Abstract
Non-dipping nocturnal blood pressure (BP) pattern is a predictor of the future decline of renal function; however, it is unclear whether it is still a risk for chronic kidney disease (CKD) patients with normal BP. To solve this question, a retrospective cohort study was conducted, and 1107 CKD patients who underwent ambulatory blood pressure monitoring (ABPM) were enrolled. We divided patients into 4 groups based on their nocturnal BP dipping pattern (dipper or non-dipper) and average 24-hour BP (hypertension or normotension). The cumulative incidence of composite renal outcomes, including a 40% reduction in eGFR, the induction of renal-replacement therapy, or death from renal causes, was analyzed. Overall, 86.1% of participants were non-dippers and 48.2% of them were normotensive. During the median follow-up period of 4.72 years, the incidence of renal composite outcomes was highest in hypertensive non-dipper patients, and was similar between normotensive dipper and non-dipper patients. Multivariate regression analysis revealed that the 24-hour systolic BP, amount of urinary protein, and hemoglobin values were associated with the incidence of renal outcomes. In conclusion, our ABPM-based analysis revealed that a non-dipping BP pattern with normotension does not predict the future incidence of composite renal outcomes in CKD patients.
Similar content being viewed by others
Introduction
The number of chronic kidney disease (CKD) patients is increasing worldwide because of the aging society1. Epidemiological studies have reported that high blood pressure (BP) is one of the strongest predictors of CKD onset in the general population and of altered kidney function in CKD patients2,3,4,5,6. Based on these findings, international guidelines have highlighted that the reducing the BP in CKD patients is essential to prevent the progression of CKD and cardiovascular diseases7,8,9. However, a target BP is still under debate due to the low reliability and reproductivity of conventional clinical BP measurements.
BP measured outside the hospital, including ambulatory BP monitoring (ABPM), was more strongly correlated with the decline in kidney function and the incidence of cardiovascular diseases than that measured at the clinic10,11,12. Furthermore, a recent large scale cohort of 63910 patients demonstrated that ABPM was superior to home BP measurements for predicting mortality13. ABPM enables the detection not only of white-coat or masked hypertension, but also BP circadian rhythms14. In general, BP decreases during the night, termed the “dipper” pattern; however, some individuals exhibit less dipping in BP during the night, termed as “non-dippers”. Previous reports demonstrated that the non-dipper BP profile pattern, which is common in patients with CKD12,15,16,17 predicted renal damage, including urinary albumin excretion18, the doubling of serum creatinine, and end-stage renal disease (ESRD)19.
However, due to the small number of interventional clinical studies with ABPM-based BP controls, the target BP in ABPM has not been established. Furthermore, in CKD patients, though the non-dipper BP pattern is regarded as a risk for the decline of kidney function and progression of cardiovascular diseases20,21,22,23, it is currently unknown whether a non-dipper BP pattern with normotension can predict the future progression of CKD. Our recent observational report demonstrated that the decline in eGFR over 2 years was correlated with the 24-hour average BP, but not its circadian rhythm24. However, the observation period in our recent study was insufficient to conclude that the 24-hour average BP, rather than its circadian rhythm, is an important predictor of the future incidence of ESRD. To overcome this limitation, we conducted a retrospective cohort study to elucidate the impact of actual BP values or its circadian rhythm on the long-term incidence of renal outcomes, including a 40% reduction in eGFR, the induction of renal-replacement therapy, or death from renal causes. In the present study, we divided CKD patients into four categories according to ABPM findings, i.e., 24-hour BP values and BP dipping patterns, and then compared the cumulative incidence of composite renal outcomes.
Methods
Patients and study protocol
We conducted a retrospective cohort study at the Omihachiman Community Medical Center in Shiga Prefecture, Japan. We enrolled 1107 CKD patients who underwent ABPM from October 2006 to July 2016. CKD was diagnosed according to the criteria of the National Kidney Foundation defined as eGFR <60 ml/min/1.73 m2 (Fig. 1)25. All clinical parameters were obtained from medical records, including age, gender, complications, and laboratory tests, which were performed within 2 weeks of the ABPM procedure. Creatinine (Cr), blood urea nitrogen (BUN), and hemoglobin were measured by a standard procedure. eGFR was calculated according to the following formula26: “194 × [age (years)]−0.287 × [serum creatinine (mg/dl)]−1.094 × [0.739 if female]”. Urine was collected for 24 hours and daily protein excretion was measured. The co-existence of DM was defined as a history of glucose reduction treatment or HbA1c value above 6.5% by the National Glycohemoglobin Standardization Program (NGSP). The body mass index (BMI) was defined as [weight (kg)]/[height (meters)]2. The protocol for the present study was approved by the ethics committee of the Omihachiman Community Medical Center (Approval number 28–46). The entire protocol of the present study was designed in accordance with the Declaration of Helsinki. Due to the retrospective design and its low risk to the patients, the ethical committee approved the use of the following opt-out methodology: The requirement for verbal informed consent was waived and informed consent was obtained by generally accessible information as well as easy methods to opt out, which were displayed on the website and at the outpatient clinic of Omihachiman Community Medical Center. Patient data were anonymized and de-identified prior to analysis.
Patient recruitment and distribution of dipper/non-dipper patients with high/controlled BP, and the relationship between the average 24-hour sBP and nocturnal BP dipping rate. (a) Flowchart of the process of patient recruitment. A patient with a less than 10% nocturnal decline in systolic BP was defined as a non-dipper. Hypertension was defined as an average 24-hour BP higher than 130/80 mmHg. In total, 86.1% of patients were categorized as non-dippers. (b) The average 24-hour sBP correlated with the nocturnal sBP dipping rate (r = 0.1962, p < 0.0001).
ABPM procedure and definition of BP profiles
ABPM devices (FB-270, Fukuda Denshi Co., Ltd., Tokyo, Japan) were fit between 15:00 and 16:00 and removed at 15:30 the next day. The cuff size was selected based on the arm circumference and applied to the non-dominant arm. During the night (22:00–07:00), measurements were taken in 1-hour intervals, whereas daytime measurements (07:00–22:00) were taken in 30-minutes intervals. During the ABPM, patient activities were not restricted, but they were asked to stay motionless at the time of measurement. Diurnal and nocturnal BP were defined arbitrarily as 7:00–22:00 and 22:00–7:00, respectively.
The average 24-hour BP was measured by ABPM. Nocturnal BP dipping was quantified as the difference between the average diurnal systolic BP (sBP) and average nocturnal sBP, and expressed as the percentage of average diurnal sBP. The non-dipper pattern was defined as an average diurnal sBP that was not 10% higher than the average nocturnal sBP. Hypertension was defined as an average 24-hour sBP higher than 130/80 mmHg (Fig. 1).
Outcomes
We analyzed composite renal outcomes, including a 40% reduction in eGFR sustained for at least two consecutive measurements, the induction of renal-replacement therapy (maintenance dialysis or renal transplantation), and death from renal causes (defined as death with a proximate renal cause, typically hyperkalemia), which were used in a recent clinical trial27. The date of 40% reduction in eGFR was defined as the latter date of two consecutive measurements, as mentioned above. The date of the induction of renal replacement therapy was the day of the first dialysis session and day of renal transplantation. The dates of major renal outcomes were ascertained from patient records. All major renal outcomes were confirmed by at least two board-certified nephrologists of the Japanese Society of Nephrology.
Statistical analysis
Data are expressed as the median (interquartile range (IQR)). Comparisons of two groups were performed using Welch’s t-test for continuous variables and Fisher’s exact test for categorical variables. Multiple comparisons among the four groups were performed by the Steel-Dwass test for continuous variables and by Bonferroni corrections for categorical variables. We examined the relationship between the average 24-hour sBP and the nocturnal sBP dipping rate using a linear correlation model.
Death before the incidence of composite renal outcomes was considered to be a competing risk event. We compared the cumulative incidence of renal outcomes using Kaplan-Meier curves plotted according to previous recommendations28 and by a competing adjusted model. Significant differences among groups were assessed by Wilcoxon’s test.
Cox’s regression analyses were performed in order to assess variables associated with the incidence of composite outcomes using gender, age, the nocturnal sBP dipping rate, and factors considered to have a significant relationship. A P-value less than 0.05 was considered to be significant. Statistical analyses were performed using JMP version 9.0.3 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
High prevalence of the non-dipping BP profile in CKD patients
The mean (+SD) age of all participants was 69.2 ± 11.5 years, and 68.9% were men (Table 1). The 1107 patients were divided into the following four groups: non-dipper with high BP (NDH 44.6%), non-dipper with controlled BP (NDC 41.5%), dipper with high BP (DH 5.1%), and dipper with controlled BP (DC 8.9%) (Fig. 1a). The average nocturnal decrease in BP was 2.2 ± 7.7%, and a high prevalence of the non-dipping BP pattern (86.1%) was observed (Fig. 1a, Table 2).
Regarding the clinical characteristics of participants, there was not significant differences in gender age, hemoglobin, or the usage rates of diuretics, or β-blockers among the groups (Table 1). The percentage of patients with DM, serum creatinine levels, eGFR at baseline, amount of urinary protein, and usage of calcium channel blockers significantly differed between NDC and NDH patients.
Based on BP profiles in each group (Table 2), the average 24-hour sBP was similar in NDH and DH patients (142 mmHg vs. 140 mmHg). The average 24-hour sBP was also similar in DC and NDC patients (116 mmHg vs. 118 mmHg). Due to the lack of a decrease in BP during the night in non-dipper patients, the average nocturnal sBP was significantly higher in NDH and NDC patients than in DH and DC patients, respectively. The linear correlation analysis demonstrated a weak but significant relationship between the average 24-hour sBP and BP dipping rate (p < 0.0001, r = 0.1962, Fig. 1b).
Renal outcomes
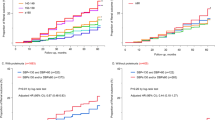
During the follow-up period (median, 4.72 years), 13.5, 32.5, and 46.1% of patients had the composite outcomes of a sustained 40% reduction in eGFR, renal-replacement therapy, or death from renal causes at 1, 3, and 5 years after recruitment, respectively (Fig. 2a). Patients were lost due to the incidence of outcomes, stopping of hospital visits, and death from non-renal causes, and the detailed profiles of annual incidences are summarized in Supplementary Table 1. In a separate analysis according to BP and its circadian rhythm, renal composite outcomes were less frequent among patients with NDC and DC than among those with NDH and DH (Fig. 2b, Supplementary Table 1). The incidence of ESRD was also lower among patients with NDC and DC than among those with NDH and DH (Supplementary Fig. 1a,b, Supplementary Table 2). These results suggest that the average 24-hour sBP value is more important for predicting the future incidence of composite renal outcomes. Of note, in contrast to previous studies reporting the risk of the non-dipping BP profile for the progression of kidney disease20, our study suggested that the normotensive non-dipper BP profile is not a risk factor for the incidence of renal outcomes.
Kaplan-Meier plots of the cumulative incidence of composite renal outcomes in this cohort. (a) Among all patients, the cumulative incidence rates of renal events 1, 3, and 7 years after recruitment were 13.5, 32.5, and 58.1%, respectively. (b) The cumulative incidence of renal events was greater in patients with hypertension than in those with normotension. *Wilcoxon’s test p < 0.05.
To investigate relevant factors for developing kidney disease, we performed a Cox regression analysis adjusted by gender, age, the nocturnal BP decline rate, and factors considered to have a significant relationship with the incidence of renal composite outcomes. The analysis identified average 24-hour sBP (HR 1.19, 95% CI: 1.14 to 1.26), proteinuria (HR 1.16, 95% CI: 1.11 to 1.20), eGFR at baseline (HR 0.60, 95% CI: 0.55 to 0.66), and hemoglobin values (HR 0.84, 95% CI: 0.79 to 0.89) as significant factors associated with the incidence of renal composite outcomes (Fig. 3). We additionally performed Cox regression analysis of the incidence of ESRD, which resulted in similar results to those for the composite renal outcomes, demonstrating that the average 24-hour sBP (HR 1.28, 95% CI: 1.14 to 1.43), proteinuria (HR 1.31, 95% CI: 1.17 to 1.46), eGFR at baseline (HR 0.38, 95% CI: 0.31 to 0.45), and hemoglobin values (HR 0.85, 95% CI: 0.76 to 0.94) are significant factors associated with the incidence of ESRD (Supplementary Fig. 2).
BP dipping pattern and the incidence of renal composite outcomes in early and advanced CKD patients
One concern in our analysis was the difference in baseline eGFR among groups, and our multivariate analysis confirmed its strong correlation with the incidence of renal outcomes. In order to exclude the impact of baseline eGFR on the future incidence of renal composite outcomes, we performed a separate analysis on advanced (eGFR < 30) and early (eGFR > 30) CKD patients. In early CKD patients, the baseline eGFR was not significantly different among groups (Supplementary Table 3). In advanced CKD patients, it was the lowest and the amount of urinary protein was the highest in NDH patients (Supplementary Table 4).
Regarding the BP profile in early CKD patients (Supplementary Table 5), the nighttime average sBP was higher in NDH patients than in DH patients (139 mmHg vs. 123 mmHg, p < 0.0001), whereas the average 24-hour sBP was similar (139 mmHg vs. 134 mmHg). Regarding BP profiles in advanced CKD patients (Supplementary Table 6), the nighttime average sBP was higher in NDH patients than in DH patients (144 mmHg vs. 133 mmHg, p < 0.0001), whereas the average 24-hour sBP was similar (144 mmHg vs. 143 mmHg).
In the analysis of early CKD patients, renal composite outcomes were less frequent among patients with NDC or DC than among those with NDH or DH (Fig. 4a, Supplementary Table 7). Similar to early CKD patients, in the analysis of advanced CKD patients, renal composite outcomes were less frequent among patients with NDC or DC than among those with NDH or DH (Fig. 4b, Supplementary Table 8). Regarding the incidence of ESRD, in both analyses of early and advanced CKD patients, ESRD was less common among patients with NDC or DC than among those with NDH or DH (Supplementary Fig. 3).
Kaplan-Meier plots of a separate analysis of the cumulative incidence of composite renal outcomes in early and advanced CKD patients. In early (a) and advanced (b) CKD patients, the cumulative incidence of renal outcomes was greater in patients with hypertension than in those with normotension. *Wilcoxon’s test p < 0.05.
Discussion
Based on previous studies demonstrating the non-dipping BP pattern to increase the risk of developing ESRD19, our clinical investigation was performed in order to clarify the impact of the non-dipping pattern of BP on the incidence of composite renal outcomes in normotensive CKD patients. A major result of this retrospective cohort study using ABPM was that the normotensive non-dipper pattern was not a risk for the future incidence of renal composite outcomes. Multivariate analysis demonstrated that a high average 24-hour BP more strongly correlated with the incidence of renal composite outcomes than the BP circadian rhythm.
Although ABPM provides extensive information, including BP circadian rhythms and BP during sleep, which are not monitored by other modalities12, the number of large-scale cohorts using ABPM is limited due to its inconvenience for patients. The number of interventional trials conducted to date is also small, and only one study has used ABPM-based BP control for pediatric hypertensive patients29. Digital health innovations are emerging in the BP monitoring field by wearable devices using cuffless BP sensors, which facilitate the continuous monitoring of BP12,30. The reliability and reproductivity of these products are important for clinical practice12, and medical professionals, hypertensive patients, and the general public are interested in these technologies30. Therefore, based on the increased demand for clinical evidence of a relationship between long-term outcomes and ABPM, the present study suggested that patients should consider BP values over 24 hours, not its circadian rhythm, in order to prevent the progression of kidney disease.
In contrast to several previous investigations demonstrating the risk of the non-dipper pattern for CKD progression, our study revealed that the non-dipping BP pattern is not associated with the incidence of renal outcomes. One possible explanation for this discrepancy is that most previous analyses were performed by the categorization of dipper vs. non-dipper, not by the BP decline, as continuous variables19,20,31,32. The BP of the study group with the non-dipping BP pattern in previous reports was often higher than that of dipping groups19,20,31,32, and it may have affected their results. Indeed, the impact of BP on renal outcomes was lost or diminished by adjusting for the BP value in some reports21,33 Regarding the impact of normotensive non-dipper pattern of renal outcomes, few studies including our previous one have been reported, but the results were not conclusive20,24,34,35. In contrast to our previous study24, a normotensive non-dipping BP can predict renal function decline in CKD patients20,34,35. However, the number of patients in these studies was small and the outcome was only the short-term renal function decline rate, not the incidence including ESRD. As we used composite renal outcomes, including ESRD and 40% eGFR reduction, in our study, our analysis provides stronger evidence for predicting the future renal prognosis, especially in normotensive non-dipping patients.
However, whether shifting the BP from the non-dipper to dipper pattern by the anti-hypertensive drugs administration has beneficial effects on renal outcomes is uncertain. In order to elucidate the impact of nocturnal BP dipping on renal outcomes, a prospective investigation of shifting the BP pattern by bedtime administration of antihypertensives may be ideal. Bedtime antihypertensive treatment for CKD patients reduced the average nocturnal BP and the percentage of non-dipper patients36. In addition, normalizing the BP circadian rhythm by administering anti-hypertensive drugs reduced not only the urinary albumin excretion in diabetic kidney disease37,38, but also the incidence of cardiovascular diseases39,40,41. However, in our observational study, the circadian rhythm of the BP profile did not correlate with the future incidence of renal outcomes in both hypertensive and normotensive individuals, suggesting that the benefit of shifting from the non-dipper to dipper pattern to prevent the progression of kidney disease is limited.
The present study has several limitations. We only assessed single-point ABPM at recruitment. Sleep-disrupting cuff inflation during the night may increase the average nocturnal BP. The daily lifestyles of patients, e.g. food intake, may be affected during ABPM in order to obtain a better profile, resulting in a different BP from baseline. Previous studies demonstrated that some patients showed a different BP pattern in serial ABPM, even with short-term intervals42,43. In addition, as we obtained no BP measurements at later time points, including ABPM or other methods, the impact of BP control on the future incidence of renal outcomes remains uncertain.
The second limitation was the low prevalence of dipper patients, particularly hypertensive dippers. The small sample size in these populations may have influenced the significance of differences, particularly in the separate analysis according to eGFR. Therefore, the incidence of renal outcomes in DH and DC patients at a later time point needs to be carefully evaluated.
Furthermore, though we revealed that the non-dipping pattern with normotension was not a risk for the incidence of renal outcomes, we did not provide an actual value for the risk threshold for the 24-hour average BP. As this was an observational study on the prognostic value of ABPM, there was no direct intervention regarding the benefits of baseline patient treatments or BP control. In addition, we selected only the average 24-hour sBP, not diurnal or nocturnal average sBP for the analysis; therefore, which BP value among these is most strongly associated with the renal outcomes remains unclear. In order to establish a target BP, more substantial evidence from a large-scale prospective interventional study that includes multiple tests on ABPM is needed. In addition, this was a study on an Asian population at a single center, and a recent nationwide analysis demonstrated variations in ABPM profiles among races and countries44. Thus, these results need to be carefully applied to individuals of other races or countries.
The last limitation was the lack of data regarding the office BP and home BP measurement. We were unable to collect information on the situation when the BP was measured from patient records, which is essential for evaluating not only home BP but also office BP.
In conclusion, our ABPM-based analysis demonstrated that a non-dipping BP pattern with normotension does not predict the incidence of renal composite outcomes in CKD patients. These results suggest that the control of BP, rather than its circadian rhythm, is essential to potentially prevent ESRD. A larger scale study to establish the target BP and interventional trials on ABPM-based BP control are required in order to obtain more conclusive evidence.
Data availability
Data are available from the corresponding author upon reasonable request.
References
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. Jama 298, 2038–2047, https://doi.org/10.1001/jama.298.17.2038 (2007).
Yamagata, K. et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney international 71, 159–166, https://doi.org/10.1038/sj.ki.5002017 (2007).
Klag, M. J. et al. Blood pressure and end-stage renal disease in men. The New England journal of medicine 334, 13–18 (1996).
Sinha, A. D. & Agarwal, R. The complex relationship between CKD and ambulatory blood pressure patterns. Advances in chronic kidney disease 22, 102–107, https://doi.org/10.1053/j.ackd.2015.01.003 (2015).
Velasquez, M. T., Beddhu, S., Nobakht, E., Rahman, M. & Raj, D. S. Ambulatory Blood Pressure in Chronic Kidney Disease: Ready for Prime Time? Kidney Int Rep 1, 94–104, https://doi.org/10.1016/j.ekir.2016.05.001 (2016).
Garofalo, C. et al. Hypertension and Prehypertension and Prediction of Development of Decreased Estimated GFR in the General Population: A Meta-analysis of Cohort Studies. American journal of kidney diseases: the official journal of the National Kidney Foundation 67, 89–97, https://doi.org/10.1053/j.ajkd.2015.08.027 (2016).
Mancia, G. et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European heart journal 34, 2159–2219, https://doi.org/10.1093/eurheartj/eht151 (2013).
Group, K. D. I. G. O. K. B. P. W. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2, 337–414 (2012).
James, P. A. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). Jama 311, 507–520, https://doi.org/10.1001/jama.2013.284427 (2014).
Gaborieau, V., Delarche, N. & Gosse, P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. Journal of hypertension 26, 1919–1927, https://doi.org/10.1097/HJH.0b013e32830c4368 (2008).
Bliziotis, I. A., Destounis, A. & Stergiou, G. S. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. Journal of hypertension 30, 1289–1299, https://doi.org/10.1097/HJH.0b013e3283531eaf (2012).
Thomas, G. & Drawz, P. E. BP Measurement Techniques: What They Mean for Patients with Kidney Disease. Clinical journal of the American Society of Nephrology: CJASN 13, 1124–1131, https://doi.org/10.2215/CJN.12551117 (2018).
Banegas, J. R. et al. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. The New England journal of medicine 378, 1509–1520, https://doi.org/10.1056/NEJMoa1712231 (2018).
Gorostidi, M. et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: A 5,693-patient cross-sectional analysis from Spain. American journal of kidney diseases: the official journal of the National Kidney Foundation 62, 285–294, https://doi.org/10.1053/j.ajkd.2013.03.025 (2013).
Andersen, M. J., Khawandi, W. & Agarwal, R. Home blood pressure monitoring in CKD. American journal of kidney diseases: the official journal of the National Kidney Foundation 45, 994–1001 (2005).
Mojon, A. et al. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiology international 30, 145–158, https://doi.org/10.3109/07420528.2012.703083 (2013).
Pogue, V. et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 53, 20–27, https://doi.org/10.1161/HYPERTENSIONAHA.108.115154 (2009).
Lurbe, E. et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. The New England journal of medicine 347, 797–805, https://doi.org/10.1056/NEJMoa013410 (2002).
Wang, C. et al. Prognostic Value of Reverse Dipper Blood Pressure Pattern in Chronic Kidney Disease Patients not Undergoing Dialysis: Prospective Cohort Study. Scientific reports 6, 34932, https://doi.org/10.1038/srep34932 (2016).
Davidson, M. B., Hix, J. K., Vidt, D. G. & Brotman, D. J. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Archives of internal medicine 166, 846–852, https://doi.org/10.1001/archinte.166.8.846 (2006).
Minutolo, R. et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Archives of internal medicine 171, 1090–1098, https://doi.org/10.1001/archinternmed.2011.230 (2011).
Agarwal, R. & Andersen, M. J. Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. American journal of nephrology 26, 503–510, https://doi.org/10.1159/000097366 (2006).
Salles, G. F. et al. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) Meta-Analysis. Hypertension 67, 693–700, https://doi.org/10.1161/HYPERTENSIONAHA.115.06981 (2016).
Kado, H., Kusaba, T., Matoba, S., Hatta, T. & Tamagaki, K. Normotensive non-dipping blood pressure profile does not predict the risk of chronic kidney disease progression. Hypertension research: official journal of the Japanese Society of Hypertension, https://doi.org/10.1038/s41440-018-0155-9 (2018).
National Kidney, F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American journal of kidney diseases: the official journal of the National Kidney Foundation 39, S1–266 (2002).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53, 982–992, https://doi.org/10.1053/j.ajkd.2008.12.034 (2009).
Neal, B. et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The New England journal of medicine 377, 644–657, https://doi.org/10.1056/NEJMoa1611925 (2017).
Pocock, S. J., Clayton, T. C. & Altman, D. G. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet 359, 1686–1689, https://doi.org/10.1016/S0140-6736(02)08594-X (2002).
Group, E. T. et al. Strict blood-pressure control and progression of renal failure in children. The New England journal of medicine 361, 1639–1650, https://doi.org/10.1056/NEJMoa0902066 (2009).
Goldberg, E. M. & Levy, P. D. New Approaches to Evaluating and Monitoring Blood Pressure. Current hypertension reports 18, 49, https://doi.org/10.1007/s11906-016-0650-9 (2016).
Timio, M. et al. “Non-dipper” hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol 43, 382–387 (1995).
Farmer, C. K. et al. Progression of diabetic nephropathy–is diurnal blood pressure rhythm as important as absolute blood pressure level? Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 13, 635–639, https://doi.org/10.1093/ndt/13.3.635 (1998).
Agarwal, R. & Andersen, M. J. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney international 69, 406–411, https://doi.org/10.1038/sj.ki.5000081 (2006).
Timio, M. et al. Circadian blood pressure changes in patients with chronic renal insufficiency: a prospective study. Ren Fail 15, 231–237 (1993).
Csiky, B., Kovacs, T., Wagner, L., Vass, T. & Nagy, J. Ambulatory blood pressure monitoring and progression in patients with IgA nephropathy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 14, 86–90, https://doi.org/10.1093/ndt/14.1.86 (1999).
Crespo, J. J. et al. Administration-time-dependent effects of hypertension treatment on ambulatory blood pressure in patients with chronic kidney disease. Chronobiology international 30, 159–175, https://doi.org/10.3109/07420528.2012.701459 (2013).
Minutolo, R. et al. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. American journal of kidney diseases: the official journal of the National Kidney Foundation 50, 908–917, https://doi.org/10.1053/j.ajkd.2007.07.020 (2007).
Hermida, R. C., Calvo, C., Ayala, D. E. & Lopez, J. E. Decrease in urinary albumin excretion associated with the normalization of nocturnal blood pressure in hypertensive subjects. Hypertension 46, 960–968, https://doi.org/10.1161/01.HYP.0000174616.36290.fa (2005).
Hermida, R. C., Ayala, D. E., Mojon, A. & Fernandez, J. R. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. Journal of the American Society of Nephrology: JASN 22, 2313–2321, https://doi.org/10.1681/asn.2011040361 (2011).
Hermida, R. C., Ayala, D. E., Mojon, A. & Fernandez, J. R. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol 58, 1165–1173, https://doi.org/10.1016/j.jacc.2011.04.043 (2011).
Hermida, R. C., Ayala, D. E., Mojon, A. & Fernandez, J. R. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiology international 27, 1629–1651, https://doi.org/10.3109/07420528.2010.510230 (2010).
Agarwal, R., Light, R. P., Bills, J. E. & Hummel, L. A. Nocturia, nocturnal activity, and nondipping. Hypertension 54, 646–651, https://doi.org/10.1161/HYPERTENSIONAHA.109.135822 (2009).
Elung-Jensen, T., Strandgaard, S. & Kamper, A. L. Longitudinal observations on circadian blood pressure variation in chronic kidney disease stages 3–5. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 23, 2873–2878, https://doi.org/10.1093/ndt/gfn126 (2008).
Drawz, P. E. et al. Variations in 24-Hour BP Profiles in Cohorts of Patients with Kidney Disease around the World: The I-DARE Study. Clinical journal of the American Society of Nephrology: CJASN 13, 1348–1357, https://doi.org/10.2215/CJN.13181117 (2018).
Author information
Authors and Affiliations
Contributions
I.T., T.K., H.K. and T.T. analyzed data. I.T. and T.K. wrote the manuscript. I.T., T.K., H.K., T.T., S.M., T.H. and K.T. participated in discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ida, T., Kusaba, T., Kado, H. et al. Ambulatory blood pressure monitoring-based analysis of long-term outcomes for kidney disease progression. Sci Rep 9, 19296 (2019). https://doi.org/10.1038/s41598-019-55732-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55732-4
This article is cited by
-
Non-dipping blood pressure pattern is associated with cardiovascular events in a 21-year follow-up study
Journal of Human Hypertension (2024)
-
Short-term blood pressure variability as a potential therapeutic target for kidney disease
Clinical Hypertension (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.