low Nottingham grade) and poorly-differentiated NE carcinoma (NEC) (high Nottingham grade). Other IBC with NE differentiation are diagnosed based on solely the non-NEN component. Due to the changes in diagnostic criteria, variable results were obtained in the previous studies on NE breast cancers. Hence, the clinical value of NE differentiation in breast cancers is not well investigated and understood. In this review, the current understanding in the pathogenesis, clinical, prognostic, immunhistochemical, and molecular features of “NE breast cancers” is summarized. Controversial issues in their diagnosis and classification are also discussed.
Similar content being viewed by others
Introduction
Neuroendocrine (NE) differentiation in cancers, conceptually, is defined as the presence of neurosecretory granules in neoplastic cells, resembling synaptic vesicles. Histologically, they are characterized by architectural patterns and cytologic features reminiscent of nonneoplastic NE cells (coarsely stippled nuclear chromatin without prominent nucleoli, granular cytoplasm, and nesting or trabecular growth pattern) and expression of NE markers. Breast cancers with NE differentiation are heterogeneous, comprising a broad spectrum that includes not only cancers morphologically similar to NE tumors (NET) of the lung and gastrointestinal tract (GI), but also infiltrating breast carcinomas, no special type (IBC-NST) and other special subtypes (solid papillary carcinomas (SPC) and mucinous carcinomas type B (MC type B) [1]), all of which show variable morphologic NE features and/or NE markers expression. Depending on the classification scheme [2,3,4], these different cancers have been loosely classified as “NE breast cancers”. Breast cancers showing NE morphology and NE markers expression are very rare while IBC-NST showing only NE markers expression without NE morphology are more common. However, the precise incidence, particularly for the latter group, is not certain as immunohistochemical (IHC) staining for NE markers is not performed routinely, leading to potential underestimation. Also, with the inconsistent findings due to the different diagnostic criteria, to date, there is no consensus on the clinical significance of NE differentiation in breast cancers. Therefore, the clinical relevance for identifying NE breast cancers is still a matter of debate. The latest WHO classification has aligned the classification of NE breast cancers with NE neoplasms (NEN) in other organs, providing a more detailed guideline on their classification [2]. In this review, the current understanding in the pathogenesis, clinical, prognostic, IHC, and molecular features of “NE breast cancers” is summarized.
WHO classification
NE breast cancers were first recognized as a separate entity in the 3rd Edition of the WHO classification (2003) (Table 1) [4]. They were defined by morphologic NE features similar to those of NET of GI/lung and expression of NE markers (chromogranin (CG) and/or synaptophysin (SYN)) in more than 50% of the cell populations. Based on morphologic features, the cancers were further classified into solid NE carcinomas (NEC), small cell/oat cell carcinomas and large cell carcinomas. Some solid NEC overlapped with SPC and alveolar pattern of invasive lobular carcinoma (ILC). The small cell and large cell subtypes were morphologically similar to their counterparts in the lung. IBC-NST with only focal NE features, revealed only by IHC NE markers expression, were excluded from this classification. In the 4th edition (2012) (Table 1) [3], NE breast cancers were termed as “carcinomas with NE features” and the 50% NE markers cutoff was removed. Carcinomas with NE features were defined by morphologic features similar to NE cancers of lung/GI and NE markers expression of any degree. Three subtypes were classified, including NET, well-differentiated; NEC, poorly-differentiated/small cell carcinomas; and invasive carcinomas with NE differentiation. The former two groups displayed similar morphologic features as their counterparts in the lung and GI. In this classification there was no clear criterion to differentiate NET from low Nottingham histologic grade IBC with NE differentiation; the described histologic features of poorly-differentiated NEC classification was mainly based on SmCC, but not large cell NEC (LCNEC); and the invasive carcinomas of NE differentiation included special subtypes (mostly MC type B and SPC) and IBC-NST with some degree of NE differentiation.
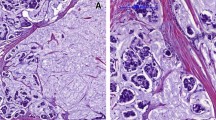
The recent WHO classification (2019) has harmonized the nomenclature for different organ systems with a unified classification of NEN aiming to reduce the inconsistencies and contradictions in their nomenclature, classifications, criteria for histologic grading and staging [2] (Fig. 1). Under this framework, the term NEN was used to encompass all tumor classes with predominantly NE differentiation (presence of histologic NE features in >90% of the tumor), and NEN were divided into well and poorly differentiated (Fig. 2A–F). NET correspond to well-differentiated NEN, while NEC correspond to poorly-differentiated NEN. NET and NEC of breast are characterized by diffuse NE marker expression and respectively low/intermediate or high Nottingham histologic grade NE morphology [2]. However, NEN grading, which encompassed mitotic count, Ki67 index and necrosis, as applied in other organs, was not advocated in breast as there was no evidence of its prognostic significance. Tumor stage and Nottingham grading remain the main prognostic parameters in breast cancer, including breast NEN. IBC-NST with NE differentiation are diagnosed when the tumors do not demonstrate histologic NE features and NE marker expression is not distinctive, uniform or extensive enough to allow classification as NEN. The diagnostic labels are based principally on the non-NEN components and the extent of NE features; when there are 10–90% tumor cells showing NE features, they are called mixed IBC-NST (or other special subtype) and NEC/NET; and when there are <10% tumor cells showing NE features, they are called IBC-NST (or other special subtypes) with an optional comment on the focal NE pattern (Fig. 2G, H). Furthermore, SPC and MC type B are classified under papillary and mucinous histotypes and are excluded from the NEN categorization.
A H&E of Nottingham histologic grade 1 NET (×200); B H&E of Nottingham histologic grade 2 NET (×200); C H&E of small cell carcinoma of breast, and D corresponding synaptophysin staining (×100); E H&E of Large cell carcinoma of the breast and F corresponding synaptophysin staining (×400); G H&E of Invasive ductal carcinoma of NE differentiation and H corresponding synaptophysin staining (×100).
Clinical features
The clinical presentation of NE breast cancers is similar to IBC-NST. Clinical syndromes related to specific hormone production in NE breast cancers have not been reported [2]. Many studies using WHO 2003 criteria showed that NET affected mostly patients with older age (Table 2) [5,6,7,8,9,10]. Small cell NEC (SmCNEC) have been reported to be more advanced at diagnosis, with a reported distant metastatic rate of 19–30% [11, 12].
Histopathology
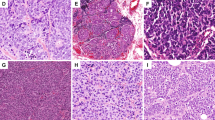
Basing on the current WHO classification (2019) [2], NET are low/intermediate Nottingham histologic grade invasive tumors with NE morphology, supported by the presence of NE granules and diffuse NE markers expression (Fig. 2A, B). Morphologically, the tumors consist of densely cellular solid nests and trabeculae of tumor cells, which may be spindled, plasmacytoid, polygonal with eosinophilic and granular or clear cytoplasm separated by delicate fibrovascular stroma (Fig. 3). The classic features of NET in lung/GI such as ribbons, cords and rosettes are not prominent NE features in breast; instead, papillary or insular patterns and alveolar like structures may be seen [13, 14]. In breast, NET constitute most NEN [15], and account for <1 to 3.7% of all breast cancers [16, 17]. They show high ER/PR level and CK7 positivity, but HER2 negativity and low Ki67 [13]. They are also mostly GATA3 positive [18, 19].
A Spindled tumor cells in ductal carcinoma-in situ (×200). B Plasmacytoid tumor cells in Nottingham histologic grade 1 NET (×400). C Tumor cells with polygonal with eosinophilic and granular cytoplasm in Nottingham histologic 1 NET (×400). D Tumor cells with large and clear cytoplasm in Nottingham histologic grade 2 NET (×400).
NEC are defined by high Nottingham histologic grade NE morphology and diffuse NE markers expression, and include SmCNEC and LCNEC. Both SmCNEC and LCNEC are very rare, with SmCNEC accounting for ~0.1% of all breast cancers [11]; LCNEC are even rarer and usually reported as single cases. The histologic and IHC profiles are sometimes indistinguishable from their lung counterparts. SmCNEC show an infiltrative growth pattern and are composed of densely packed, fairly uniform, small dark hyperchromatic nuclei with a high N:C ratio, nuclear molding, scant cytoplasm, inconspicuous nucleoli and poorly defined cytoplasmic border. Mitotic count is high with apoptosis, and there may be areas of necrosis (Fig. 2C, D). ER and PR are expressed in 30–50% of cases [20, 21]; BCL2 is frequently expressed but not HER2 [21]. TTF-1 expression in breast SmCNEC has been reported only very rarely, thus TTF-1 is still useful to differentiate a metastatic SmCC of lung origin from a primary breast origin [22,23,24,25]. The tumor cells in LCNEC possess highly pleomorphic nuclei with coarse chromatin and moderate amount of cytoplasm (Fig. 2E, F). Diagnosing LCNEC can be challenging, more so than in lung or GI, as they may resemble high Nottingham histologic grade IBC-NST in H&E sections, and hence IHC staining for NE markers might not been performed, leading to significant underreporting. Indeed, to date, only less than ten LCNEC cases have been reported in the literature and their diagnoses were mainly hinged on NE markers expression [26].
As NEN are rare in breast, before a primary NEN diagnosis is made, it is prudent to exclude the possibility of metastases. Conversely, metastatic well-differentiated NEN of the breast involving visceral organs can mimic primary visceral NEN [27]. Careful clinical and radiographic evaluations are helpful; the presence of accompanying in situ carcinoma, axillary node metastases, an absence of history of extra-mammary NEN and positive reactivity for breast markers (GATA3, GCDFP15, and Mammaglobin) support a breast origin. Negativity for pulmonary (TTF-1), GI (CDX-2), or pancreatic (PDX-1) markers may provide further support of primary mammary NEN.
In breast, NE markers expression is not unique or limited to NEN. Some special subtypes particularly MC type B and SPC are known to express NE markers, which were reported in 20% and 70% of cases respectively [28]. Less commonly, ILC and IBC with medullary pattern may also express NE markers [5, 6, 28]. Up to 10–20% of IBC-NST may express NE markers but they lack the typical NE morphology [6]. Compared to NET, these cancers were of higher Nottingham histologic grade, demonstrated basal CK expression, lower ER and PR positivity, higher HER2 positivity, and lower NE markers expression. Compared to IBC without NE differentiation, these cancers were of lower Nottingham histologic grade and higher ER positivity, but more tumor lymphovascular invasion was observed. Thus their biologic profile appeared to be intermediate between NET and IBC without NE differentiation [17].
Neuroendocrine markers
Historically, histochemical argyrophilic staining and electron microscopic demonstration of neurosecretory granules in tumor cells was the gold standard for identification of NE differentiation. However, the argyrophilic granules reflected merely a secretory feature rather than specifically neurosecretory granules [29, 30]. Now IHC staining for NE markers (mostly CG (usually CG-A) and SYN) is universally adopted for NE differentiation. CG is a glycoprotein secreted by neurons and NE cells and is sequestered in secretory granules. SYN is a membrane protein of small vesicles present in the presynaptic vesicles in nerve terminals and NE cells. The expression level of CG depends on the number of secretory granules [31]; therefore, poorly-differentiated NEC may show only focal CG staining [18]. In comparison, SYN has a higher sensitivity but is not as specific as CG (SYN expression has been reported in adrenal cortical adenoma and carcinoma). In NE breast cancers, CG and SYN showed an overall expression rates of 30–72% and 74–100% respectively [16,17,18, 28]. It should be noted that breast tumors with morphology suggestive of NE differentiation may not always demonstrate CG/SYN staining. The lack of immunoreactivity could be caused by the rapid release or degradation of these antigens as high mRNA levels had been demonstrated in some of the IHC negative cases [32]. Similar phenomenon has been observed in SmCC of lung—up to two-third could be negative for CG and SYN [33]. Thus additional NE markers for diagnosis are needed.
CD56 (also known as neural cell adhesion molecule, NCAM) and neuron-specific enolase (NSE) has also been used as NE markers. However, their reliability in breast is questionable. CD56 is a typical adhesion molecule of neuronal cells and a NE marker particularly useful for SmCC of lung [34]. However, it is also expressed in non-NE tissues such as renal tubules and thyroid follicular cells [35]. In IBC, CD56 expression did not correlate with CG and SYN [17, 36]; moreover, IBC expressing only CD56 (but not CG or SYN) showed a biologic profile different from most other NEN. The CD56 positive only IBC are mostly non luminal, and expressed high level of basal markers [17]. Thus it is uncertain whether the biologic behavior of CD56 positive only IBC will be the same as the conventional NEN, casting doubt to the validity of using CD56 as the sole marker for NE differentiation in IBC.
NSE, a glycolytic enzyme, is present in different isoforms in all cells. The enolase has a dimeric structure formed by three different subunits: α, β, and γ. Only the γγ isoform is specific for NE differentiation and neuronal cells [35]. Given its localization in cytosol rather than neurosecretory granules, it also stains dedifferentiated and degranulated NET. NSE expression was observed in majority of NET [36]. However, antibody against NSE could not discriminate γγ isoform from others, thus it frequently cross-reacts to non-NE cells (such as smooth muscle cells, myoepithelial cells, and lymphocytes); hence NSE has now fallen out of favor and is not recommended for diagnostic application.
Recently, Insulinoma-associated Protein 1 (INSM1) has been suggested as a superior NE marker. INSM1 is a zinc finger transcriptional factor crucial for NE differentiation and drives the transcription of other NE molecules (like CG, SYN, and CD56) during NE tissues development [37]. Its expression is highly restricted to nuclei of NE cells and tissues [38]. High INSM1 positivity has been found in NE cancers of various sites including lung, GIT, pancreas, genitourinary tract, and the head and neck region [38,39,40,41,42,43,44,45]. Importantly, it detected 75% of SmCC of lung that were negative for other NE markers [45]. In a large consecutive series of IBC, INSM1 showed positive association with SYN and CG expression and 35% of SYN/CG expressing IBC co-expressed INSM1 [46]. Another smaller series showed that INSM1 was expressed in five out of seven IBC with NE differentiation [47]. Human achaete-scute homolog-1 (hASH-1) and doublecortin-like kinase 1 (DCLK1) are investigative NE markers. hASH-1, a transcriptional factor involved in mammalian neural development, was expressed in 63 and 38% of IBC with high and low NE differentiation, respectively. It was found to be restricted to tumor cells having low proliferative potential [48]. DCKL1, a microtubule-associated protein that regulates neuronal development, was found to be expressed in 65% IBC with NE differentiation defined by SYN/CG expression [5]. As experiences with these novel markers in breast were limited, further works need to be done to evaluate the diagnostic value, especially their role in identifying NE differentiation in IBC not expressing conventional NE markers (CG, SYN).
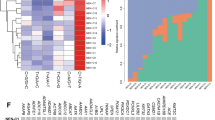
In routine practice these NE markers are frequently used in combination to identify NE differentiation. In a large TMA series, we found 239/1217 (19.6%) of IBC-NST expressed at least one NE markers, in descending order SYN (60.7%), INSM1 (36.4%), CD56 (36.4%), and CG (24.2%) (Fig. 4). Using both SYN and CG as NE markers showed only a slightly higher sensitivity (64.9%) than SYN alone; thus SYN was the best single NE marker. Using INSM1 will detect an additional 15.0% of NE cancers; and using CD56 could detect another 20% of cancers with NE differentiation. However, as CD56 only positive IBC may have a different biologic make up, and until further information is available, this group is best considered separately from the usual NE breast cancers.
The expression of NE markers (Synpatophysin (SYN), Chromogranin (CG), Insulinoma-associated protein 1 (INSM1) and CD56) on a large local cohort of IBC-NST (N = 1217) were analysed by immunohistochemistry on tissue microarray. A cutoff of 1% was applied to define positivity. In total, 239 cases expressed either one of the four markers (NE marker positive case). The percentage in the rectangle represented the expression rate of each marker in the NE marker positive cases. The embedded table showed the expression rate of different NE marker combinations in all IBC-NST or in cases with at least NE marker positivity.
Molecular features
The molecular features of NE breast cancers are yet to be fully deciphered. Most studies usually involved few cases, and comparison of results was difficult as the precise definition, diagnostic criteria and composition of NE breast cancers were not uniform; some emphasizing on extensive morphologic NE features and others on diffuse NE markers expression as diagnostic criteria. A more consistent finding was NE breast cancers were of mostly luminal subtypes by transcriptional profiling [18, 49, 50]; majority of poorly differentiated, NEC were luminal B, whereas the well differentiated, NET were about equally split into luminal A and B subtypes [18]. NE breast cancers showed downregulation of genes associated with connective tissue/extracellular matrix compared to IBC-NST [49]. Their gene expression was distinct but clustered with MC, particularly MC type B which frequently display NE features [49, 50], supporting NE breast cancers as a discrete molecular subtype distinct from IBC-NST and sharing a common profile with MC type B. NE breast cancers also demonstrated different mutational profiles from other ER+HER2− breast cancers, having a lower proportion of PIK3CA but a higher mutation rate in other genes (e.g., ARID1A). A 254 genes targeted sequencing analysis of 15 NE breast cancers (defined by WHO 2003 classification, but without SmCNEC in the cohort) demonstrated high frequency mutations in GATA3, FOXA1, TBX3, and ARID1A (17%), followed by mutations in PIK3CA, AKT1, and CDH1 (11%), but not TP53 mutations [51]. The repertoire of alterations shared some similarities with MC, ILC as well as NET of other sites. Similar to MC [52,53,54], NE breast cancers harbored lower frequency of PIK3CA mutations and concurrent 1q gains/16q losses. They also had frequent mutation in chromatin-remodeling gene, ARID1A, which are common in lung carcinoids [55]. A small series reported NE breast cancers were enriched with FOXA1 and TBX3 mutations and reduced E-cadherin expression, sharing the same genomic profile as ILC [56]. Another 15 cases (excluding papillary and MC) study demonstrated recurrent mutations in FGFR family members (13.3%) and PIK3CA (20%) while KDR and HRAS mutations were found only in single incident [57]. Separately in a larger study of 43 NE breast cancers of various histologic types, TP53 and PIK3CA mutations were found in 3/43 (7%) cases for both. Interestingly most (2/3) TP53 mutations were found in the poorly-differentiated carcinomas while all (3/3) PIK3CA mutations were found in the well-differentiated carcinomas [18]. A similar high frequency (75%) of TP53 mutations has been reported in a series of SmCNEC of breast, in addition to PIK3CA mutations (38%) [20]. The presence of PIK3CA mutations in NE breast cancers corroborated with their association with luminal cancers. A high rate of TP53 mutations particularly found in SmCNEC/poorly-differentiated NE cancers echoed the findings of its frequent mutations in high Nottingham histologic grade breast cancers. The results also echoed the findings in SmCC of lung, which showed more frequent TP53 alterations (>75%) than lung carcinoids (NET) (10%) [58, 59]. Thus at a molecular level, the poorly-differentiated NEC are distinct genetically from NET and may arise through different molecular mechanisms. Even though they are grouped together as part of the same spectrum under the umbrella of NEN, they are distinct entities. To date there is still a large gap in our knowledge in NE breast cancers, as these cases (particularly SmCNEC and LCNEC) are very rare; and only few studies focused on the under-recognized IBC-NST with NE features.
Prognosis
As of now, there is no consensus on the prognostic significance of NE features in breast cancers. Published studies showed mixed results (Table 2). This apparent discrepancy could be attributed to the relatively small cohorts, the different diagnostic criteria applied for NE differentiation and the heterogeneity within NE breast cancers. Notably, SPC or MC type B were excluded as NE breast cancers only in the most recent WHO 2019 classification, but not in the previous classifications. As these tumors were associated with more indolent clinical behavior and longer survival time [13], their inclusion could mask the findings in the prognostic analysis. Most recent publications demonstrated adverse outcomes for NE breast cancers [5, 7,8,9,10, 16,17,18, 28], however most of these reported studies considered NE breast cancers as a whole without stratification into different subtypes (as stipulated in the WHO classification). Thus information on the prognostic implication of NE features in breast cancers is insufficient to be conclusive. Among all NE breast cancers, SmCNEC showed the worst prognosis. Based on SEER data, the reported 5-year OS and DFS rates for SmCNEC were 50.5 and 32.2%, compared to the respective rates of 62.4 and 74.0% in NET and 68.9 and 73.3% in IBC-NST with NE differentiation [15]. Nevertheless, in interpreting these results, one has to remember that the study was a registry-based analysis. Therefore, there could be inaccuracies in coding and abstracting. An interesting series comparing SmCC from breast and lungs found better outcome for the former [21].
The more favorable outcome of NET compared to SmCNEC in breast is partly attributed to their lower histologic Nottingham histologic grade. Although in other anatomic sites, well-differentiated NET had much better long term outcome compared to non-NE cancers, so far there was no data supporting similar conclusion in breast. One study using WHO 2019 criteria for NEN showed no survival difference between NET and other IBC with no NE differentiation [17]. Among NEN, similar to IBC, Nottingham histologic grading and staging are prognostically relevant [18, 60]. Ki67 proliferative index, although useful in NE cancers of other sites, is not prognostically useful in NE breast cancers [7, 60].
Prognosis is most uncertain for IBC-NST with NE differentiation. One reason is that this subgroup is quite heterogeneous as it includes cases of variable NE marker expression and level of NE morphologic differentiation. Many studies demonstrated worse outcome in NE breast cancers (defined by NE markers expression) might have possibly included many cases of IBC-NST with NE differentiation.
Whether or not the expression level of NE markers has any prognostic implication is controversial. Focal (compared to diffuse) NE markers expression, in particular CG, appeared to be an adverse outcome indicator [17, 61]. Others studies using a higher NE marker cutoff or TMA analysis reported no survival differences [16, 28], but their case selection might under-report the very low expression cases. It is interesting to note that SYN/CG+ breast cancers co-expressing additional NE markers, namely DCLK1 and INSM1, demonstrated a better survival [5, 46]. Taken together, it appears that higher level of NE differentiation may be associated with a better prognosis. Among the individual markers, focal CG expression, but not other NE markers, was found to be an independent unfavorable prognostic indicator [17]. As additional comprehensive studies are lacking, these observations were preliminary but interesting.
At this juncture, it may be reasonable to suggest that in diagnosing NEN, it is prudent to differentiate between NET and NEC, and note the expression level of NE markers; using additional markers (e.g., INSM1) may further stratify patients into different prognostic groups. With the standardization of the diagnostic criteria and classification, future studies are more likely to demonstrate more robust prognostic information in NE breast cancers.
Treatment
Currently, there is a lack of clinical trials specifically on NE breast cancers, and surgery remains the mainstay of treatment, with supplementary hormonal therapy. Chemotherapy is reserved for those with high risk of recurrence. The choice of treatment for NE breast cancers is based on the main prognostic factors (including TNM stage, Nottingham histologic grade, ER, PR and HER2 status) as for the other types of breast cancer. NE breast cancers appear to have a worse prognosis than other breast cancers. Data on treatment response of NE breast cancers are scarce. A trend of better outcome for NE breast cancers (basing on WHO 2003 classification) receiving hormonal therapy and radiotherapy has been demonstrated, but an opposite trend for chemotherapy has been shown in one report [9]. However, the data did not reach statistical significance. Recent data suggested the contribution of ARID1A mutation to endocrine resistance in luminal breast cancers [62]. This finding added evidence to the poor prognosis of NE breast cancers which showed more frequent ARID1A mutations and highlighted the requirement for refinement in NE cancer treatment. More specific approaches may be tailored for these breast cancers. Under current classification, NET and NEC are both classified under NEN; yet they are of different biology and Nottingham histologic grade; thus requiring different treatment strategies. For SmCNEC, some evidence suggested they may response to hormonal therapy [63] but there is no improvement in their survival after radiotherapy [11]. In the metastatic SmCNEC setting, regimens for SmCC of lung are usually attempted, but the outcome was still dismal.
The information on molecular analysis helps to identify novel potential treatment targets. Several candidates deserve specific investigations. PIK3CA mutations were commonly reported alterations in NE breast cancers, suggesting potential therapeutic target. Inactivation of ARID1A, another reported alteration in NE breast cancers, could sensitize cancer cells to AKT1-mTOR pathway inhibitors [64]. The efficacies of inhibitors targeting this pathway have been demonstrated in breast cancers [65] and pancreatic NET [66]. It may be a worthwhile therapeutic option to explore. The CDK4/6 inhibitors have been actively investigated in breast cancers. Results from a phase II clinical trial showed that over half of patients with PIK3CA-mutated HR+HER2− advanced breast cancers showed no disease progression for over 6 months after treatment with CDK4/6 inhibitor together with aromatase inhibitor [67]. Others reported dramatic response to CDK4/6 inhibitor in a single case of refractory poorly-differentiated NEC [68]. CDK4/6 inhibitors could be another possible treatment option in NE breast cancers.
Future perspective
Due to the changes in diagnostic criteria and the variable results, the clinical value of NE differentiation in IBC has not been well investigated and understood. Current management of NE breast cancers did not differ from conventional IBC and routine NE marker analysis is not recommended. However, recent findings generally supported a poor prognosis for NE breast cancers. The latest WHO classification (2019) provides better defined diagnostic criteria, in line with NEN of other organ systems, and this may refine the prognostic value of NE differentiation. IBC-NST with NE differentiation (NE marker expression but lacking NE morphology) would likely be under-diagnosed in the usual clinical settings. However, this group seemed to have poorer clinical outcomes. Judicious use of NE markers is essential. Although SYN and CG are the most commonly used, there are still some missing cases when used alone; other novel complementary NE markers will be required, and INSM1 seems to hold promise. Molecular analysis on NE breast cancers, due to their rarity, frequently included assorted cases with different histologies, including NET, NEC, SPC, ILC, MC, and IBC-NST. Given the limited cohort size in these reported studies, their findings could be biased. The true molecular features of different NE subgroups still need to be defined. Another issue is that NET and NEC are put together under NEN in the current classification. Evidence from NEN of other anatomic sites and available data obtained from breast cancers suggested that they may behave differently and develop via different pathogenic pathways. Although they are put commonly under NEN category, one should be aware of their differences in biology and prognosis.
References
Capella C, Eusebi V, Mann B, Azzopardi JG. Endocrine differentiation in mucoid carcinoma of the breast. Histopathology. 1980;4:613–30.
WHO classification of tumors editorial board editor. WHO classification of tumors of the breast. Lyon: IARC 2019.
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. World Health Organisation classification of tumors of the Breast. 4th ed. Lyon: IARC Press; 2012.
Tavassoli FA, Devilee P. World Health Organisation classification of tumors. Pathology and genetics of tumors of the breast and female genital organs. London: IARC Press; 2003.
Liu YH, Tsang JYS, Ni YB, Hlaing T, Chan SK, Chan KF, et al. Doublecortin-like kinase 1 expression associates with breast cancer with neuroendocrine differentiation. Oncotarget. 2016;7:1464–76.
Miremadi A, Pinder SE, Lee AH, Bell JA, Paish EC, Wencyk P, et al. Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology. 2002;40:215–22.
Roininen N, Takala S, Haapasaari KM, Jukkola-Vuorinen A, Mattson J, Heikkila P, et al. Primary neuroendocrine breast carcinomas are associated with poor local control despite favourable biological profile: a retrospective clinical study. BMC Cancer. 2017;17:72. https://doi.org/10.1186/s12885-017-3056-4.
Wang J, Wei B, Albarracin CT, Hu J, Abraham SC, Wu Y. Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer. 2014;14:147.
Wei B, Ding T, Xing Y, Wei W, Tian Z, Tang F, et al. Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer. 2010;116:4463–73.
Zhang Y, Chen Z, Bao Y, Du Z, Li Q, Zhao Y, et al. Invasive neuroendocrine carcinoma of the breast: a prognostic research of 107 Chinese patients. Neoplasma. 2013;60:215–22.
Hare F, Giri S, Patel JK, Hahn A, Martin MG. A population-based analysis of outcomes for small cell carcinoma of the breast by tumor stage and the use of radiation therapy. Springerplus. 2015;4:138.
Wong YN, Jack RH, Mak V, Henrik M, Davies EA. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970-2004. BMC Cancer. 2009;9:209.
Sapino A, Righi L, Cassoni P, Papotti M, Gugliotta P, Bussolati G. Expression of apocrine differentiation markers in neuroendocrine breast carcinomas of aged women. Mod Pathol. 2001;14:768–76.
Tang F, Wei B, Tian Z, Gilcrease MZ, Huo L, Albarracin CT, et al. Invasive mammary carcinoma with neuroendocrine differentiation: histological features and diagnostic challenges. Histopathology. 2011;59:106–15.
Cloyd JM, Yang RL, Allison KH, Norton JA, Hernandez-Boussard T, Wapnir IL. Impact of histological subtype on long-term outcomes of neuroendocrine carcinoma of the breast. Breast Cancer Res Treat. 2014;148:637–44.
Kwon SY, Bae YK, Gu MJ, Choi JE, Kang SH, Lee SJ, et al. Neuroendocrine differentiation correlates with hormone receptor expression and decreased survival in patients with invasive breast carcinoma. Histopathology. 2014;64:647–59.
Lai BS, Tsang JY, Poon IK, Shao Y, Chan SK, Tam FK, et al. The clinical significance of neuroendocrine features in invasive breast carcinomas. Oncologist. 2020;25:e1318–29.
Lavigne M, Menet E, Tille JC, Lae M, Fuhrmann L, Bonneau C, et al. Comprehensive clinical and molecular analyses of neuroendocrine carcinomas of the breast. Mod Pathol. 2018;31:68–82.
Mohanty SK, Kim SA, DeLair DF, Bose S, Laury AR, Chopra S, et al. Comparison of metastatic neuroendocrine neoplasms to the breast and primary invasive mammary carcinomas with neuroendocrine differentiation. Mod Pathol. 2016;29:788–98.
McCullar B, Pandey M, Yaghmour G, Hare F, Patel K, Stein K, et al. Genomic landscape of small cell carcinoma of the breast contrasted to small cell carcinoma of the lung. Breast Cancer Res Treat. 2016;158:195–202.
Shin SJ, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol. 2000;24:1231–8.
Boutrid H, Kassem M, Tozbikian G, Morgan E, White J, Shah M, et al. TTF-1 positive primary small cell carcinoma of the breast: a case report and review of the literature. Front Endocrinol. 2020;11:228.
Christie M, Chin-Lenn L, Watts MM, Tsui AE, Buchanan MR. Primary small cell carcinoma of the breast with TTF-1 and neuroendocrine marker expressing carcinoma in situ. Int J Clin Exp Pathol. 2010;3:629–33.
Ersahin C, Bandyopadhyay S, Bhargava R. Thyroid transcription factor-1 and “basal marker”–expressing small cell carcinoma of the breast. Int J Surg Pathol. 2009;17:368–72.
Ni YB, Tsang JY, Shao MM, Chan SK, Tong J, To KF, et al. TTF-1 expression in breast carcinoma: an unusual but real phenomenon. Histopathology. 2014;64:504–11.
Kawasaki T, Hasebe T, Oiwa M, Sugiyama K, Muramatsu C, Ueda S, et al. Invasive carcinoma with neuroendocrine differentiation of the breast showing triple negative, large and basal cell-like features. Pathol Int. 2019;69:502–4.
Cloutier J, Thompson ED, Cimino-Mathews A, Rooper LM, Matoso A, Argani P. Metastatic breast cancer simulating well-differentiated neuroendocrine neoplasms of visceral organs. Hum Pathol. 2018;82:76–86.
Bogina G, Munari E, Brunelli M, Bortesi L, Marconi M, Sommaggio M, et al. Neuroendocrine differentiation in breast carcinoma: clinicopathological features and outcome. Histopathology. 2016;68:422–32.
Ferguson DJ, Anderson TJ. Distribution of dense core granules in normal, benign and malignant breast tissue. J Pathol. 1985;147:59–65.
McCutcheon J, Walker RA. The significance of argyrophilia in human breast carcinomas. Virchows Arch A Pathol Anat Histopathol. 1987;410:369–74.
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72.
Pagani A, Papotti M, Hofler H, Weiler R, Winkler H, Bussolati G. Chromogranin A and B gene expression in carcinomas of the breast. Correlation of immunocytochemical, immunoblot, and hybridization analyses. Am J Pathol. 1990;136:319–27.
Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol. 2012;25S:18–30.
Zheng G, Ettinger DS, Maleki Z. Utility of the quantitative Ki-67 proliferation index and CD56 together in the cytologic diagnosis of small cell lung carcinoma and other lung neuroendocrine tumors. Acta Cytol. 2013;57:281–90.
Bussolati G, Volante M, Papotti M. Classic and recent special stains used in differential diagnosis of endocrine tumors. Endocr Pathol. 2001;12:379–87.
Mjones P, Sagatun L, Nordrum IS, Waldum HL. Neuron-specific enolase as an immunohistochemical marker is better than its reputation. J Histochem Cytochem. 2017;65:687–703.
Fujino K, Motooka Y, Hassan WA, Ali Abdalla MO, Sato Y, Kudoh S, et al. Insulinoma-associated protein 1 is a crucial regulator of neuroendocrine differentiation in lung cancer. Am J Pathol. 2015;185:3164–77.
Rosenbaum JN, Guo Z, Baus RM, Werner H, Rehrauer WM, Lloyd RV. INSM1: a novel immunohistochemical and molecular marker for neuroendocrine and neuroepithelial neoplasms. Am J Clin Pathol. 2015;144:579–91.
Chen JF, Yang C, Sun Y, Cao D. Expression of novel neuroendocrine marker insulinoma-associated protein 1 (INSM1) in genitourinary high-grade neuroendocrine carcinomas: An immunohistochemical study with specificity analysis and comparison to chromogranin, synaptophysin, and CD56. Pathol Res Pract. 2020;216:152993.
Gonzalez I, Lu HC, Sninsky J, Yang C, Bishnupuri K, Dieckgraefe B, et al. Insulinoma-associated protein 1 expression in primary and metastatic neuroendocrine neoplasms of the gastrointestinal and pancreaticobiliary tracts. Histopathology. 2019;75:568–77.
Kim D, Viswanathan K, Goyal A, Rao R. Insulinoma-associated protein 1 (INSM1) is a robust marker for identifying and grading pancreatic neuroendocrine tumors. Cancer Cytopathol. 2020;128:269–77.
McHugh KE, Mukhopadhyay S, Doxtader EE, Lanigan C, Allende DS. INSM1 is a highly specific marker of neuroendocrine differentiation in primary neoplasms of the gastrointestinal tract, appendix, and pancreas. Am J Clin Pathol. 2020;153:811–20.
Mukhopadhyay S, Dermawan JK, Lanigan CP, Farver CF. Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: an immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod Pathol. 2019;32:100–9.
Rooper LM, Bishop JA, Westra WH. INSM1 is a sensitive and specific marker of neuroendocrine differentiation in head and neck tumors. Am J Surg Pathol. 2018;42:665–71.
Sakakibara R, Kobayashi M, Takahashi N, Inamura K, Ninomiya H, Wakejima R, et al. Insulinoma-associated Protein 1 (INSM1) is a better marker for the diagnosis and prognosis estimation of small cell lung carcinoma than neuroendocrine phenotype markers such as chromogranin A, synaptophysin, and CD56. Am J Surg Pathol. 2020;44:757–64.
Razvi H, Tsang JY, Poon IK, Chan SK, Cheung SY, Shea KH, et al. INSM1 is a novel neuroendocrine marker of good prognosis in luminal B breast cancer. Pathology. 2021;53:170–8.
Roy M, Buehler DG, Zhang R, Schwalbe ML, Baus RM, Salamat MS, et al. Expression of insulinoma-associated protein 1 (INSM1) and orthopedia homeobox (OTP) in tumors with neuroendocrine differentiation at rare sites. Endocr Pathol. 2019;30:35–42.
Righi L, Rapa I, Votta A, Papotti M, Sapino A. Human achaete-scute homolog-1 expression in neuroendocrine breast carcinoma. Virchows Arch. 2012;460:415–21.
Weigelt B, Geyer FC, Horlings HM, Kreike B, Halfwerk H, Reis-Filho JS. Mucinous and neuroendocrine breast carcinomas are transcriptionally distinct from invasive ductal carcinomas of no special type. Mod Pathol. 2009;22:1401–14.
Weigelt B, Horlings HM, Kreike B, Hayes MM, Hauptmann M, Wessels LF, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–50.
Marchio C, Geyer FC, Ng CK, Piscuoglio S, De Filippo MR, Cupo M, et al. The genetic landscape of breast carcinomas with neuroendocrine differentiation. J Pathol. 2017;241:405–19.
Horlings HM, Weigelt B, Anderson EM, Lambros MB, Mackay A, Natrajan R, et al. Genomic profiling of histological special types of breast cancer. Breast Cancer Res Treat. 2013;142:257–69.
Kehr EL, Jorns JM, Ang D, Warrick A, Neff T, Degnin M, et al. Mucinous breast carcinomas lack PIK3CA and AKT1 mutations. Hum Pathol. 2012;43:2207–12.
Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010;222:282–98.
Fernandez-Cuesta L, Peifer M, Lu X, Sun R, Ozretic L, Seidal D, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun. 2014;5:3518.
Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive molecular portraits of invasive lobular breast. Cancer Cell 2015;163:506–19.
Ang D, Ballard M, Beadling C, Warrick A, Schilling A, O’Gara R, et al. Novel mutations in neuroendocrine carcinoma of the breast: possible therapeutic targets. Appl Immunohistochem Mol Morphol. 2015;23:97–103.
Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res. 2016;22:3618–29.
Simbolo M, Mafficini A, Sikora KO, Fassan M, Barbi S, Corbo V, et al. Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J Pathol. 2017;241:488–500.
Tian Z, Wei B, Tang F, Wei W, Gilcrease MZ, Huo L, et al. Prognostic significance of tumor grading and staging in mammary carcinomas with neuroendocrine differentiation. Hum Pathol. 2011;42:1169–77.
Rovera F, Lavazza M, La Rosa S, Fachinetti A, Chiappa C, Marelli M, et al. Neuroendocrine breast cancer: retrospective analysis of 96 patients and review of literature. Int J Surg. 2013;11S:79–83.
Xu G, Chhangawala S, Cocco E, Razavi P, Cai Y, Otto JE, et al. ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer. Nat Genet. 2020;52:198–207.
Alkaied H, Harris K, Brenner A, Awasum M, Varma S. Does hormonal therapy have a therapeutic role in metastatic primary small cell neuroendocrine breast carcinoma? Case report and literature review. Clin Breast Cancer. 2012;12:226–30.
Samartzis EP, Gutsche K, Dedes KJ, Fink D, Stucki M, Imesch P. Loss of ARID1A expression sensitizes cancer cells to PI3K- and AKT-inhibition. Oncotarget. 2014;5:5295–303.
Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–40.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23.
Rugo HS, Lerebours F, Ciruelos E, Drullinsky P, Borrego MR, Neven P, et al. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDKi) + aromatase inhibitor (AI): BYLieve study results. J Clin Oncol. 2020;38:1006.
Shanks A, Choi J, Karur V. Dramatic response to cyclin D-dependent kinase 4/6 inhibitor in refractory poorly differentiated neuroendocrine carcinoma of the breast. Proceedings. 2018;31:352–4.
Makretsov N, Gilks CB, Coldman AJ, Hayes M, Huntsman D. Tissue microarray analysis of neuroendocrine differentiation and its prognostic significance in breast cancer. Hum Pathol. 2003;34:1001–8.
van Krimpen C, Elferink A, Broodman C A, Hop WC, Pronk A, Menke M. The prognostic influence of neuroendocrine differentiation in breast cancer: results of a long-term follow-up study. Breast 2004;13:329–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsang, J.Y., Tse, G.M. Breast cancer with neuroendocrine differentiation: an update based on the latest WHO classification. Mod Pathol 34, 1062–1073 (2021). https://doi.org/10.1038/s41379-021-00736-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00736-7
This article is cited by
-
Decoding the basis of histological variation in human cancer
Nature Reviews Cancer (2024)
-
Clinical and genomic analyses of neuroendocrine neoplasms of the breast
Modern Pathology (2022)
-
Genetic and immunohistochemical profiling of small cell and large cell neuroendocrine carcinomas of the breast
Modern Pathology (2022)