Abstract
Euchromatic segments of the X chromosomes of placental mammals are the most conservative elements of the karyotype, only rarely subjected to either inter- or intrachromosomal rearrangements. Here, using microdissection-derived set of region-specific probes of Terricola savii we detailed the evolutionary rearrangements found in X chromosomes in 20 vole species (Arvicolinae, Rodentia). We show that the evolution of X chromosomes in this taxon was accompanied by multiple para- and pericentric inversions and centromere shifts. The contribution of intrachromosomal rearrangements to the karyotype evolution of Arvicolinae species was approximately equivalent in both the separate autosomal conserved segments and the X chromosomes. Intrachromosmal rearrangements and structural reorganization of the X chromosomes was likely accompanied by an accumulation, distribution, and evolution of repeated sequences.
Similar content being viewed by others
Introduction
Most eutherians have two sex chromosomes (gonosomes)—X and Y. The gonosomes are thought to have emerged from a pair of autosomes with the advent of the sex-determining gene1. Between 166–105 million years ago a number of Robertsonian translocations between the sex chromosomes and autosomes occurred2. The gonosomes soon diverged and became heteromorphic due to the absence of recombination in all but a small pseudoautosomal region on both chromosomes. Over the last 70 years cytogenetists have documented the morphology, centromere position, heterochromatin content and distribution of X and Y chromosomes of various mammalian species. One important conclusion was that the X chromosomes were often highly conserved even between distantly related species3. This conservatism of the X chromosome even in phylogenetically distant mammals, such as humans, pigs, horses, dogs, and cats, was amply confirmed by the study of genetic marker order4. Comparative cytogenetic studies often show that the X chromosome remains conserved even when almost all autosomes are highly rearranged, for example, dogs have highly rearranged autosomes compared to other carnivores, but the X chromosome is conserved5,6. It is well appreciated that rodents are generally characterized by highly rearranged genomes, but nonetheless many rodents (beavers, squirrels) have a conserved X chromosome7. It is thought that the X chromosome is conserved as a result of a dose compensation mechanism that imposes evolutionary restrictions on rearrangements8,9.
However, there are well known cases when the X chromosome, both with respect to gene content and marker order10, is not conserved. The X chromosomes of a significant number of mouse-like rodents (Myomorpha) and cetartiodactyls are clearly rearranged and subject to both intrachromosomal and interchromosomal rearrangements11,12,13,14. The reasons why some taxa escape X chromosome conservatism are not clear.
Myomorpha is the largest placental suborder and it is characterized by high rates of karyotypic evolution. The mouse-like rodents have the highest number of species among mammals with rearranged sex chromosomes15. It appears that sex chromosomes in these species are most often subject to translocation with autosomes and the Y chromosome is often lost. Frequent variations in centromere positions, even in closely related species, indicate that pericentric inversions and/or the emergence of evolutionarily new centromeres (ENC) are common. Cases of the emergence of ENC on gonosomes have been confirmed for species of the genus Tokudaia16. Tokudaia tokunoshimensis has the same localization of centromere as Rattus norvegicus, while T. osimensis has an ENC, that presumably appeared after the divergence of the genus Tokudaia and their common ancestor with R. norvegicus16. Some populations of Microtus agrestis have a so-called “Lu-Y” chromosome formed due to pericentric inversion of the Y chromosome17.
The huge variety of sex chromosome systems described for myomorphs makes them unique among mammals and even rodents from other suborders. Moreover, autosomal sets of mouse-like rodents also underwent a mega reorganization during evolution due to numerous intra- and interchromosomal rearrangements18,19.
Recently, it was shown that autosomal syntenic blocks in Arvicolinae karyotypes were subjected to multiple evolutionary rearrangements. Apparently, the number of intrachromosomal rearrangements exceeded interchromosomal rearrangements20,21. It is important to note that the autosomes of voles often have some amount of pericentromeric heterochromatin. The accumulation of heterochromatin and duplications of tandem repeats can significantly affect the morphology of sex chromosomes in mouse-like rodents. Some arvicolines (M. agrestis, M. cabrerae, M. chrotorrhinus, M. epiroticus, and M. transcaspicus) have so-called "giant" sex chromosomes, representing up to 20% of the genome. Variation in length and morphology (from acrocentric to metacentric) of the gonosomes, in this case, could be caused by the inclusion of inhomogeneous heterochromatic blocks. Previously it has been shown that such blocks were capable of forming whole heterochromatic arms of chromosomes22. C-banding reveals heterochromatic blocks that make up more than half the length of the X chromosome in many species. In addition, some species exhibit hypervariability in the amount and distribution of heterochromatin (e.g. Lasiopodomys mandarinus23).
Unfortunately, the evolution of the X chromosomes of rodents as well as other mammals is not well understood. Previously, X chromosomes of only five species of the genus Microtus (M. arvalis, M. kirgisorum, M. rossiaemeridionalis, M. transcaspicus, M. agrestis) were investigated using region-specific probes of the species M. rossiaemeridionalis. The study revealed differences in X chromosomes resulted from inversions or intrachromosomal translocations (exchange of chromosomal segments within the same chromosome). The authors reconstructed the possible evolution of the X chromosome during karyotype divergence, underlying the presence of repeated sequences and their possible participation in intrachromosomal rearrangements24.
Here, on a large sample of arvicoline species, we report the stability of the euchromatic regions of the X chromosomes and show a momentous contribution of intrachromosomal rearrangements and accumulation of repeated sequences to the evolution of their X chromosomes.
Methods
Compliance with ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experiments were approved by the Ethics Committee on Animal and Human Research of the Institute of Molecular and Cellular Biology, Siberian Branch of the Russian Academy of Sciences, Russia (order No. 32 of May 5, 2017). This article does not contain any studies with human participants performed by any of the authors.
Species sampled
We used chromosome suspensions obtained from cell lines in the Laboratory of Animal Cytogenetics, the IMCB SB RAS, Russia. All cell lines were retrieved from the IMCB SB RAS cell bank (“The general collection of cell cultures”, No 0310-2016-0002). The list of species is presented in Table 1: the origin of each sample, the establishment of cell lines, karyotype description for each studied species were previously reported25,26,27.
Chromosome preparation and chromosome staining
Chromosome suspensions were obtained from cell lines according to earlier published protocols35,36. G-banding was performed on chromosomes of all species prior to FISH using the standard trypsin/Giemsa treatment procedure37. C-banding was performed as described previously36,38.
Microdissection, probe amplification, and labeling
We decided to generate microdissected probes from Terricola savii for a number of reasons. It is known that T. savii populations differ for the morphology of the X chromosome39. Here we utilized individuals from Imola, Italy. The X chromosome was clearly distinguishable in metaphases plates because it is the only metacentric in the karyotype . Further, we concluded that that the X chromosome of T. savii individuals from Imola do not have large C-positive blocks. The X-chromosome is small even compared to the X chromosomes of other arvicoline species known not to have large additional heterochromatic blocks. Addtionally, previously published reports on differential staining of chromosomes of T. savii confirmed that the metacentric form of this chromosome does not have large heterochromatic blocks39.
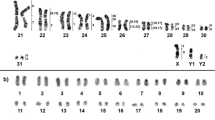
Glass needle-based microdissection was performed as described earlier40. Seven copies of each X chromosome region from T. savii were collected. Chromosomal DNA was amplified and labeled using WGA kits (Sigma) according to the manufacturer's protocol. In total, we obtained 5 region-specific painting probes covering the whole X chromosome of T. savii (Fig. 1).
Metaphase chromosomes of T. savii. (a) Localization of microdissection-derived probes A, B, C, D, and E on T. savii DAPI-banded X chromosome, (b) T. savii X chromosome: C-banding shown on the left, and GTG-banding on the right. Black arrows mark pericentromeric and interstitial heterochromatic regions. Vertical lines indicate the localization of the region-specific probes used in the work. The continuous line indicates the location of the main signal of the probe, the dotted line – the additional signal. Black dots mark the position of centromere. Localization of region-specific probes on T. savii chromosomes: (c) probe A, (d) probe B, (e) probe C, (f) probe D, (g) probe E.
Fluorescence in situ hybridization (FISH)
FISH was performed following previously published protocols6,41. Images were captured using VideoTest-FISH software (VideoTesT) with a JenOptic CCD camera mounted on an Olympus BX53 microscope. Hybridization signals were assigned to specific chromosome regions defined by G-banding pattern captured by the CCD camera prior to FISH. All images were processed using Corel Paint Shop Pro X2 and X3 (Corel).
Data analysis
When analyzing the results, we used a combination of different approaches. First, we identified the most common combinations of the structure of the ancestral X chromosome. Secondly, comparative chromosome painting data were compared with the previously established and published phylogenies of Arvicolinae42,43,44. Here, a comparison with outgroup group at the level of individual genera and tribes was made. The reconstruction of the likely structure of the ancestral arvicoline X chromosome was carried out in accordance with the principles of cladistics: the most likely evolution scenario is the one that includes the smallest number of rearrangements (presence of synapomorphies, avoidance of homoplasies)45.
Results
Using microdissection, a set of 5 region-specific painting DNA-probes, covering the whole X chromosome of the Savi's vole (T. savii), was established. To clarify the boundaries of probes localization, fluorescence in situ hybridization of the probes to T. savii chromosomes was performed (Fig. 1c–g). It is important to note that additional signals of the probe C were localized on the centromeric regions of all autosomes and in the p-arm of the X chromosome (Fig. 1e). We performed C-banding of T. savii chromosomes and found a heterochromatic block in the p-arm of the X chromosome, which corresponded well to the location of this additional signal (Fig. 1b). Also, the probes D and E partially overlapped with this heterochromatic block (Fig. 1a,b).
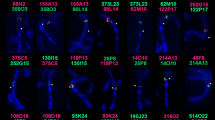
The set of probes was used for the comparison of chromosomes of the wide range of Arvicolinae species (Table 1). Hybridization efficiency varied between species, but it was sufficient for probe mapping. The difference in size between the localization areas of the probes in different species might be caused by the amplification of repeated sequences. Examples of fluorescence in situ hybridizations are shown in Figs. 2 and S1.
Results of localization of microdissection-derived probes on the chromosomes of some species of voles: (a) A (red) and B (green), (b) A (green) and D (red), (c) B (green), (d) B (green) and E (red), (e) C (green), (f) D (green) and E (red). GTG-banding shown on the left. Abbreviated names of species correspond to Table 1.
In the case of probe C, a clear signal was perceived for most species, however, on chromosomes of five species (Alexandromys evoronensis, Chionomys gud, L. gregalis, M. arvalis, M. schidlovskii) the probe had a discrete signal, which made it difficult to establish the boundaries of the hybridization. This probe also did not label centromeres in any of the species, except for those of the Terricola group where the probe C had a discrete signal in pericentromeric regions of X chromosomes. Probes D and E, when localized to the chromosomes of some species, had additional signals. Because these additional signals were often covered by the probe C, we assumed that they might also coincide with heterochromatic blocks, as it is with T. savii. Moreover, in karyotypes of most species (mostly Microtus) probe D had a discrete signal (Fig. 2b). The result of the localization of the full set of probes on the chromosomes of all studied species is presented in Fig. 3.
X chromosomes of the investigated arvicoline species. From left to right: C-banding (present data for ATUV, BJUL, MSCH, TDAG, TMAJ, other from previously published works46,47,48,49), G-banding, probe localization. The continuous line indicates the location of the main signal of the probe, the dotted line—the additional signal. Black dots mark centromere positions. Black arrows mark heterochromatic regions. Grey arrows mark regions that were not labeled by any probes.
C-banding of the chromosomes of most of the species used in the study was previously performed46,47,48,49,50. Here, in addition to T. savii, we carried out C-banding of chromosomes of six more species of voles (A. mujanensis, Alticola tuvinicus, Blanfordimys juldaschi, M. dogramacii, T. daghestanicus, and T. majori), which allowed to visualise not only centromeric, but interstitial heterochromatic blocks on X chromosomes of four of these species—A. tuvinicus, B. juldaschi, T. daghestanicus, and T. majori (Fig. 3).
The analysis of the obtained patterns of localization of region-specific probes revealed two predominant types of X chromosome configuration differing in centromere position only (Fig. 4). Of the 20 species analyzed, four species had an acrocentric X chromosome, and five had a metacentric X chromosome with the same order of probes. We assumed that one of the morphological types represent a putative ancestral variant of the arvicoline X chromosome. The reconstruction of possible transformation paths that led to the formation of the X chromosomes of modern species of voles was made (Fig. 4).
Diagram of rearrangements of X chromosomes in voles. The dotted line circles the presumptive ancestral versions of the X chromosome. Black dots mark centromere positions. The scheme does not reflect variations caused by the number and distribution of repeated sequences. The scheme does not reflect the phylogenetic relationships between species.
The analysis of the painting data and the previously obtained pattern of phylogenetic relationships in Arvicolinae subfamily suggests that the ancestral X chromosome of the voles was probably acrocentric with probe order from the centromere A-B-C-D-E. To better visualize the results and determine the number and distribution of intrachromosomal rearrangements in different groups of voles, the rearrangements were plotted on a previously published phylogenetic tree of Arvicolinae42,43,44 (Fig. 5).
Phylogenetic tree of the Arvicolinae42,43,44 with additions: intrachromosomal rearrangements are indicated above the branch, the alleged ancestral X chromosome is placed at the base of the tree. An asterisk denotes the same pericentric inversions (convergent event). Red exclamation marks indicate the possible places of ambiguous development of the scenario of karyotypic evolution (see text). The tree shows only branching, the relative scale and length of the branches are not informative.
Discussion
Arvicolinae is a multi-species and rapidly evolving taxon. Recent molecular studies have clarified phylogenetic relationships in the subfamily, and cytogenetic studies were able to distinguish morphologically similar species and reconstruct the ancestral karyotype of the subfamily based on the analysis of interchromosomal rearrangements25,26,29,30,51. It was also shown that vole karyotype evolution was accompanied by intrachromosomal rearrangements: at least three ancestral autosomal conservative segments underwent significant reorganization due to inversions and centromere shifts20,21. Recent work has raised questions about the prevalence and importance of intrachromosomal rearrangements in vole karyotype evolution.
Intrachromosomal rearrangements in the evolution of the X chromosomes of voles
To date, studies of the evolution of vole sex chromosomes were mainly limited to the descriptions of morphology and localization of repeated sequences. However, region-specific X chromosome probes of M. rossiaemeridionalis showed that differences in the X chromosomes of five species from the genus Microtus could be due to inversions or intrachromosomal translocations24.
In this research, having localized the set of region-specific microdissected probes of the Savi's vole on chromosomes of 19 species of voles belonging to different tribes, we assumed that the ancestral X chromosome of voles was acrocentric. This type of X chromosome morphology is concordant with the previously proposed version of the ancestral karyotype of the voles, consisting of 56 acrocentric chromosomes52. We were able to map multiple intrachromosomal rearrangements including 9 paracentric inversions, 6 pericentric inversions, and 6 centromere shifts. Although there was no indication of any prevailing type of rearrangements between groups, the results showed that X chromosomes of voles, not only of the genus Microtus, frequently undergo intrachromosomal rearrangements. Such high variability in X chromosome morphology generated by intrachromosomal rearrangements was previously documented only for some ruminants11,12. As for arvicoline rodents, ruminants are also characterized by an increased rate of karyotype evolution among mammals.
In the evolution of the X chromosomes of modern species of voles a single case of potential convergence was identified, a convergent pericentric inversion in C. gud and A. amphibius. It is noteworthy that the number of convergent events recorded in the autosomes was significantly higher20,21.
The X chromosome of the putative ancestor of almost all species of the Microtina subtribe, except for C. gud, is characterized by a centromere shift. Centromere shifts in the X chromosomes have been found in other species. For example, the X chromosomes of elephant and humans differ by the position of the centromere but maintain the same gene order. A similar situation was observed in two species of the genus Tokudaia, Ryukian spiny mice16. It is also known that the X chromosome of the squirrel monkey (genus Saimiri) differs from human`s only in the formation of ENC53. Further, cladistic analysis shows that a reverse shift of the centromere back to its original position might have occurred in the evolutionary branch leading to the genus Microtus, specifically in M. dogramacii, M. guentheri, and M. schidlovskii. However, an alternative hypothesis is that multiple repeated events of convergence have also affected sex chromosomes in these species. It is impossible to rule out that centromere repositioning occurred repeatedly and independently in the phylogenetic lineages leading to the genera Lasiopodomys, Alexandromys, Blanfordimys, and Terricola.
The number of intrachromosomal rearrangements varies significantly in different branches of the vole phylogenetic tree. In general, rearrangements affecting the localization areas of probes A and B were observed in representatives of the basal branches, i.e. the tribes Ellobiini and Myodini, as well as in species C. gud, A. amphibius, and M. rossiaemeridionalis. For the remaining species of the Arvicolini tribe, the preservation of this segment in its ancestral form was shown.
Earlier, in the study of bird genomes, it was suggested that inversions are more often fixed in sex chromosomes than in autosomes54. Among eutherian mammals, there are several examples of significant rearrangement of sex chromosomes compared to autosomes11,24, although in general X chromosomes are remarkably conserved3,4,7. In case of arvicoline species we were unable to confirm or disprove that intrachromosomal rearrangements are more frequent in sex chromosomes than in autosomes. Firstly, the study of intrachromosomal rearrangements was carried out on the example of only three autosomal conservative segments of the ancestral karyotype, not on the entire autosomal set. Secondly, the uneven frequency of occurrence of rearrangements even in the three analyzed segments led us to suggest that the analysis of a larger number of segments could significantly change our ideas about the contribution of intrachromosomal rearrangements to the evolution of autosomal sets20,21. We found a higher occurrence of intrachromosomal rearrangements of the X chromosome only in the genus Ellobius21. In some species (C. gud, B. afghanus) intrachromosomal rearrangements in X chromosomes were found, while previous analyses showed that the autosomes were intact. The opposite situation was observed in three species of the genus Terricola and L. gregalis where conserved ancestral status of X chromosomes was accompanied by a great number of rearrangements in three previously analyzed segments of autosomes.
The contribution of repeated sequences to the evolution of sex chromosomes
Conventional cytogenetic technique, such as C-banding, is able to detect and descript regions of accumulation of constitutive heterochromatin in karyotype55. Characteristically, constitutive heterochromatin consists largely of highly repetitive DNA. The use of AT-/GC-specific fluorochromes discovered great variability in the heterochromatin composition56. Simple repetitive sequences (e.g., microsatellites) are often accumulated in high copy numbers on the sex chromosomes in many taxa57,58,59, although the same repeats can be distributed throughout the genome in low copy numbers55. But in some species moderately repetitive sequences rather than highly repetitive DNA represent blocks of heterochromatin.
In voles, autosomal heterochromatin is mainly centromeric and contains dissimilar, repeated families in different species50. The blocks of constitutive heterochromatin on sex chromosomes are highly heterogeneous52 and also contain varying repeated DNA50. It was believed that heterochromatic variation does not appear to play a role in the speciation of arvicoline rodents60. But the results of this research and recently published studies indicate that the accumulation of repeated sequences could play a significant role in the evolution of X chromosomes of the voles24.
In most cases, our set of probes completely covered the entire X chromosome, however, for some species we encountered difficulties in analyzing the results. Some X chromosome regions were not hybridized by the probes, or, conversely, individual probes apparently had additional signals. We expected to get additional signals from probes C, D, and E because they partly overlapped the heterochromatic region of p-arm of T. savii X chromosome. Indeed, additional signals from probe C were observed on sex chromosomes of almost all species, but their localization did not always correlate to the distribution of heterochromatin.
Probe C marked the pericentromeric regions of all autosomes and sex chromosomes in the T. savii karyotype which might be due to a species-specific amplification and accumulation of repeats (Fig. 1e). The probe also slightly hybridized with pericentromeric regions of X chromosomes of all representatives of the genus Terricola and had a weak background signal in pericentromeric regions of X chromosomes of M. arvalis and A. evoronensis (Figs. 2e, 3). Within the genus Terricola, the size of hybridized areas varied greatly. C-banding shows that this variation in signal size is associated with the size of the heterochromatic regions (Fig. 3). In the karyotype of T. savii, the pericentromeric region of the X chromosome is C-positive, but no distinct blocks were found in the pericentromeric regions of karyotypes of the rest of the species listed above. Apparently, repeated sequences may be both species-specific and heterogeneous within the same chromosome50.
The pericentromeric regions of the X chromosomes of other species were not labeled with any of the probes, which may be explained by the fact that during the hybridization, repetitive sequences were suppressed using Cot DNA isolated from tissues of different species of voles (mainly, Microtus), or that there is little homology in pericentromeric repeats found in different species. However, it should be noted that three species (M. dogramacii, M. guentheri, M. schidlovskii), with large unlabeled pericentomeric regions (Fig. 3), belong to the same branch of the phylogenetic tree (Fig. 5). This may indicate the main role of accumulation of repeats in the evolution of sex chromosomes of these species. Moreover, C-banding did not reveal any large blocks of heterochromatin in the pericentromeric region of the X chromosomes of M. dogramacii (Fig. 3).
Previous research26 showed that M. dogramacii used here has an acrocentric X chromosome, but a metacentric X chromosome was described for this species by other authors61. However, this difference is not so surprising because interspecies chromosome polymorphism has been widely reported for voles, which affects both the centromere positions and the number of chromosomes (for example, for A. mujanensis in Lemskaya et al.18) and for M. dogramacii in Lemskaya et al.26).
Clear additional signals in the localization of probes D and E were detected only in M. rossiaemeridionalis, and their correspondence to the heterochromatic regions was established (Figs. 2b,d,3). There was a region on the q-arm of X chromosome of T. daghestanicus between signals from probes D and E, corresponding to a heterochromatic block (Figs. 2b,d,3). In this region we observed an additional signal from probe C, but there were no signals from probes D and E. This result may indicate similarity or convergence of repeated sequences in T. savii and M. rossiaemeridionalis and distinguishing them from other species used in this study.
Probe B provided an unusual result on chromosomes of C. gud and A. amphibius. Although the arrangement of the signals was the same for these species, in C. gud the additional signal was weaker than the main signal and corresponded to the dark heterochromatic region on the C-banded X chromosome but in A. amphibius both signals had the same intensity (Fig. 2c,d). This difference may be due to variations in the amount and accumulation of repeated sequences46,48.
In some cases, only cell cultures established for males were available for analysis. This allowed us to detect that probes C, D, and E provided signals on the Y chromosome of the species A. evoronensis, A. mujanensis, B. juldaschi, M. guentheri, M. rossiaemeridionalis, M. schidlovskii, and L. gregalis (Fig. S1). On the Y chromosome of M. rutilis, probe D provided a strong signal while signals of probes C and E were weak. No signals were found on the Y chromosome of A. tuvinicus and C. gud. In the case of the XX-male E. talpinus, no additional signals were observed on any other chromosomes. The results obtained are consistent with earlier studies in which the heteromorphism of X chromosome of the male E. talpinus was detected only in the analysis of meiosis47. The localization of the C, D, and E probes on the Y chromosomes of some vole species suggests the similarity of repeated sequences in their X and Y chromosomes. We did not find any relationship between the localization of probes on the Y chromosome and the synaptic or asynaptic behavior of sex chromosomes in meiosis in males62.
Recently it was shown that accumulation and expansion of microsatellites and DNA transposons might involve heterochromatinization and initiate sex chromosome differentiation in various taxa59,63,64. Thus, chromosomes having similar morphology and G-banding pattern can accumulate different repeated sequences in heterochromatic regions, i.e. repeated sequences can be species-specific and, conversely, variation in repeated sequences can give different variants of sex chromosomes in one species22,50. It was suggested that the karyotype of a common ancestor of modern arvicoline species contained varying repetitive families, and that descendants selectively amplified or deleted different repeats on different chromosomes50. This led to interspecific variability in the chromosomal distribution and number of copies of repeats. The result of the present work tested previously mentioned hypotheses on the particular lability of the arvicoline sex chromosomes in relation to C-band modification60 and also suggests a significant heterogeneity of the heterochromatic regions of the X chromosomes of voles46,47,48,49,50,60.
Conclusions
Studies of the evolution of the genomes of non-mammalian species show that the euchromatic portion of the X chromosome is the fastest evolving by genomic rearrangements65,66, and sex chromosomes more often than autosomes drive speciation and hybrid incompatibilities67. Apparently in this case the evolution of repeated sequences has played a major role in incompatibilities, which could help to maintain reproductive isolation between species68. The same mechanisms may operate in mammalian species. In voles, we observe that evolution of the sex chromosomes was accompanied by multiple, not previously identified intrachromosomal rearrangements. As in the case of autosomes, para- and pericentric inversions and centromere shifts were common in the evolution of X chromosomes. Apparently identical types of rearrangements sometimes arose independently in different branches of the phylogenetic tree of voles. Unlike other taxa, it seems that the contribution of intrachromosomal rearrangements to the formation of karyotypes of the modern arvicoline species was approximately equivalent for the separate conservative segments of autosomes and X chromosomes. Moreover, the apparent diversity of X chromosomes of the voles by the presence, location, and size of heterochromatic blocks indicates that further study of intrachromosomal rearrangements in this taxon requires the study of repeated sequences in order to assess their contribution to the evolution of sex chromosomes.
References
Zhou, Q. et al. Neo-sex chromosomes in the black muntjac recapitulate incipient evolution of mammalian sex chromosomes. Genome Biol.9, R98 (2008).
Graves, J. A. M. Did sex chromosome turnover promote divergence of the major mammal groups?. BioEssays38, 734–743 (2016).
Pathak, S. & Stock, A. D. The X chromosomes of mammals: karyological homology as revealed by banding techniques. Genetics78, 703–714 (1974).
Murphy, W. J. et al. Evolution: dynamics of mammalian chromosome evolution inferred from multispecies comparative maps. Science (80-.).309, 613–617 (2005).
Perelman, P. L. et al. Comparative chromosome painting in carnivora and pholidota. Cytogenet. Genome Res.137, 174–193 (2012).
Yang, F. et al. A complete comparative chromosome map for the dog, red fox, and human and its integration with canine genetic maps. Genomics62, 189–202 (1999).
Beklemisheva, V. R. et al. Reconstruction of karyotype evolution in core Glires. I. The genome homology revealed by comparative chromosome painting. Chromosom. Res.19, 549–565 (2011).
Ohno, S., Beçak, W. & Beçak, M. L. X-autosome ratio and the behavior pattern of individual X-chromosomes in placental mammals. Chromosoma15, 14–30 (1964).
Ohno, S., Wolf, U. & Atkin, N. B. Evolution from fish to mammals by gene duplication. Hereditas59, 169–187 (1968).
Kim, J. et al. Reconstruction and evolutionary history of eutherian chromosomes. Proc. Natl. Acad. Sci. USA114, E5379–E5388 (2017).
Proskuryakova, A. A. et al. X chromosome evolution in cetartiodactyla. Genes (Basel)8, E216 (2017).
Proskuryakova, A. A. et al. Comparative chromosome mapping of musk Ox and the X chromosome among some bovidae species. Genes (Basel)10, E857 (2019).
Robinson, T. J., Harrison, W. R., De León, F. A. P., Davis, S. K. & Elder, F. F. B. A molecular cytogenetic analysis of X chromosome repatterning in the Bovidae: transpositions, inversions, and phylogenetic inference. Cytogenet. Cell Genet.80, 179–184 (1998).
Graphodatsky, A. S. Conserved and variable elements of mammalian chromosomes. in Cytogenetics of animals (ed. Halnan CRE) 95–124 (CAB International Press, 1989).
Romanenko, S. A. & Volobouev, V. Non-Sciuromorph rodent karyotypes in evolution. Cytogenet. Genome Res.137, 233–245 (2012).
Kobayashi, T. et al. Centromere repositioning in the X chromosome of XO/XO mammals, Ryukyu spiny rat. Chromosom. Res.16, 587–593 (2008).
Fredga, K. & Jaarola, M. The origin and distribution of the Lund Y chromosome in Microtus agrestis (Rodentia, Mammalia). Hereditas126, 25–34 (1997).
Lemskaya, N. A. et al. Chromosome polymorphism in microtus (Alexandromys) mujanensis (Arvicolinae, Rodentia). Cytogenet. Genome Res.146, 238–242 (2015).
Romanenko, S. A. et al. Chromosome translocations as a driver of diversification in mole voles Ellobius (Rodentia, mammalia). Int. J. Mol. Sci.20, E4466 (2019).
Romanenko, S. A. et al. Intrachromosomal rearrangements in rodents from the perspective of comparative region-specific painting. Genes (Basel)8, E215 (2017).
Romanenko, S. A. et al. Multiple intrasyntenic rearrangements and rapid speciation in voles. Sci. Rep.8, 14980 (2018).
Rovatsos, M. T. et al. Extensive sex chromosome polymorphism of Microtus thomasi/Microtus atticus species complex a associated with cryptic chromosomal rearrangements and independent accumulation of heterochromatin. Cytogenet. Genome Res.151, 198–207 (2017).
Kovalskaya, J. M. & Smorkatcheva, A. V. Overview of geographical variability of karyotype of Chinese vole Lasiopodomys mandarinus Milne-Edwards 1871 (Rodentia, Arvicolinae)—Another group of close species? in Teriofauna of Russia and adjacent territories 227 (KMK Scientific Press Ltd, 2011).
Rubtsov, N. B. et al. Reorganization of the X chromosome in voles of the genus Microtus. Cytogenet. Genome Res.99, 323–329 (2002).
Sitnikova, N. A. et al. Chromosomal evolution of Arvicolinae (Cricetidae, Rodentia). I. The genome homology of tundra vole, field vole, mouse and golden hamster revealed by comparative chromosome painting. Chromosom. Res.15, 447–456 (2007).
Lemskaya, N. A. et al. Chromosomal evolution of Arvicolinae (Cricetidae, Rodentia). III. Karyotype relationships of ten Microtus species. Chromosom. Res.18, 459–471 (2010).
Romanenko, S. A. et al. Genome-wide comparative chromosome maps of Arvicola amphibius, Dicrostonyx torquatus, and Myodes rutilus. Chromosom. Res.24, 145–159 (2016).
Gladkikh, O. L. et al. Rapid karyotype evolution in Lasiopodomys involved at least two autosome—Sex chromosome translocations. PLoS ONE11, e0167653 (2016).
Romanenko, S. A. et al. Chromosomal evolution of Arvicolinae (Cricetidae, Rodentia). II. the genome homology of two mole voles (genus Ellobius), the field vole and golden hamster revealed by comparative chromosome painting. Chromosom. Res.15, 891–897 (2007).
Bakloushinskaya, I. Y. et al. A comparative analysis of the mole vole sibling species Ellobius tancrei and E. talpinus (Cricetidae, Rodentia) through chromosome painting and examination of synaptonemal complex structures in hybrids. Cytogenet. Genome Res.136, 199–207 (2012).
Pavlinov, I. Y. & Khlyap, L. A. Order Rodentia Bowdich, 1821. in The mammals of Russia: a taxonomic and geographic reference (eds. Pavlinov, I. Y. & Lissovsky, A. A.) 142–312 (KMK Scientific Press Ltd, 2012).
Pavlinov, I. Y. Systematics of recent mammals. (Moscow University, 2003).
Bannikova, A. A., Lebedev, V. S. & Golenishchev, F. N. Taxonomic position of Afghan vole (Subgenus Blanfordimys) by the sequence of the mitochondrial cytb gene. Russ. J. Genet.45, 91–97 (2009).
Wilson, D. E. & Reeder, D. M. Mammal species of the world. Taxon. Geograph. Ref. https://doi.org/10.2307/4498724 (2005).
Stanyon, R. & Galleni, L. A rapid fibroblast culture technique for high resolution karyotypes. Bolletino di Zool.58, 81–83 (1991).
Romanenko, S. A. et al. Segmental paleotetraploidy revealed in sterlet (Acipenser ruthenus) genome by chromosome painting. Mol. Cytogenet.8, 90 (2015).
Seabright, M. A rapid banding technique for human chromosomes. Lancet2, 971–972 (1971).
Sumner, A. T. A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res.75, 304–306 (1972).
Galleni, L., Stanyon, R., Tellini, A., Giordano, G. & Santini, L. Karyology of the Savi pine vole, Microtus savii (De Sélys-Longchamps, 1838) (Rodentia, Arvicolidae): G-, C-, DA/DAPI-, and AluI-bands. Cytogenet. Genome Res.59, 292 (1992).
Yang, F., Trifonov, V., Ng, B. L., Kosyakova, N. & Carter, N. P. Generation of paint probes from flow-sorted and microdissected chromosomes. Fluoresc. In Situ Hybrid. (FISH) https://doi.org/10.1007/978-3-662-52959-1_6 (2017).
Graphodatsky, A. S. et al. Dog chromosome-specific paints reveal evolutionary inter- and intrachromosomal rearrangements in the American mink and human. Cytogenet. Cell Genet.90, 275–278 (2000).
Buzan, E. V., Krystufek, B., Hänfling, B. & Hutchinson, W. F. Mitochondrial phylogeny of Arvicolinae using comprehensive taxonomic sampling yields new insights. Biol. J. Linn. Soc.94, 825–835 (2008).
Abramson, N. I., Lebedev, V. S., Tesakov, A. S. & Bannikova, A. A. Supraspecies relationships in the subfamily Arvicolinae (rodentia, cricetidae): an unexpected result of nuclear gene analysis. Mol. Biol.43, 897–909 (2009).
Martínková, N. & Moravec, J. Multilocus phylogeny of arvicoline voles (Arvicolini, Rodentia) shows small tree terrace size. Folia Zool.61, 254–267 (2012).
Dobigny, G., Ducroz, J. F., Robinson, T. J. & Volobouev, V. Cytogenetics and cladistics. Syst. Biol.53, 470–484 (2004).
Borodin, P. M., Sablina, O. V. & Rodionova, M. I. Pattern of X-Y chromosome pairing in Microtine rodents. Hereditas123, 17–23 (1995).
Matveevsky, S., Bakloushinskaya, I. & Kolomiets, O. Unique sex chromosome systems in Ellobius: How do male XX chromosomes recombine and undergo pachytene chromatin inactivation?. Sci. Rep.6, 29949 (2016).
Euarchontoglires. in Atlas of Mammalian Chromosomes (eds. OBrien, S. J., Menninger, J. C. & Nash, W. G.) 95–355 (Wiley, Hoboken, 2006). https://doi.org/10.1086/513346.
Arslan, A., Toyran, K., Gözütok, S., Yorulmaz, T. & Zima, J. Comparison of the chromosome banding patterns in three species of social voles (Microtus Irani karamani, M. Schidlovskii, M. Anatolicus) from Turkey. Turkish J. Zool.40, 910–916 (2016).
Modi, W. S., Serdyukova, N. A., Vorobieva, N. V. & Graphodatsky, A. S. Chromosomal localization of six repeated DNA sequences among species of Microtus (Rodentia). Chromosom. Res.11, 705–713 (2003).
Bakloushinskaya, I. et al. Rapid chromosomal evolution in enigmatic mammal with XX in both sexes, the Alay mole vole Ellobius alaicus Vorontsov et al., 1969 (Mammalia, Rodentia). Comp. Cytogenet.13, 147–177 (2019).
Marchal, J. A., Acosta, M. J., Bullejos, M., Díaz De La Guardia, R. & Sánchez, A. Sex chromosomes, sex determination, and sex-linked sequences in Microtidae. Cytogenet. Genome Res.101, 266–73 (2003).
Rocchi, M., Archidiacono, N., Schempp, W., Capozzi, O. & Stanyon, R. Centromere repositioning in mammals. Heredity (Edinb).108, 59–67 (2012).
Hooper, D. M. Range overlap drives chromosome inversion fixation in Passerine birds. Nat. Ecol. Evol.1, 1526–1534 (2017).
Ezaz, T. & Deakin, J. E. Repetitive sequence and sex chromosome evolution in vertebrates. Adv. Evol. Biol.1, 1–9 (2014).
Lemskaya, N. A. et al. A combined banding method that allows the reliable identification of chromosomes as well as differentiation of AT- and GC-rich heterochromatin. Chromosom. Res.26, 307–315 (2018).
Conde-Saldaña, C. C., Barreto, C. A. V., Villa-Navarro, F. A. & Dergam, J. A. An unusual accumulation of ribosomal multigene families and microsatellite DNAs in the XX/XY sex chromosome system in the trans-andean catfish Pimelodella cf. chagresi (Siluriformes:Heptapteridae). Zebrafish15, 55–62 (2018).
Pokorná, M., Kratochvíl, L. & Kejnovský, E. Microsatellite distribution on sex chromosomes at different stages of heteromorphism and heterochromatinization in two lizard species (Squamata: Eublepharidae: Coleonyx elegans and Lacertidae: Eremias velox). BMC Genet.12, 90 (2011).
Singchat, W. et al. Chromosome map of the Siamese cobra: Did partial synteny of sex chromosomes in the amniote represent ‘a hypothetical ancestral super-sex chromosome’ or random distribution?. BMC Genom.19, 939 (2018).
Modi, W. S. C-Banding analyses and the evolution of heterochromatin among Arvicolid rodents. J. Mammal.62, 142–148 (1987).
Albayrak, İ, Baydemir, N. A. & Gözütok, S. C bands and Nucleolar Organizer Regions (NORs) of Microtus dogramacii Kefelioğlu & Krystufek, 1999 (Rodentia: Cricetidae) from Black Sea Region. Munis Entomol. Zool.7, 742–745 (2012).
Borodin, P. M. et al. Multiple independent evolutionary losses of XY pairing at meiosis in the grey voles. Chromosom. Res.20, 259–268 (2012).
Schemberger, M. O. et al. DNA transposon invasion and microsatellite accumulation guide W chromosome differentiation in a Neotropical fish genome. Chromosoma128, 547–560 (2019).
de Freitas, N. L. et al. Early stages of XY sex chromosomes differentiation in the fish Hoplias malabaricus (Characiformes, Erythrinidae) revealed by DNA repeats accumulation. Curr. Genom.19, 216–226 (2017).
Jiang, X. et al. Genome analysis of a major urban malaria vector mosquito, Anopheles stephensi. Genome Biol.15, 459 (2014).
Neafsey, D. E. et al. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science (80-.)347, 1258522 (2015).
Deakin, J. E. et al. Chromosomics: Bridging the gap between genomes and chromosomes. Genes (Basel)10, E627 (2019).
Ferree, P. M. & Barbash, D. A. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol.7, e1000234 (2009).
Acknowledgements
This study was entirely funded by Russian Science Foundation No 19-14-00034 (ASG). We are very grateful to N.A. Lemskaya, O.L. Gladkikh (Institute of Molecular and Cellular Biology, SB RAS, Novosibirsk, Russia) and N.V. Rubtsova (Institute of Cytology and Genetics, SB RAS, Novosibirsk, Russia), who took part in some cell line establishment. We are very grateful to F.N. Golenishchev and V.G. Malikov (Zoological Institute, RAS, Saint-Petersburg, Russia), I.V. Kartavtseva (Institute of Biology and Soil Science, FEB RAS, Vladivostok, Russia), N.Sh. Bulatova and S.V. Pavlova (A.N. Severtzov Institute of Ecology and Evolution, RAS, Moscow, Russia), I.Yu. Baklushinskaya (Koltzov Institute of Developmental Biology RAS, Moscow, Russia), A.V. Smorkatcheva (Department of Vertebrate Zoology, Saint Petersburg State University, Saint Petersburg, Russia), N.V. Lopatina and S.A. Abramov (Institute of Systematics and Ecology of Animals, SB RAS, Novosibirsk, Russia) for help in collecting material. The authors gratefully acknowledge the resources provided by the “Molecular and Cellular Biology” core facility of the IMCB SB RAS (0310-2018-0011 grant).
Author information
Authors and Affiliations
Contributions
S.A.R. established cell lines, made chromosome suspensions, carried out microdissection, probe amplification, some microscopy analysis, and wrote the manuscript. Y.E.F. made some chromosome suspensions, carried out main FISH experiments, C-banding, microscopy analysis, and revised the manuscript. N.A.S. extracted Cot DNA, labeled and prepared probes for FISH, carried out some FISH experiments. M.Z. collected materials from some rodents, including T. savii. R.S. and M.Z. provided a cell line of T. savii and contributed to writing the manuscript. A.S.G. conceived and supervised the project, discussed the results, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Romanenko, S.A., Fedorova, Y.E., Serdyukova, N.A. et al. Evolutionary rearrangements of X chromosomes in voles (Arvicolinae, Rodentia). Sci Rep 10, 13235 (2020). https://doi.org/10.1038/s41598-020-70226-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70226-4
This article is cited by
-
The emergence of a new sex-system (XX/XY1Y2) suggests a species complex in the “monotypic” rodent Oecomys auyantepui (Rodentia, Sigmodontinae)
Scientific Reports (2022)
-
Sex differences in the meiotic behavior of an XX sex chromosome pair in males and females of the mole vole Ellobius tancrei: turning an X into a Y chromosome?
Chromosoma (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.