Abstract
The Kunitz/BPTI-type peptides are ubiquitous in numerous organisms including marine venomous animals. The peptides demonstrate various biological activities and therefore they are the subject of a number of investigations. We have discovered a new HCIQ subfamily belonging to recently described multigene HCGS family of Heteractis crispa Kunitz-peptides. The uniqueness of this subfamily is that the HCIQ precursors contain a propeptide terminating in Lys-Arg (endopeptidase cleavage site) the same as in the neuro- and cytotoxin ones. Moreover, the HCIQ genes contain two introns in contrast to HCGS genes with one intron. As a result of Sanger and amplicon deep sequencings, 24 HCIQ isoforms were revealed. The recombinant peptides for the most prevalent isoform (HCIQ2c1) and for the isoform with the rare substitution Gly17Glu (HCIQ4c7) were obtained. They can inhibit trypsin with Ki 5.2 × 10−8 M and Ki 1.9 × 10−7 M, respectively, and interact with some serine proteinases including inflammatory ones according to the SPR method. For the first time, Kunitz-peptides have shown to significantly increase neuroblastoma cell viability in an in vitro 6-OHDA-induced neurotoxicity model being a consequence of an effective decrease of ROS level in the cells.
Similar content being viewed by others
Introduction
Kunitz-type proteinase inhibitors are present in various living organisms and in viruses. They are widely distributed and well-characterized in animals including marine invertebrates, snakes, spiders, ticks, flies and mammals1. In spider, snake, scorpion, cone snail and sea anemone venoms Kunitz-peptides may exist in multiple isoforms possessing conserved BPTI-like fold but exhibit different biological activities2,3,4,5,6,7,8,9. This phenomenon is associated with gene duplication and their diversification throughout adaptive evolution leading to the formation of families of evolutionarily related but functionally distinct genes10. Among sea anemone transcriptomes, such multigene families have been discovered in Anemonia viridis11, Stichodactyla haddoni12, and Heteractis crispa5. HCGS multigene family of H. crispa has been found to be divided in four distinct subfamilies (GS, RG, GG, and GN) forming the combinatory library of Kunitz/BPTI peptides5. The group of HCGN peptides was presented by one sequence, different from other sequences that are characterized by a propeptide insertion containing the cleavage site Lys-Arg, and additional residues Ile-Gln at the N-terminus of a mature peptide. Its full-length homolog HMIQ3c1, containing a mature peptide with the same residues at N-terminus, was derived from the cDNA of the sea anemone Heteractis magnifica13. The most abundant HCGS and HCRG peptides are being actively studied nowadays14,15,16,17. The main targets of Kunitz-peptides are serine proteinases, such as trypsin and α-chymotrypsin. Both HCGS and HCRG peptides characterized have been shown to inhibit trypsin. Some of them possess different biological activities besides serine proteinases inhibition. HCGS1.19, HCGS1.20 and HCGS1.36 suppress the increase of the Ca2+ response under the influence of histamine in vitro14,17 and provide analgesic action in vivo18; APHC119 and HCRG2116 inhibit TRPV1. RmInI and RmInII exhibit anti-inflammatory activity by weakening rat inflammatory reactions in response to histamine injection20 while HCRG1 and HCRG2 reduce secretion of tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6), as well as expression level of IL-1β precursor (proIL-1β) in LPS-activated macrophages15. Moreover, HMIQ3c1 decreases β-amyloid-induced neurotoxicity on murine neuroblastoma cells13. Thus, Kunitz-peptides can be seen as potential therapeutic compounds preventing some inflammatory processes.
It is known that inflammation in cells can be triggered by oxidative stress, which also plays a major role in a number of neurodegenerative disorders including Parkinson’s disease (PD)21,22. One of the most widely used models for investigation of neuroprotective activity of different compounds is 6-hydroxydopamine (6-OHDA)-induced cytotoxicity. 6-Hydroxydopamine is a neurotoxin which destroys catecholaminergic systems by oxidative stress induced by non-enzymatically auto-oxidation to hydrogen peroxide and p-quinone23. At present, mainly low molecular compounds have been shown to exhibit neuroprotective activity24,25,26,27. Some peptides and proteins have been found to protect dopamine neurons and mitigate their destruction28,29,30. Recently, Kunitz-like peptide PcKuz3 from the zoanthid Palythoa caribaeorum has been shown to exert a neuroprotective effect in a 6-OHDA-induced neurotoxicity model on zebrafish larvae31. However, the ability of the sea anemone Kunitz-peptides to protect neurons against 6-OHDA toxicity has not been studied yet. Here we have investigated a new HCIQ gene subfamily encoding Kunitz-type proteinase inhibitors of H. crispa. We show that the recombinant HCIQ peptides inhibit serine proteinases as well as possess protective effect in a 6-OHDA-induced cytotoxicity model.
Results
Sequence identification and analysis of H. crispa Kunitz IQ peptide diversity
To determine the full-length HCIQ coding sequences, we designed a pair of primers, SIG_all_F and Inh_XhoI_R, complementary to highly conserved regions encoding signal and mature parts of HCGS peptides, respectively. After PCR amplification, cloning, and sequencing we obtained cDNAs encoding HCGS precursors (HCGS2c2 and HCGS2c4), HCRG precursors (HCRG2c8 and HCRG2c10) and HCIQ precursors (HCIQ2c1 and HCIQ2c9) (Fig. 1). Deduced HCIQ2с1 of 85 aa comprises a signal peptide (22 aa), a propeptide (5 aa), and a mature peptide (58 aa). Deduced HCIQ2c9 differs from HCIQ2c1 by lacking a C-terminal part of the signal peptide. The first fifteen residues of the signal part are identical to those of HCGS and HCRG precursors, while the main differences are found in the remaining signal peptide (Fig. 1). The propeptide contains the proteolytic Lys-Arg cleavage site (at the positions 26–27). Thus, based on the high identity of an N-terminal part of the signal sequences of HCIQ, HCGS, and HCRG peptides, they belong to the same family. The differences in C-terminal signal regions and the presence of propeptide sequences allow us to single out the HCIQs as a distinct subfamily.
Multiple alignment of the precursor sequences of sea anemone Kunitz-type peptides. InhVJ20,49, APHC119,48, HCIQ1c9-HCIQ6c38, HCRG2c8, HCRG2c10, HCGS2c2, HCGS2c4 from H. crispa; HMIQ3c1 from H. magnifica13; SHPI-1 from Stichodactyla helianthus54. The asterisks (*) below the sequences indicate the contact sites with serine proteinases. Р1 – amino acid residue of the inhibitor reactive center. Signal peptide is shown in italic. The pro-part is underlined. Identical and conservative residues are shown in light and dark grey respectively. Horizontal arrows indicate the primer directions.
To evaluate the sequence diversity of HCIQ peptides, two approaches were applied. Firstly, we designed an additional primer (SIG_GN_F) complementary to the region encoding to the C-terminal part of the signal HCIQ peptide. As a result of PCR amplification and sequencing, we were able to obtain nine additional HCIQ isoforms (Fig. 1) which were similar to HMIQ3c1 (96–99% of identity). The most prevalent isoforms were HCIQ2c1 (68.75%) and HCIQ5c1 (12.5%); the rest of the sequences occurred in a single copy. The HCIQ4c9 precursor does not contain a propeptide similar to HCGSs, and has a mature peptide identical to HCIQ2c1. We suggest that HCIQ4c9 is a transitional gene form between genes encoding HCGS and HCIQ peptides.
The second approach was to use next-generation amplicon deep sequencing. In total, 25201 reads were obtained. Among these 965 reads were HCIQ transcripts (3.83%); 764 reads with correct reading frame (79.1%), 174 reads with frameshift (18.1%), and 27 short reads (2.8%). All amplicon sequences encoded highly conserved signal and propeptides; only 68 reads contained synonymous substitutions in corresponding regions. The HCIQ6c37 amplicon contained repeats of a propeptide-coding region (Fig. 1). In total, 96 HCIQ mature peptide isoforms were deduced. The isoforms occurring less than in three copies (14.0%) were not taken into further analysis. The most abundant isoforms were HCIQ6c38 (520 amplicons), HCIQ6c15 (17 amplicons) and HCIQ6c39 (8 amplicons), the rest sequences included less seven copies (Fig. 2a). Comparison of the peptide sequences revealed that HCIQ peptides determined by Sanger sequencing except HCIQ4c8 and HCIQ5c10 were also identified by amplicon deep sequencing (Fig. 1).
Diversity of isoforms derived from amplicon deep sequencing and charge distribution of HCIQ peptides. (a) Circular diagram of frequency of HCIQ isoforms derived from amplicon deep sequencing. The image was obtained by Microsoft Excel 14.0 program. (b) Distribution of HCIQ isoforms with different charge values. (c) Distribution of HCIQ-transcripts encoding the peptides with different charge values.
All mature HCIQ peptides taken into analysis (22 isoforms, obtained by amplicon deep sequencing, nine of which were also derived by Sanger sequencing, and two isoforms obtained by Sanger sequencing only, Fig. 1) share high identity from 87 to 99% including six conservative Cys residues (except HCIQ1c5 and HCIQ4c8). The HCIQ peptides have two contact sites with serine proteinases: the main contact site (positions 11–19) and the weak contact site (positions 35–40). The HCIQ peptides contain Arg at the position P1 (named by HCIQ-R) unlike HCGS peptides with Arg (HCGS-R), Lys (HCGS-K), or Thr (HCGS-T) residues at this position (Fig. 1). The amino acid substitutions are concentrated near both contact sites but most of them are close to the weak contact site. The point substitutions Val12Leu in HCIQ6c12, Gly17Arg in HCIQ1c9 and Gly17Glu in HCIQ4c7 were found in the main contact site (Fig. 1). The substitutions such as Ile35 to Thr (HCIQ4c8) or Leu (HCIQ6c2), Cys39 to Arg (HCIQ4c8) as well as Gly40 to Glu (HCIQ6c10) or Lys (HCIQ6c2, HCIQ6c9 and HCIQ6c23) were at the weak contact site positions (Fig. 1). Compared to HCGS peptides, HCIQs contain unique substitutions such as Ile1, Gln2, Asn4, and Ser7.
HCIQ peptides have a narrow range of molecular masses (6258.09–6557.46 Da) and pI values (9.78–10.74) but a wide charge range (+ 2.82–+ 6.09) (Supplementary Table S1). The most prevalent HCIQ isoforms are those with the charge value + 4 (60%), the rest have the charge value + 3 (10%), + 5 (20%), and + 6 (10%) (Fig. 2b), while at the transcriptional level the number cDNAs encoding HCIQ peptides with charge value + 4 increases up to 95% (Fig. 2c).
Determination of exon-intron gene structure
The exon-intron structure of HCIQ genes was determined by PCR amplifications of H. crispa genomic DNA (gDNA) with primer sets 1 (SIG_all_F and PRO_GN_R) and 2 (SIG_GN_F and Inh_XhoI_R) (Fig. 3). H. crispa cDNA was used as a template control. The lengths of PCR fragments obtained from gDNA were longer with an extra 1 kb for the primer set 1 and about 600 bp for the primer set 2 compared to those from cDNA. We compared the sequences and found that the HCIQ genes consisted of three exons. Intron 1 interrupts the conservative exons 1 and 2 encoding the signal peptide while intron 2 separates exon 3 coding the propart and mature peptide (Fig. 3). The intron/exon boundaries follow the GT/AG rule. The content of AT in the exons and introns oscillated slightly from 54.4% (exon 3) to 67.5% (exon 1). The intron identity was about 60%. The sequences of the first HCIQ and HCGS introns shared 93–96% identity.
Scheme of HCIQ gene structure determination. The lengths of PCR fragments are shown below the lines. Horizontal arrows indicate the primer directions. The exons coding for signal peptide are displayed in white boxes; the exon coding for the pro-part and the mature peptide is shown in striped and black boxes respectively. The lengths of HCIQ gene elements are shown above the lines and boxes. The image was carried out using the Paint.net 4.0 program.
Phylogenetic analysis and electrostatic potential of HCIQ-peptides
To explore the evolutionary relationships between HCIQ and HCGS precursors, we have analyzed their nucleotide sequences, containing more informative sites than the amino acid sequences. BPTI and SHTX-III were used as an out-group to construct a NJ phylogenetic tree. HCIQ and HCGS sequences share a common monophyletic origin (Fig. 4). The division into two groups is correlated with polymorphisms of the residue P1: the first group comprises HCIQ-R and HCGS-R clusters, while the second group includes HCGS-K and HCGS-T clusters. At the bottom of HCIQ-R cluster the precursor of HCIQ4c9 is located. Similarly to HCGS precursors, HCIQ4c9 has no propart region, which implies transition from HCGS to HCIQ through gene duplication. Therefore, in accordance with the monophyletic origin and the presence of the transitional gene form, we propose that the HCIQ peptides belong to the HCGS family. At the same time, we can assign the HCIQ-R cluster to a separate subfamily within the HCGS family, based on the high bootstrap values, the presence of the propart, and differences in the signal and mature sequences.
Phylogenetic analysis and electrostatic potential destribution of HCIQ and HCGS peptides. NJ phylogenetic tree was constructed by MEGA 6 using p-distance model and pairwise deletion with bootstrap support of 1000 replications. Nodes with confidence values greater than 50% are indicated. The abundant transcripts are shown with asterisks. Models of representative peptides are shown by green ribbon diagram, with an isopotential surface (blue color for positive charges and red for negative charges). The image was carried out using the SPDBV 4.1 (http://spdbv.vital-it.ch/) program78.
In order to determine how sequence divergence might influence functional specialization, we generated 3D-models of mature HCIQ peptides and calculated their electrostatic potential. The results of electrostatic potential clustering of HCIQ and HCGS peptides showed that both peptide groups were divided into the same four clusters as on the NJ phylogenetic tree, except HCIQ1c5 and HCIQ4c8, which fall into the BPTI cluster, and HCIQ1c9 that belongs to the HCGS-R cluster (Supplementary Fig. S1). Despite the high sequence and spatial structure similarity between HCIQ and HCGS peptides, they have diverse equipotential surfaces (Fig. 4). The majority of HCIQ peptides have a large positive electrostatic potential at the proteinase-binding loop. However, HCIQ4c7 has a local negative charge at the loop, due to Gly17Glu, which may imply a decrease in its proteinase inhibitory activity. In contrast, Gly17Arg in HCIQ1c9 results in an increase of the positive charge and falls into the HCGS-R cluster (Supplementary Fig. S1). Furthermore, the substitutions of Cys31Arg in HCIQ1c5 and Cys39Arg in HCIQ4c8 contribute to a high positive electrostatic potential intrinsic to BPTI cluster. Such electrostatic peculiarities of distinct HCIQ peptides may suggest the functional specialization.
Expression and purification of recombinant HCIQ-peptides
To study the biological activity, we have chosen HCIQ2c1 and HCIQ4c7 with Gly17Glu at the proteinase-binding site. The recombinant HCIQ2c1 and HCIQ4c7 peptides were produced as fusion proteins with thioredoxin in Escherichia coli BL21 (DE3) cells. The fusion proteins with molecular masses about 23 kDa were isolated by metal-affinity chromatography and cleaved by CNBr. Recombinant HCIQ2c1 and HCIQ4c7 were purified by RP-HPLC. Retention times of the peptides on a reverse-phase column amounted to 34.9 min for rHCIQ2c1 and 27.3 min for rHCIQ4c7 (Supplementary Fig. S2). The final yields of rHCIQ2c1 and rHCIQ4c7 were 9.89 mg/l and 13.05 mg/l, respectively. The molecular masses were determined by MALDI-TOF/TOF and amounted to 6330 (rHCIQ2c1) and 6404 (rHCIQ4c7) Da, consistent with the predicted molecular masses. The N-terminal amino acid sequences (15 aa) determined by the automated Edman degradation matched well with amino acid sequences deduced from cDNA.
Inhibitory activity of rHCIQ2c1 and rHCIQ4c7
The peptides rHCIQ2c1 and rHCIQ4c7 inhibit trypsin. The value of the inhibition constant (Ki) of trypsin for rHCIQ2c1 (5.2 × 10−8 M) is closer to Ki values for the peptides in the groups with Arg and Lys at the position P1 (Table 1). At the same time, the value of Ki for rHCIQ4c7 (1.9 × 10−7 M) was the highest in the group with Arg but comparable with those of the peptides with Thr at the position P1.
Interaction of rHCIQ2c1 and rHCIQ4c7 with serine proteinases
The interaction of the peptides with serine proteinases (trypsin, α-chymotrypsin, human neutrophil elastase, pancreatic kallikrein and cathepsin G) was studied using the surface plasmon resonance (SPR) method. The peptides were immobilized on a CM4 sensor chip. It was determined that rHCIQ2c1 specifically binds to all target proteinases (Supplementary Fig. S3a), whereas rHCIQ4c7 bounds to trypsin and elastase only (Supplementary Fig. S3b). Kinetic parameters were calculated at 25 °C. Obtained data indicated the formation of tight complexes and eliminated non-specific binding (Table 2). The association constant (KA) magnitudes of the rHCIQ2c1/proteinase complexes were in the following order: trypsin >cathepsin G > α-chymotrypsin > elastase > kallikrein. The dissociation constant (KD) magnitudes of the complexes were in the range of 10−10–10−7 M; KD of rHCIQ4c7 with trypsin being higher by three orders than for rHCIQ2c1, while binding with elastase differed insignificantly (Table 2).
Thermodynamic parameters of intermolecular interactions were determined for the complexes of rHCIQ2c1 with trypsin and α-chymotrypsin in temperature range of 10–40 °С at an interval of 5 °С (Table 3). The magnitudes of free energy change (∆G) of the complexes differed slightly from the same complexes of HCRG1 and HCRG2 and were lower than for the InhVJ ones.
Effect of rHCIQ2c1 and rHCIQ4c7 on Kv channels
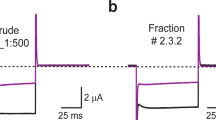
The electrophysiological assays of rHCIQ2c1 and rHCIQ4c7 effects on eight cloned Kv channels (rKv1.1 – rKv1.6, Shaker IR, and hERG) were carried out. It was found that neither 10 μM of rHCIQ2c1 nor rHCIQ4c7 exerted any activity on the Kv channels (Fig. 5).
Differential effects of rHCIQ2c1 and rHCIQ4c7 on Kv isoforms expressed in Xenopus laevis oocytes. Representative whole cell K+ current traces of oocytes expressing cloned Kv isoforms Kv1.1-Kv1.6, hERG (Kv11.1) and the Drosophila channel Shaker IR. The dotted line indicates zero-current level. The asterisk (*) indicates the steady-state current peak amplitude in the presence of 10 µM rHCIQ2c1 and rHCIQ4c7. The image was carried out by pClamp Clampfit 10.0 (Molecular Devices, Downingtown, PA, USA) and Origin 7.5 software (Originlab, Northampton, MA, USA).
Effect of rHCIQ2c1 and rHCIQ4c7 on 6-OHDA-induced cell death
Effects of rHCIQ2c1 and rHCIQ4c7 were studied using murine neuroblastoma Neuro2a cells in the presence of 6-OHDA (25 µM). After incubation of Neuro2a cells with recombinant peptides and 6-OHDA for 24 h, the cell viability was evaluated using MTT assay. As shown in Fig. 6a, rHCIQ4с7 and rHCIQ2с1 at concentrations of 10 μM were non-toxic and increased the cell viability by 14% and 47%, relative to cells treated with 6-OHDA respectively. The reliable effect was revealed for rHCIQ2c1. The cytoprotective effect of rHCIQ2с1 had a linear dose-dependent character and the maximum activity was achieved at concentration of 10 μM (Fig. 6b).
Effects of recombinant peptides on neuroblastoma cell viability with 6-OHDA. (a) The effect of 10 µM peptides on the viability of 6-OHDA-treated Neuro2a cells. (b) Dose-response effect of rHCIQ2c1 on Neuro2a cell viability. (c) Dose-response effect of rHCIQ2c1 on ROS formation into 6-OHDA-treated murine neuroblastoma cells. The influence of peptides on cell viability without 6-OHDA is shown in the white bar. * – p < 0.05.
Detection of intracellular ROS formation level
We estimated whether rHCIQ2c1 is able to inhibit reactive oxygen species (ROS) production. After incubation of Neuro2a cells with rHCIQ2c1 (up to 10 µM) and 6-OHDA (25 µM), ROS content was evaluated by measurement of dichlorofluorescin intensity. The highest ROS level was observed in the cells with 6-OHDA alone while the pretreatment with rHCIQ2c1 reduced ROS production by 34% to concentration of 0.001–0.01 µM. An increase in ROS formation when the concentration of rHCIQ2c1 increases from 0.01 to 1 µM, and its decrease again at 10 µM is probably due to complicated bell-shaped character of peptide influence upon intracellular ROS level (Fig. 6c).
Discussion
The sea anemone H. crispa Kunitz-peptides form a combinatorial library, consisting of HCGS, HCRG, HCGG, and HCGN peptides coded by the HCGS multigene family5. The HCGN group was represented by only one sequence with propeptide insertion. The pro-part contained the subtilisin/kexin-like endoproteinase cleavage site (Lys-Arg) also found in precursors of actinoporins32,33,34 and neurotoxins35,36,37 involved in sea anemone venoms. Obviously, the mature HCIQ sequences start with Ile-Gln- (IQ-), rather than with Gly-Asn- (GN-) as suggested earlier, so, we designated the peptides as HCIQ where “HC” means H. crispa (Fig. 1). On the basis of PCR cloning and amplicon deep sequencing, we deduced 24 HCIQ peptides differing by both the precursor structure and point substitutions at the mature peptide. The most prevalent isoforms were HCIQ2c1, HCIQ6c15 and HCIQ5c1. The HCIQ precursors shared 88–98% identity to HMIQ3c1 derived from H. magnifica cDNA13. We revealed that HCIQs had four precursor structures differing by the presence of the propeptide (HCIQ2c1) or its tandem repeats (HCIQ6c37) and lacking the propeptide (HCIQ4c9) or C-terminal signal region (HCIQ2c9) (Fig. 1). Kunitz-type toxin genes of spider Ornithoctonus huwena also contain repeats in the precursor which, together with hypermutation and post-translational modifications, form the spider toxin diversity38. Importantly, a propeptide sequence has not been found in the known Kunitz-precursors of sea anemones while it has been described for their toxin precursors. The main function of a propeptide in the toxin precursors is the protection of its own tissues from crucial damages by these toxins. In conopeptides, the pro-part may be involved in folding and modification39,40. In addition, a short propeptide (6 aa) was also discovered in Kunitz-peptide precursors of Conus californicus6 and several spider species4,41,42 while the Lys-Arg cleavage site was found only in C. californicus precursors. We suggest that the appearance of the propeptide-coding region indicates that the HCIQ peptides could be involved in the sea anemone venom.
There is little information on gene organization of sea anemone Kunitz-homologs. Therefore, we determined the exon-intron structure of the HCIQ genes. We found that they included two introns in contrast to HCGS genes with one intron5. The first intron divided the sequences coding the signal peptide and the second intron separated the sequence coding the propeptide and the mature peptide (Fig. 3). Both HCIQ introns displayed low conservation between each other. Therefore, the first HCIQ intron shares high identity to HCGS intron including the presence of CA-repeat elements of different lengths. The CA-repeats can affect gene expression and participate in splicing regulation; long elements act as splicing enhancers while the short ones do as silencers43,44. The second HCIQ intron has no CA-repeats, and it is more variable than the first one. It strongly suggests that the second intron appeared because of the duplication of the first intron throughout the evolution of HCIQ genes.
According to the phylogenetic tree, HCIQ and HCGS genes originated from a common ancestor (Fig. 4). More precisely, HCIQ genes evolved following duplication of HCGS-R genes. This can be explained by the presence of a transitional gene form (HCIQ4c9), which is placed at the bottom of HCIQ-R cluster and does not code pro-part similarly to the HCGS genes. Therefore, we assert that HCIQ genes belong to the HCGS multigene family because of an absolute identity of N-terminal signal peptide encoding sequences. The presence of the second intron and pro-part encoding region in HCIQ genes as well as the variation of C-terminal signal and N-terminal mature regions of the precursors are likely to result from an adaptive evolution. This allows us to assign HCIQ genes to a separate subfamily within the HCGS multigene family.
To understand the functional specialization of HCIQ peptides, their electrostatic potential distribution was calculated. This characteristic is important to determine the specificity and kinetics of peptide molecular binding to the target45. Generally, the results of electrostatic potential clustering of HCIQ and HCGS peptides coincide with the phylogenetic data. Notably, two additional Ile-Gln residues on N-terminus do not contribute greatly to the charge distribution. The majority of HCIQ peptides have equipotential surfaces, similar to abundant HCIQ2c1 (Fig. 4). The peptide keeps a large positive charge at the proteinase-binding loop, typical for efficient Kunitz-type serine proteinase inhibitors46,47. Interestingly, HCIQ4c7 differed from HCIQ2c1 by a local negative charge in the loop (Gly17Glu) that may influence its biological activity.
Previous studies showed that Kunitz-peptides from H. crispa exhibit inhibitory activity against trypsin13,14,17,20,48,49. The HCIQ and HCGS peptides share high identity. The major differences are localized on N-terminus of the peptides (1, 2, 4, and 7 residues of HCIQs). The main and weak contact sites of HCIQ peptides have high residue conservation (Fig. 1). Both sites take part in the interaction with serine proteinases and define inhibitory specificity50. The main contact site is located on a relatively conserved binding loop and contains the residue P1, which makes a large part of enzyme-inhibitor contacts47,48,49,50,51. The residues of a weak contact site make additional electrostatic interactions and H-bonds with the proteinase active site49. The main contact site of HCIQ peptides completely coincides with the HCGS-R group whose representative, rHCGS1.19, inhibits trypsin (Ki 3.0 × 10−8 M)17. Furthermore, a high percentage of identity is observed between HCIQs and SHPI-1 from S. helianthus (87–94%), which inhibits serine, cysteine and aspartic proteinases along with blocking Kv channels47. Therefore, we suppose HCIQ peptides have to possess similar activities. At the same time, differences may indicate the direction to other targets.
For the investigation of biological activity, rHCIQ2c1 (the prevalent isoform) and rHCIQ4c7 (Gly17Glu) were obtained. Previous studies showed strong inhibitory activity of Kunitz-peptides was associated with a high positive charge at the proteinase-binding loop51. Therefore, the negatively charged amino acid residue at the main contact site of rHCIQ4c7 may decrease the ability to inhibit proteinases. Indeed, the inhibition constant value of trypsin for rHCIQ4c7 (Ki 1.9 × 10−7 M) is higher than those for rHCIQ2c1 (Ki 5.2 × 10−8 M) (Table 2). Despite high sequence identity of rHCIQ2c1 and rHCIQ4c7 with SHPI-1 (91–93%) from S. helianthus47, Ki for SHPI-1 is lower because there are differences in the main contact site residues including position P1.The study of the interaction of peptides with five serine proteinases by the SPR method showed that rHCIQ2c1 binds to all targeting proteinases (Supplementary Fig. S3a) in contrast to the characterized InhVJ, HCRG1 and HCRG2 from H. crispa15,52. rHCIQ4c7 is more specific than rHCIQ2c1; it binds to trypsin and human neutrophil elastase only (Supplementary Fig. S3b). The values of the entropic factor at both cases contributed to complex formation while the values of enthalpy indicated possible breakage of hydrogen and electrostatic bonds or changes of molecule conformation during the complex formation process38. Similar entropy-dependent complex formation was also observed for HMIQ3c153, InhVJ52, HCRG1 and HCRG215. According to the dissociation constant values, the most stable complexes are rHCIQ2c1/trypsin, rHCIQ2c1/cathepsin G and rHCIQ2c1/α-chymotrypsin. Both recombinant peptides interact with human neutrophil elastase with dissociation constant values around 10−7 M while other known Kunitz-peptides from H. crispa do not bind to the inflammatory proteinase15,52. Currently, the weak inhibitory activity against elastase has been shown also for SHPI-154. Interestingly, rHCIQ2c1 binds to three inflammatory proteinases (cathepsin G, kallikrein and neutrophil elastase) indicating a potential anti-inflammatory activity.
We examined the effect of rHCIQ2c1 and rHCIQ4c7 in an in vitro model of 6-OHDA-induced cytotoxicity on murine Neuro2a cells. 6-OHDA is able to penetrate cells using dopamine (DAT) or norepinephrine (NET) transporters55 and stimulate ROS formation leading to an oxidative stress and cell death56,57,58. We revealed the peptides are capable to increase cell viability in the presence of 6-OHDA; the reliable effect is displayed for rHCIQ2c1 (Fig. 6a). This peptide decreased toxic activity of 6-OHDA in a dose-dependent manner (Fig. 6b) and effectively inhibited intracellular ROS formation (Fig. 6c). It was shown earlier that the Kunitz-like peptide, PcKuz3, from P. caribaeorum was able to suppress 6-OHDA-induced neurotoxicity in zebrafish larvae31. The authors hypothesized the effect is caused by blocking Kv1 channels31. Indeed, Kv channels are actively involved in the regulation of neuronal processes and considered potential therapeutic targets for some neurodegenerative diseases, such as PD59,60. Thus, 4-aminopyridine, the blocker of Kv1 and Kv3 channel subfamily, suppresses MPTP-induced neurodegeneration and rat behavioral disturbances61; ShK-170 from S. helianthus is able to mitigate radiation-induced brain injury via Kv1.3 blocking62. Moreover, Kunitz-type inhibitor SHPI-1 is also able to block Kv1.1, Kv1.2, and Kv1.647. However, neither rHCIQ2c1 nor rHCIQ4c7 exerted Kv1 channel activity (Fig. 5). Consequently, the further investigation of the action mechanism of rHCIQ2c1 against 6-OHDA-induced neurotoxicity has to focus on the determination of the peptide interaction with dopamine transporter or enzymes involved in the synthesis and/or degradation of ROS.
In conclusion, here we have shown that paralogous genes coding Kunitz-peptides in H. crispa genome form distinct subfamilies within a great multigene family. The appearance of a propeptide in HCIQ precursors may be necessary for the employment of the peptides in sea anemone venom in enhancing toxic effects. It has been revealed for the first time that Kunitz-peptides are able to decrease the neurotoxic effect of 6-OHDA on neuroblastoma cells by reducing the ROS level. Taking into account their specificity to target proteinases, including inflammatory proteinases, we suppose that sea anemone Kunitz-peptides possess a great therapeutic potential in the complex treatment of neurodegenerative diseases.
Materials and Methods
Animal collection
Heteractis crispa specimens were collected near Vietnam during a marine expedition aboard the research vessel “Academik Oparin”. Dr. E. Kostina (National Scientific Center of Marine Biology of the Far Eastern Branch of the Russian Academy of Sciences, Vladivostok, Russia) confirmed the identity of the species.
Precursor and gene structure determination
PCR amplification of sequences encoding HCIQ peptides was carried out using gene specific forward primers SIG_all_F (5′- CAA AGA CAA GAT AAC AAG ATG AAG GGA-3′) and SIG_GN_F (5′-AGG TTT CTA TTT CAG AAG CAT TCA AGG T-3′) complementary to 5′- and 3′-tereminal sequences coding signal peptide, respectively, and reverse primer Inh_XhoI_R (5′-ACT CGA GTT ACG CCC TGC ATA TAG CTC GGC AT-3′) complementary to 3′-terminal sequence coding mature peptide. PCR was conducted using GoTaq DNA Polymerase (Promega, USA) under the following conditions: 95 °C for 2 min; 30 cycles of 94 °C 30 s; 60 °C 30 s; 72 °C 1 min; 72 °C 3 min. Complementary DNA isolated earlier63 was used as a template. PCR-fragments (∼250 bp) were analyzed by gel electrophoresis, purified, cloned into the pTZ57R/T vector (Thermo Fisher Scientific, USA), and transformed into Top 10 E. coli cells (Invitrogen, Life Technologies) according to standard protocols64. Positive colonies were screened by amplifications with M13 universal primers. Plasmids containing inserts were sequenced using the ABI 3130xl Genetic Analyzer (Applied Biosystems, USA) according to the manufacturer protocol.
Genomic DNA (gDNA) was isolated from H. crispa tentacles by homogenization in guanidinium thiocyanate followed by phenol-chloroform extraction64 and used as a template to identify introns into coding region of HCIQ genes. PCR amplifications were conducted using two gene specific primer sets: first one, Inh_SIG_all_F and Inh_PRO_GN_R (5′-ATG TTA CCT TGA ATC CTT TTG-3′) complementary to sequences encoding propart; the second one, SIG_GN_F and Inh_XhoI_R. Complementary DNA was used as control. PCR amplification, cloning, and sequencing were carried out as described above.
Amplicon deep sequencing
The sequence diversity of HCIQ-peptides of sea anemone H. crispa was investigated with amplicon deep sequencing using a ‘One-Way Reads’ experimental design according to Guidelines for Amplicon Experimental Design (Roche/454 Life Sciences). To generate amplicons, cDNA sample isolated from H. crispa was amplified by PCR with fusion primers A_Mid1sig_allF (5′-CGT ATC GCC TCC CTC GCG CCA TCA GAC ACG ACG ACT CAA AGA CAA GAT AAC AAG ATG AAG GGA-3′) and B_inh_R (5′-CTA TGC GCC TTG CCA GCC CGC TCA GCT AAC AAG ATT ACG CCC TGC ATA-3′), including a 21-mer adaptor sequences, sequencing key’TCAG‘, 11-base molecular barcode RL001 and template-specific primer complementary to signal part of HCGS-peptides, and GoTaq DNA Polymerase (Promega, USA) under the following conditions: 95 °C for 3 min; 35 cycles of 94 °C 30 s, 55 °C 30 s, 72 °C 1 min; 72 °C 15 min. Amplicons were analyzed by gel electrophoresis, purified and quantified using the Quant-iT PicoGreen dsDNA reagent (Invitrogen, Life Technologies). Emulsion PCR, bead recovery, bead enrichment and sequencing on a Genome Sequencer Junior (Roche/454 Life Sciences) were performed using the GS Junior Titanium series sequencing reagents and corresponding protocols. Data analysis was performed using the GS Amplicon Variant Analyzer (AVA) version 2.7 (Roche/454 Life Sciences) and HCIQ2с1 sequence was used as the alignment reference.
Sequence, phylogenetic analysis and modeling
Determined nucleotide and amino acid sequences were aligned by MEGA 665 and Vector NTI (Invitrogen, Life Technologies) programs. Searching of the Kunitz/BPTI homologs was carried out in the GeneBank database using BLASTN and BLASTP algorithms (http://www.ncbi.nlm.nih.gov/BLAST). The phylogenetic analysis of HCIQ precursor nucleotide sequences was conducted in MEGA 665, using the neighbor-joining method66 with a bootstrap test67 of 1000 replicates. The evolutionary distances were computed using the p-distance method68. BPTI from B. taurus and SHTX-III from S. haddoni were used as an out-group. Alignment gaps were excluded using function “Pairwise deletion”. The spatial structure models of HCIQ peptides were generated using Modeller 9.11 and Chimera 1.969,70 programs. The atomic coordinates of SHPI-1 (PDB ID 1SHP) from the sea anemone S. helianthus used as a template (the identity between SHPI-1 and HCIQs is 87–94%). The quality of the models was tested using a web server PROCHECK and MOE program (Chemical Computing Croup, Inc.; http://www.chemcomp.com/). The electrostatic potential distribution of Kunitz peptide models was computed and equipotential surfaces were visualized using program SPDBV 4.1 (http://spdbv.vital-it.ch/). Clustering of HCIQ and HCGS peptides based on electrostatic properties was conducted using webPIPSA server (http://pipsa.h-its.org/)45. Calculation of HCIQ peptide molecular masses was performed using the software Vector NTI 10 (Invitrogen, Life Technologies). Total net charges and pI values of these peptides were computed by PROPKA 2.0 program71 on the basis of 3D-structure models.
Cloning, expression and purification of HCIQ-peptides
To amplify sequences encoding mature HCIQ peptides gene-specific forward primer GN_EcoR_F (5′-GCG AAT TCG ATG ATT CAA GGT AAC ATT TGT TCA-3′) containing EcoRI restriction site and Met-codon for CNBr cleavage and reverse primer Inh_XhoI_R (5′-ACT CGA GTT ACG CCC TGC ATA TAG CTC GGC AT-3′) containing XhoI restriction site and stop-codon were used. PCR of the DNA-fragments built in pTZ57R/T vector (Thermo Fisher Scientific, USA) was carried out using GoTaq DNA Polymerase (Promega, USA) under the following conditions: 95 °C for 3 min; 30 cycles of 94 °C 30 s, 55 °C 30 s, 72 °C 1 min; 72 °C 15 min. The recombinant plasmids pTZ57/hciq2c1 and pTZ57/hciq4c7 were used as templates. PCR-fragments (∼200 bp) were restricted by EcoRI and XhoI endonucleases and cloned into the pET32b(+) vector (Novagen, Germany). After verification by sequencing of the recombinant plasmids were transformed into BL21 (DE3) E. coli cells (Novagen, Germany) for expression.
Cells transformed with the recombinant plasmids were cultured at 37 °C in Luria-Bertani medium containing 100 µg/ml ampicillin up to reaching the optical density (OD600) ~0.5. After induction with IPTG in concentrations of 0.2 mM for rHCIQ2c1 and 0.3 mM for rHCIQ4c7, the cells were incubated at 37 °C for 3 hours at 180 rpm, centrifuged for 6 min at 6000 rpm at 4 °C, and supernatant was removed. Occurrence of recombinant peptides was determined in 12% polyacrylamide gel by Laemmli’s SDS-PAGE method72. Precipitate was resuspended in the start buffer for affinity chromatography (400 mM NaCl, 20 mM Tris-HCl buffer, pH 8.0) and ultrasonicated on ice. Then lysed cells were centrifuged for 10 min at 10000 rpm to remove all insoluble particles. Supernatant was applied to a Ni-NTA agarose (Qiagen, Netherlands) and fusion protein was purified under native conditions according to the manufacturer’s instructions. The collected fusion protein was cleaved by CNBr overnight at room temperature with molar ratio CNBr to protein 600:1. The recombinant peptides were purified from reaction mixture on reverse-phase column Jupiter C4 10 × 250 mm (Phenomenex, USA) using a linear gradient of acetonitrile (from 0% to 70%) with 0.1% TFA in 70 min with a constant flow rate of 1.5 ml/min. The molecular masses of the purified peptides were analyzed by Ultra Flex III MALDI-TOF/TOF mass spectrometer (Bruker, Germany). The amino acid sequences of the recombinant peptides were determined on an automated sequencer protein Procise 492 Clc (Applied Biosystems, Foster City, CA, USA).
Trypsin inhibitory activity
The trypsin inhibitory activity of HCIQ peptides was estimated according to the standard procedure using N-α-benzoyl-D,L-arginine p-nitroanilide (BAPNA) as a substrate73. The trypsin inhibition constants of rHCIQ2c1 and rHCIQ4c7 were determined by the method of Dixon74 using substrate (BAPNA) concentrations of 0.6 and 1.2 mM. The trypsin concentration in the reaction mixture was 208 nM. The range of peptide concentrations was 0–26.6 µM for rHCIQ2c1 and 0–24 µM for rHCIQ4c7. The constants (Ki) were calculated based on the results of three parallel experiments. Computational error limits were in the range of 0.1–0.5%.
SPR measurements
The study of the interaction of serine proteinases with rHCIQ2c1 and rHCIQ4c7 was performed on SPR biosensor Biacore 3000 (GE Healthcare, USA) running under the program “Biacore 3000 Control Software v.1.0”. The evaluation of data was carried out using “Biacore Evaluation v.4.1”. Peptides were covalent immobilized on carboxymethylated surface of the Biacore CM4 sensor chip (GE Healthcare, USA) activated by EDC/NHS (N-hydroxysuccinimide/1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride) mixture by the injection of peptide solution (100 µg/ml) in 10 mM sodium acetate (pH 5.5) during 10 min with flow rate 5 µl/min. Quantities of immobilized peptides were 970 RU (resonance units, 1 RU corresponds 1 pg peptide) for rHCIQ2c1 and 460 RU for rHCIQ4c7. In all of the experiments, the flow cell 1 (Fc1) without the immobilized polypeptides was considered as the reference cell for the correction of the signal responses. HBS (HEPES buffered saline-NaCl): 0.15 M NaCl, 0.01 M HEPES, pH 7.4 (cat. no. BR-1003-69, GE Healthcare, USA) was used as the running buffer for the SPR assays. Protease solutions at concentrations from 1 nM to 5 μM were passed through measuring and control biosensor cells at 25 °C during 5 min with a flow rate 5 µl/min. Further complex breakdown was recorded during at most 10 min. After each cycle of SPR measurement, the sensing surface was regenerated by the injection of 50 mM NaOH for 0.5 min at a flow rate of 50 μl/min. Changes in Gibbs free energy (ΔG) at 25 °C were calculated by the following equation:
where KD is the dissociation constant. Changes in enthalpy (ΔH) and entropic term (−TΔS) were calculated from the linear equation:
using the liner approximation of temperature dependence of ΔG (Van’t Hoff diagram).
Expression of voltage-gated ion channels in X. laevis oocytes
For the expression of the voltage-gated potassium channels (rKv1.1, rKv1.2, hKv1.3, rKv1.4, rKv1.5, rKv1.6, Shaker IR and hERG) in X. laevis oocytes, the linearized plasmids were transcribed using the T7 or SP6 mMESSAGE-mMACHINE transcription kit (Ambion, USA). The harvesting of stage V–VI oocytes from an anaesthetized female X. laevis frog was previously described75. Oocytes were injected with 50 nl of cRNA at a concentration of 1 ng/nl using a micro-injector (Drummond Scientific, USA). The oocytes were incubated in a solution containing (in mM): NaCl, 96; KCl, 2; CaCl2, 1.8; MgCl2, 2; HEPES, 5 (pH 7.4), supplemented with 50 mg/l gentamycin sulfate.
Electrophysiological recordings
Two-electrode voltage-clamp recordings were performed at room temperature (18–22 °C) using a Geneclamp 500 amplifier (Molecular Devices, USA) controlled by a pClamp data acquisition system (Axon Instruments, USA), as described in47. Whole cell currents from oocytes were recorded 1–4 days after injection. Bath solution composition was ND96 (in mM): NaCl, 96; KCl, 2; CaCl2, 1.8; MgCl2, 2 and HEPES, 5 (pH 7.4). Voltage and current electrodes were filled with 3 M KCl. The resistances of both electrodes were kept between 0.7 and 1.5 MΩ. The elicited currents were filtered at 0.5 kHz and sampled at 2 kHz using a four-pole low-pass Bessel filter. Leak subtraction was performed using a -P/4 protocol. Kv1.1–Kv1.6 and Shaker currents were evoked by 250 ms depolarizations to 0 mV followed by a 250 ms pulse to −50 mV, from a holding potential of −90 mV. Current traces of hERG channels were elicited by applying a + 40 mV prepulse for 2 s followed by a step to −120 mV for 2 s. Comparison of two sample means was made using a paired Student’s test (p < 0.05). All data represent at least three independent experiments (n ≥ 3) and are presented as mean ± standard error. All data were analysed using pClamp Clampfit 10.0 (Molecular Devices, Downingtown, PA, USA) and Origin 7.5 software (Originlab, Northampton, MA, USA).
6-Hydroxydopamine-induced cytotoxicity model
The cytoprotective activities of peptides was examined, as described previously76. Neuro2a murine neuroblastoma cells (ATCC CCL-131) (American Type Culture Collection, Virginia, USA) were cultivated in DMEM medium (BioloT, Russia) with 10% of fetal bovine serum (FBS, BioloT, Russia) and 80 µg/ml gentamicine (BioloT, Russia). The cells were seeded onto 96-well plate at concentration of 1 × 103 cells per well. Substances were added at different concentrations, and then cells were incubated for 30 min at 37 °C and 5% CO2. Cells were treated with 6-OHDA (25 μM) for 24 h at 37 °C with 5% CO2. Thereafter, medium was replaced with clean serum-free medium, and cells were treated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for 4 h77. Then the medium was collected and whole cells were lysed in 100% dimethylsulfoxide (DMSO), the absorbance was measured at 570 nm using Multiscan FC (Thermo Fisher Scientific, USA). Cytotoxic activity of substance was calculated as concentration of 50% metabolic cell activity inhibition (IC50) . The results were expressed as the mean ± SD of three independent experiments.
Analysis of ROS production
The intracellular ROS level was detected using 2,7-dichlordihydrofluorescein diacetate (H2DCF-DA) solution (Molecular Probes, final concentration 10 µM). In this study, mouse neuroblastoma Neuro2a cells seeded on 96-well plates were washed twice with serum-free DMEM and thereafter incubated for 1 h in serum-free MEM medium in the presence of compounds in concentrations from 10 pM to 10 µM. Cell were treated with 6-OHDA (25 μM) for 30 min at 37 °C with 5% CO2. Followed by treatment with 6-OHDA, the cells were washed once with PBS. After washing with serum-free DMEM medium, the cells were loaded with H2DCF-DA for 30 min. The intensity of dichlorofluorescin fluorescence was measured with plate reader PHERAstar FS (BMG Labtech, Germany) at λex = 485 nm and λem = 518 nm. The cytoprotective effect of peptides and the increase in cell viability under their action was calculated as a percentage, taking the cell viability treated with 6-OHDA as 100%.
Accession codes
sequences obtained by Sanger sequencing were submitted to GeneBank database under accession numbers MH249934–MH249943.
Data availability
The additional data that support the manuscript are available from the corresponding author upon request.
References
Mourão, C. B. F. & Schwartz, E. F. Protease inhibitors from marine venomous animals and their counterparts in terrestrial venomous animals. Mar. Drugs 11, 2069–2112 (2013).
Župunski, V., Kordiš, D. & Gubenšek, F. Adaptive evolution in the snake venom Kunitz/BPTI protein family. FEBS Lett. 547, 131–136 (2003).
Ranasinghe, S. & McManus, D. P. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev. Comp. Immunol. 39, 219–227 (2013).
Yuan, C. H. et al. Discovery of a distinct superfamily of Kunitz-type toxin (KTT) from tarantulas. PLoS One 3, e3414 (2008).
Isaeva, M. P. et al. A new multigene superfamily of Kunitz-type protease inhibitors from sea anemone Heteractis crispa. Peptides 34, 88–97 (2012).
Elliger, C. A. et al. Diversity of conotoxin types from Conus californicus reflects a diversity of prey types and a novel evolutionary history. Toxicon 57, 311–322 (2011).
Chen, Z. et al. Genomic and structural characterization of Kunitz-type peptide LmKTT-1a highlights diversity and evolution of scorpion potassium channel toxins. PLoS One 8, e60201 (2013).
Fry, B. G. et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 10, 483–511 (2009).
Casewell, N. R., Wu, W., Vonk, F. J., Harrison, R. A. & Fry, B. G. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol. Evol. 28, 219–229 (2013).
Kordiš, D. & Gubenšek, F. Adaptive evolution of animal toxin multigene families. Gene 261, 43–52 (2000).
Kozlov, S. & Grishin, E. The mining of toxin-like polypeptides from EST database by single residue distribution analysis. BMC Genomics 12, 88 (2011).
Madio, B., Undheim, E. A. B. & King, G. F. Revisiting venom of the sea anemone Stichodactyla haddoni: Omics techniques reveal the complete toxin arsenal of a well-studied sea anemone genus. J. Proteomics 166, 83–92 (2017).
Kvetkina, A. N. et al. A New IQ-peptide of the Kunitz-type from the Heteractis magnifica sea anemone exhibits neuroprotective activity in a model of Alzheimer’s Disease. Russ. J. Bioorganic Chem. 44, 416–423 (2018).
Sintsova, O. V. et al. Anti-inflammatory activity of a polypeptide from the Heteractis crispa sea anemone. Russ. J. Bioorganic Chem. 41, 657–663 (2015).
Gladkikh, I. et al. New Kunitz-type HCRG polypeptides from the sea anemone Heteractis crispa. Mar. Drugs 13, 6038–6063 (2015).
Monastyrnaya, M. et al. Kunitz-Type peptide HCRG21 from the sea anemone Heteractis crispa is a full antagonist of the TRPV1 receptor. Mar. Drugs 14, 229 (2016).
Sintsova, O. V. et al. Kunitz-type peptides of the sea anemone Heteractis crispa: Potential anti-inflammatory compounds. Russ. J. Bioorganic Chem. 43, 91–97 (2017).
Tabakmakher, V. M. et al. Analgesic effect of novel Kunitz-type polypeptides of the sea anemone Heteractis crispa. Dokl. Biochem. Biophys. 461, 80–83 (2015).
Andreev, Y. A. et al. Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1). J. Biol. Chem. 283, 23914–23921 (2008).
Sokotun, I. N. et al. Proteinase inhibitors from the tropical sea anemone Radianthus macrodactylus: isolation and characteristic. Biochem. 72, 301–306 (2007).
Dias, V., Junn, E. & Mouradian, M. M. The role of oxidative stress in Parkinson’s Disease. J. Park. Dis 3, 461–491 (2013).
Hwang, O. Role of oxidative stress in Parkinson’s Disease. Exp. Neurobiol. 22, 11–17 (2013).
Soto-Otero, R., Méndez-Álvarez, E., Hermida-Ameijeiras, Á., Muñoz-Patiño, A. M. & Labandeira-Garcia, J. L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: Potential implication in relation to the pathogenesis of Parkinson’s disease. J. Neurochem. 74, 1605–1612 (2000).
Feng, C., Hung, H., Huang, S. & Chen, C. Neuroprotective effect of the marine-derived compound 11-dehydrosinulariolide through DJ-1-related pathway in in vitro and in vivo models of Parkinson’s disease. 1–18 (2016) https://doi.org/10.3390/md14100187.
Wang, Z. et al. Neuroprotective effects of benzyloxy substituted small molecule monoamine oxidase B inhibitors in Parkinson’s disease. Bioorganic Med. Chem. 24, 5929–5940 (2016).
Kang, S. Y. et al. Autophagic modulation by rosuvastatin prevents rotenone-induced neurotoxicity in an in vitro model of Parkinson’s disease. Neurosci. Lett. 642, 20–26 (2017).
Yamazaki, M., Chiba, K. & Satoh, K. Neuro2a cell death induced by 6-hydroxydopamine is attenuated by Genipin. J. Heal. Sci. 54, 638–644 (2008).
Tunçel, N. et al. Antioxidant and anti-apoptotic activity of Vasoactive Intestinal Peptide (VIP) against 6-hydroxy dopamine toxicity in the rat corpus striatum. J. Mol. Neurosci. 46, 51–57 (2012).
Sun, S. Y., An, C. N. & Pu, X. P. DJ-1 protein protects dopaminergic neurons against 6-OHDA/MG-132-induced neurotoxicity in rats. Brain Res. Bull. 88, 609–616 (2012).
Yin, S.-M. et al. Neuroprotection by scorpion venom heat resistant peptide in 6-hydroxydopamine rat model of early-stage Parkinson’s disease. Acta Physiol. Sin. 66, 658–666 (2014).
Liao, Q. et al. Novel Kunitz-like peptides discovered in the zoanthid Palythoa caribaeorum through transcriptome sequencing. J. Proteome Res. 17, 891–902 (2018).
Leychenko, E. et al. Multigene family of pore-forming toxins from sea anemone Heteractis crispa. Mar. Drugs 16, 183 (2018).
Wang, Y., Chua, K. L. & Khoo, H. E. A new cytolysin from the sea anemone, Heteractis magnifica: isolation, cDNA cloning and functional expression. Biochem. Biophys. acta 1478, 9–18 (2000).
Pungerčar, J., Anderluh, G., MačEk, P., Franc, G. & Štrukelj, B. Sequence analysis of the cDNA encoding the precursor of equinatoxin V, a newly discovered hemolysin from the sea anemone Actinia equina. Biochim. Biophys. Acta 1341, 105–107 (1997).
Honma, T., Nagai, H., Nagashima, Y. & Shiomi, K. Molecular cloning of an epidermal growth factor-like toxin and two sodium channel toxins from the sea anemone Stichodactyla gigantea. Biochim. Biophys. Acta - Proteins Proteomics 1652, 103–106 (2003).
Honma, T. et al. Novel peptide toxins from the sea anemone Stichodactyla haddoni. Peptides 29, 536–544 (2008).
Oliveira, J. S., Fuentes-Silva, D. & King, G. F. Development of a rational nomenclature for naming peptide and protein toxins from sea anemones. Toxicon 60, 539–550 (2012).
Jiang, L., Deng, M., Duan, Z., Tang, X. & Liang, S. Molecular cloning, bioinformatics analysis and functional characterization of HWTX-XI toxin superfamily from the spider Ornithoctonus huwena. Peptides 54, 9–18 (2014).
Bandyopadhyay, P. K. et al. Conantokin-G precursor and its role in γ-carboxylation by a vitamin K- dependent carboxylase from a Conus snail. J. Biol. Chem. 273, 5447–5450 (1998).
Buczek, O., Olivera, B. M. & Bulaj, G. Propeptide does not act as an intramolecular chaperone but facilitates protein disulfide isomerase-assisted folding of a Conotoxin precursor. Biochem. 43, 1093–1101 (2004).
Tang, X. et al. Molecular diversification of peptide toxins from the tarantula Haplopelma hainanum (Ornithoctonus hainana) venom based on transcriptomic, peptidomic, and genomic analyses. J. Proteome Res. 9, 2550–2564 (2010).
Jiang, L. et al. Genomic organization and cloning of novel genes encoding toxin-like peptides of three superfamilies from the spider Orinithoctonus huwena. Peptides 29, 1679–1684 (2008).
Hui, J. et al. Intronic CA-repeat and CA-rich elements: a new class of regulators of mammalian alternative splicing. EMBO J. 24, 1988–1998 (2005).
Agarwal, A. K. et al. CA-Repeat polymorphism in intron 1 of HSD11B2: effects on gene expression and salt sensitivity. Hypertension 36, 187–194 (2000).
Richter, S., Wenzel, A., Stein, M., Gabdoulline, R. R. & Wade, R. C. webPIPSA: a web server for the comparison of protein interaction properties. Nucleic Acids Res. 36, 276–280 (2008).
Vincent, J. P. & Lazdunski, M. Trypsin-pancreatic trypsin inhibitor association. Dynamics of the interaction and role of disulfide bridges. Biochem. 11, 2967–2977 (1972).
García-Fernández, R. et al. The Kunitz-type protein ShPI-1 inhibits serine proteases and voltage-gated potassium channels. Toxins (Basel). 8, 110 (2016).
Kozlov, S. A. et al. New polypeptide components from the Heteractis crispa sea anemone with analgesic activity. Russ. J. Bioorganic Chem. 35, 789–798 (2009).
Gladkikh, I. et al. Atypical reactive center Kunitz-type inhibitor from the sea anemone Heteractis crispa. Mar. Drugs 10, 1545–1565 (2012).
Chen, Z. & Bode, W. Refined 2.5 Å X-ray crystal structure of the complex formed by porcine kallikrein A and the bovine pancreatic trypsin inhibitor. J. Mol. Biol. 164, 283–311 (1983).
Krowarsch, D., Zakrzewska, M., Smalas, A. O. & Otlewski, J. Structure-function relationships in serine protease – bovine pancreatic trypsin inhibitor interaction. Protein Pept. Lett. 12, 403–407 (2005).
Sokotun, I. N. et al. Study of the interaction of trypsin inhibitor from the sea anemone Radianthus macrodactylus with proteases. Biochem. 1, 139–142 (2007).
Kvetkina, A. N. et al. New targets of Kunitz-type peptide from sea anemone Heteractis magnifica. Dokl. Biochem. Biophys. 487, 1–4 (2019).
Delfin, J. et al. Purification, characterization and of proteinase inhibitors from Stichodactyla helianthus. Toxicon 34, 1367–1376 (1996).
Duty, S. & Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 164, 1357–1391 (2011).
Bove, J., Prou, D., Perier, C. & Przedborski, S. Toxin-induced models of Parkinson’s Disease. J. Am. Soc. Exp. Neurother. 2, 484–494 (2005).
Blesa, J., Phani, S., Jackson-Lewis, V. & Przedborski, S. Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012, 845618 (2012).
Sherer, T. B. et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 23, 10756–10764 (2003).
Redman, P. T. et al. A vital role for voltage-dependent potassium channels in dopamine transporter-mediated 6-hydroxydopamine neurotoxicity. Neurosci. 143, 1–6 (2006).
Chen, X. et al. Potassium channels: a potential therapeutic target for Parkinson’s Disease. Neurosci. Bull. 34, 341–348 (2017).
Taherian, R. & Ahmadi, M. A. 4-Aminopyridine decreases MPTP-induced behavioral disturbances in animal model of Parkinson’s disease. Int. Clin. Neurosci. J. 2, 142–146 (2015).
Peng, Y. et al. Blockade of Kv1.3 channels ameliorates radiation-induced brain injury. Neuro. Oncol. 16, 528–539 (2014).
Il’ina, A. et al. Amino acid sequence of RTX-A’s isoform actinoporin from the sea anemone, Radianthus macrodactylus. Toxicon 47, 517–520 (2006).
Sambrook, J. & Russel, D. W. Molecular Cloning. A Laboratory Manual. Cold Spring Harboc Laboratory Press vols 1–3 (2001).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Saitou, N. & Nei, M. The Neighbor-Joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987).
Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Ann. Stat. 39, 783–791 (1985).
Zuckerkandl, E. & Pauling, L. Molecules as documents of history. J. Theor. Biol. 8, 357–366 (1965).
Eswar, N. et al. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinforma. 5, (2006).
Pettersen, E. F. et al. UCSF Chimera - A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Bas, D. C., Rogers, D. M. & Jensen, J. H. Very fast prediction and rationalization of pKa values for protein-ligand complexes. Proteins Struct. Funct. Genet. 73, 765–783 (2008).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Kassell, B. Bovine trypsin-kallikrein inhibitor (Kunitz inhibitor, Basic Pancreatic Trypsin Inhibitor, polyvalent inhibitor from bovine organs). Methods Enzymol. 19, 844–852 (1970).
Dixon, M. The graphical determination of Km and Ki. Biochem. J. 129, 197–202 (1972).
Peigneur, S. et al. Where cone snails and spiders meet: design of small cyclic sodium-channel inhibitors. FASEB J. 33, 3693–3703 (2019).
Lyakhova, E. G. et al. Lissodendoric acids A and B, manzamine-related alkaloids from the far eastern sponge Lissodendoryx florida. Org. Lett. 19, 5320–5323 (2017).
Carmichael, J., Degraff, W. G., Gazdar, A. F., Minna, J. D. & Mitchell, J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Am. Assoc. Cancer Res. 47, 936–942 (1987).
Guex, N. & Peitsch, M. C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Acknowledgements
We thank Oleg Chernikov for the peptide sequencing, Stanislav Anastyuk for the determination of peptide molecular masses, and Aleksandra Litavrina for the revision of the English text. The sequence identification, analysis of the peptide diversity and obtaining of the recombinant peptides were supported by RSF № 19-74-20088. SPR study was performed within the framework of the Program of Basic Scientific Research of National Science Academies for 2013–2020 using the equipment of “Human Proteome” Core Facility of the Institute of Biomedical Chemistry. J.T. was funded by GOC2319N and GOA4919N (FWO-Vlaanderen) and CELSA/17/047 (BOF - KU Leuven). KU Leuven funding (PDM/19/164) supports S.P.
Author information
Authors and Affiliations
Contributions
M.I., E.L. and A.K. designed the experiments, wrote and edited the main manuscript text; V.C., M.I. and A.K. performed molecular parts of the experiments; N.C., E.L. and M.I. performed amplicon sequencing; K.G performed Sanger sequencing; A.K. determined trypsin inhibitory activity and prepared the Figs. 1–4; E.Z. performed molecular modeling and electrostatic potential and prepared Figure S2; E.P. and E.M. performed cytoprotective and ROS detection models; E.Y. performed MTT assay and prepared Fig. 6; L.K. and A.I. performed and analyzed biosensor assay data and prepared Figure S3; S.P. and J.T. performed and analyzed electrophysiological assay data and prepared Fig. 5; D.A., J.T., S.P., A.I. and E.K. edited manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kvetkina, A., Leychenko, E., Chausova, V. et al. A new multigene HCIQ subfamily from the sea anemone Heteractis crispa encodes Kunitz-peptides exhibiting neuroprotective activity against 6-hydroxydopamine. Sci Rep 10, 4205 (2020). https://doi.org/10.1038/s41598-020-61034-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61034-x
This article is cited by
-
Diversity analysis of sea anemone peptide toxins in different tissues of Heteractis crispa based on transcriptomics
Scientific Reports (2024)
-
Cnidarians as a potential source of antiparasitic drugs
Parasitology Research (2022)
-
Anti-epileptic Kunitz-like peptides discovered in the branching coral Acropora digitifera through transcriptomic analysis
Archives of Toxicology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.