Abstract
Ambulatory blood pressure monitoring (ABPM) can produce many variables, of which the lowest nocturnal systolic blood pressure (LNSBP) currently used in calculating morning surge is occasionally overlooked in recent kidney studies compared with other ABPM parameters. We explored the clinical effects of LNSBP in elderly patients with chronic kidney disease (CKD) in a multicenter, observational cohort study. A total of 356 elderly patients with CKD from 19 clinics were included in this analysis. We used multiple logistic regression and survival analyses to assess the associations between the lowest nocturnal systolic blood pressure and heavy proteinuria and kidney disease outcomes, respectively. The median age was 66 years, and 66.6% were men. The median eGFR was 49.2 ml/min/1.73 m2. Multivariate logistic regression analysis demonstrated that LNSBP (OR 1.24; 95% CI 1.10–1.39; P < 0.001; per 10 mmHg) was associated with heavy proteinuria. During the median follow-up of 23 months, 70 patients (19.7%) had a composite outcome; of these, 25 initiated dialysis, 25 had 40% eGFR loss, and 20 died. Cox analysis showed that the renal risk of LNSBP for CKD outcomes remained significant even after adjusting for background factors, including age, sex, medical history of hypertension and diabetes, smoking status, eGFR, 24-h proteinuria, and etiology of CKD (HR 1.18; 95% CI 1.06–1.32; P = 0.002; per 10 mmHg). Concentrating on LNSBP could be valuable in guiding antihypertensive treatment to control heavy proteinuria and improve renal prognosis in elderly CKD patients.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) can arise from heterogeneous diseases affecting 11–16% of the population worldwide1, in which diabetes mellitus and hypertension are the top two causes of CKD in the majority of counties2. It is well established that elevated blood pressure (BP) strongly indicates the progression of renal function and mortality in CKD or non-CKD populations3,4,5. In the past few decades, 24-h ambulatory blood pressure monitoring (ABPM) has exerted a better impact on evaluating the status of blood pressure throughout the day, distinguishing mask or white-coat hypertension in CKD patients more effectively than office blood pressure6,7,8. Hypertension management guidelines consistently have suggested ABPM as a better method to diagnose hypertension and can identify the proper time of antihypertensive drug administration9,10. ABPM also has a prognostic effect on the incidence and progression of renal damage9,11.
Heavy proteinuria is one of the essential diagnostic factors for nephrotic syndrome, which is commonly recognized as greater than 3.5 g measured by a timed 24-h urine collection method12. Clinical and experimental data have indicated that heavy proteinuria is associated with the formation of renal interstitial fibrosis and contributes to the progression of renal failure13. The NHANES study showed that proteinuria was an independent risk factor for hypertension and independently associated with blood pressure (BP) control in CKD14. BP control reduces the incidence of albuminuria15. However, the association between 24-h proteinuria and 24-h ambulatory blood pressure in elderly CKD patients remains uncertain because of their cumbersome, time-consuming procedures, and expenditure in clinical practice. ABPM generates an abundance of data. Researchers have evaluated BP at different times, such as morning, daytime, and nighttime blood pressure. However, the lowest nocturnal systolic blood pressure (LNSBP), which is currently used to calculate the morning surge, is scarcely studied in CKD patients. We were particularly interested in the LNSBP whether it has an association with heavy proteinuria and can predict CKD outcomes, which has not been specifically addressed to the best of our knowledge.
Results
Baseline characteristic of the analyzed population
Baseline characteristics are shown in Table 1. A total of 356 patients (median age 66 years, 66.6% males) from 19 clinics were included in this analysis with a median eGFR of 49.2 ml/min per 1.73 m2. A great part of them (73.9%) had hypertension, and greater than one-third of the population had diabetes. As presented in Table 1, no differences were noted between the LNSBP tertiles in age, smoking status, BMI, serum lipids, eGFR or etiology of CKD. ABPM parameters were all increased in patients from the highest LNSBP tertile with the exception of 24-h heart rate. We also found a trend toward a higher percentage of males, hypertension, diabetes, massive proteinuria, calcium channel blocker users, and anemia in the higher LNSBP tertile.
Association between the lowest nocturnal systolic blood pressure and heavy proteinuria in elderly CKD patients
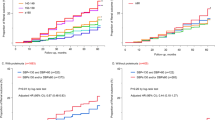
We conducted logistic regression analysis to evaluate the relationship between LNSBP and heavy proteinuria (Table 2). Higher LNSBP, which was analyzed both as a continuous and categorical variable, was associated with higher proteinuria in all models. Every 10-mmHg increment in LNSBP (P < 0.001) increased the opportunity for heavy proteinuria by 24% (95% CI 1.10–1.39) after adjusting for background factors. Figure 1 showed the influence of CKD stage and proteinuria on the LNSBP. Patients with the heaviest proteinuria and the highest CKD stage had the highest LNSBP which was greater than 130 mmHg.
Influence of CKD stage and proteinuria on the lowest nocturnal systolic blood pressure (LNSBP). Quartile 4 represented superior proteinuria, and quartile 1 represented inferior proteinuria. Quartile 1: 24-h proteinuria < 0.5 g; Quartile 2: 0.5 g ≤ 24 h proteinuria < 1.8 g; Quartile 3: 1.8 g ≤ 24 h proteinuria < 4.0 g; Quartile 4: 24 h proteinuria ≥ 4.0 g.
Associations of the lowest nocturnal systolic blood pressure with outcomes
During the median follow-up of 23 months, 70 patients (19.7%) had a composite outcome. Of those, 25 initiated dialysis, 25 had 40% eGFR loss from baseline, and 20 died. In Kaplan–Meier (KM) analysis (Fig. 2), the time functions for LNSBP predicting CKD composite outcome-free survival probability were significantly different (P < 0.01). The patients with the highest LNSBP tertile were more likely to have poor prognoses during follow-up. Cox proportional hazards models consistently showed that LNSBP was independently associated with kidney disease outcomes, including CKD progression and death, after adjusting for age, sex, current smoking, medical history of hypertension and diabetes, eGFR, 24-h proteinuria, and etiology of CKD, as illustrated in Table 3. A 10-mmHg increase in the LNSBP values conferred an 18% (95% CI 1.06–1.32; P = 0.002) increase in the risk of developing CKD composite events and a 24% (95% CI 1.08–1.41; P = 0.002) increase in CKD progression (initiation of replacement treatment therapy and 40% eGFR decline) when death was considered as censored data.
Discussion
To the best of our knowledge, this is the first study emphasizing the association between LNSBP and heavy proteinuria and the risk of LNSBP for kidney disease outcomes.
A well-established association between ABPM and microalbuminuria has been reported in some previous studies16,17,18. A cross-sectional study described that higher nighttime blood pressure indicated higher albuminuria in diabetes and CKD patients19. However, the authors defined albuminuria (albumin creatinine rate, ACR > 300 mg/g) as a very high level of proteinuria instead of using a 24-h urine test, which is a method used to assess the quantity of all kinds of urine protein. In addition, we explored the lowest systolic blood pressure as either a continuous variable or a categorical variable to predict heavy proteinuria and obtained a positive result. Several studies reported that ABPM variables, particularly nighttime blood pressure, were strongly associated with the progression of microalbuminuria in individuals at high risk of CKD17,20,21, and our data added evidence that nighttime blood pressure was also associated with heavy proteinuria.
Hypertensive nephropathy is the second leading cause of CKD in the majority of countries22. Recently, a new viewpoint contrasting with previous thinking that elevated proteinuria is secondary to the development of high BP has been justified gradually23,24,25,26. Brantsma et al.21 conducted a longitudinal study with 4635 normotensive individuals at baseline to investigate the relationship between urinary albumin excretion and the new onset of hypertension. They found that participants with higher urinary albumin excretion at baseline were more likely to develop hypertension (OR 1.18; per 1-unit In (UAE) change; 95% CI 1.03–1.36) after a mean 4.2 year of follow-up. In 2018, Haas et al.26 examined genetically bidirectional associations of proteinuria with blood pressure and suggested that elevated proteinuria and blood pressure could affect each other, which meant that increased proteinuria would lead to higher blood pressure and vice versa. It is reasonable for us to understand that sustained hypertension can lead to proteinuria. However, how can hypertension be explained by proteinuria? The mechanisms accounting for this interaction remain unclear. However, there are several speculations here. First, proteinuria was considered the result of kidney damage and seemed to be a major risk factor and pathological stimulus of renal inflammation-causing impairment in sodium homeostasis and overload, which led to hypertension27,28. Second, proteinuria might arise due to generalized endothelial dysfunction, which may also cause hypertension29,30. Third, urinary serine proteases aberrantly filtered into tubules, such as plasmin, activate the epithelial sodium channel in the distal tubule, with which sodium retention and volume overload may contribute to hypertension24,31. Combining with previous studies, we indeed think there is an underlying link between night blood pressure and heavy proteinuria, and a higher nighttime blood pressure level can be strongly associated with heavier proteinuria or the converse association.
The LNSBP is mostly used in calculating morning surges32,33. However, studies illustrating the physiological meaning of LNSBP are rare. Cesare Cuspidi et al.34 reported that the LNSBP was predictive of new-onset left ventricular mass during a 10-year follow-up period. Combining our data with published literature, on the one hand, in terms of physical and mental activity as well as body position, LNSBP represents the minimal BP needed for adequate organ perfusion and physiological activity. On the other hand, in terms of arterial structure and elasticity, LNSBP reflects the degree of vascular stiffness. A higher LNSBP indicates a greater chance of target organ damage. In our study, one-third of elderly CKD patients had a nocturnal systolic blood pressure of greater than 120 mmHg, which far exceeds 90/60 mmHg, the lowest organ perfusion requirement in a healthy population. We confirmed that in elderly CKD patients, high LNSBP may be attributed to high basal systolic blood pressure or arterial stiffness free from interference of diurnal activities, hormone changes, sympathetic nervous tone, and the renin–angiotensin system. However, further research on relative explorations and comparison is warranted.
Our study suggested an increased risk of poor prognosis in elderly CKD patients with higher LNSBP independent of conventional CKD progression risk factors. Nighttime BP has been explored in many studies, and most of them have concluded that nighttime BP is superior in indicating ESRD or death than daytime BP and office blood pressure in CKD or non-CKD patients35,36,37. Although the pathophysiological mechanism of the association between nocturnal blood pressure and worsening renal prognosis has not been confirmed, our study demonstrated that higher LNSBP was associated with heavier proteinuria, which can accelerate renal function loss. In addition, LNSBP was independently associated with prognosis, indicating that higher organ blood perfusion or the degree of arterial stiffness can affect the hemodynamics of the kidney and lead to renal function loss.
Given that heavy proteinuria and prognosis are the priorities for nephrologists, it is reasonable to measure the lowest nighttime blood pressure by ABPM devices. Proper administration of anti-night hypertensive treatment may help to control proteinuria and slow the progression of CKD in the elderly population.
Several limitations should be mentioned. First, only elderly patients were enrolled in this study, and population representation was relatively limited. Second, 70 outcomes, including only 20 all-cause deaths, occurred during follow-up, which prevented us from detecting an association between LNSBP and all-cause death. Patient number did not provide sufficient power to evaluate the antihypertensive drug effects on LNSBP. Third, some of the day and night durations were defined by wide fixed-clock intervals (6:00 AM–10:00 PM for the day and 10:00 PM–6:00 AM for the night). Some patients may be asleep, whereas others may be awake, representing a source of data inaccuracy. Some sleep durations were self-reported, which was probably misreported by patients, resulting in potential errors in some ABPM values. Fourth, 24-h urine collection and ABPM were both performed only once at baseline, and changes in blood pressure and proteinuria during follow-up may influence the results. Finally, we did not analyze the association between LNSBP and proteinuria during follow-up due to the susceptibility of proteinuria to environmental factors, drugs, physical activities, and diets. Thus, the causal relationship between LNSBP and proteinuria has not been studied. In conclusion, higher nocturnal systolic BP was independently associated with an increased risk for heavy proteinuria and poor outcomes. Concentrating on the lowest nocturnal systolic blood pressure could be valuable in guiding antihypertensive treatment to control heavy proteinuria and improve renal prognosis in elderly CKD patients.
Methods
Study design and participants
This study was a multicenter, observational cohort study that was launched in February 2017 to investigate elderly Chinese CKD patients (greater than 60 years of age) recruited from 19 clinical centers in China. All the participants conformed to the following inclusion criteria: aged ≥ 60 years old, received a diagnosis of CKD38 and provided signed informed consent. We excluded participants if they (1) had received dialysis or renal transplantation; (2) were diagnosed with acute kidney injury; (3) had active malignancy within 24 months prior to screening or metastatic cancer; (4) had a life expectancy less than 6 months; (5) had severe heart failure (New York Heart Association function class III or IV); (6) had HIV infection; (7) had isolated hematuria; and (8) were unable to communicate with examiners, unable to complete the study procedure even if assisted or otherwise unable to comply with the study protocol. A total of 356 predialysis patients with ABPM records and basic information were enrolled until February 2019. The CKD Epidemiology Collaboration (CKD-EPI) creatinine equation39 was adopted to calculate the estimated glomerular filtration rate (eGFR). This study was approved by the Ethics Committee of the Chinese PLA General Hospital (No. S2016-100-02). All participants signed informed consent before they joined this study.
24-h proteinuria and 24-h ABPM
Our patients started 24-h urine collections after their first voiding in the morning and to collect all urine continuously for 24 h, including the last void at the end of the 24-h period, or timely collected 24-h urine from 7:00 to 7:00 AM of the second day. Twenty-four-hour urine total protein was assayed by using the pyrogallol red method (Beijing Strong Biotechnologies, China). Urine volume less than 500 ml was excluded.
ABPM was performed by skilled physicians with validated devices (H-1184 Budapest, Hungary) on a routine working day after the patients provided informed consent. The monitors recorded blood pressure at 15-min intervals in the daytime and 30-min intervals in the nighttime for a 24-h period. Different sized cuffs were used based on the arm circumference of the patients. Patients with monitors were suggested to maintain their usual activities. Valid measurements were those fulfilling ≥ 80% continuous recordings. Day and night durations were defined by wide fixed-clock intervals (6:00 AM–10:00 PM for the day and 10:00 PM–6:00 AM for the night) or according to self-reports of patients. The lowest nocturnal systolic blood pressure (LNSBP) was defined as the mean of the three nighttime SBP readings, the lowest reading, and the readings immediately before and after, centered on the time of the lowest nighttime values. Morning SBP (MSBP) was defined as the average of SBP values during the first 2 h after wake-up or the mean value obtained between 06:00 AM and 08:00 AM.
Other variables and definitions
Apart from 24-h proteinuria and ABPM, we also collected data, including age, sex, smoking status, body mass index (BMI), medical history of hypertension and diabetes, serum creatinine, eGFR, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), blood urea nitrogen (BUN), uric acid, hemoglobin, and antihypertensive medication history.
CKD stages were consistent with the Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines38. Specific causes of CKD were mainly dependent on pathologic diagnosis when the biopsy result was available or otherwise dependent on the clinical diagnosis made by nephrologists. All enrolled patients were categorized into the following subgroups: CKD with uncertain reason, membranous nephropathy, IgA nephropathy, diabetic nephropathy, hypertensive nephropathy, and others. The patients with other specific renal biopsy diagnoses were categorized as “others”. Hypertension was defined as office blood pressure (BP) ≥ 140/90 mmHg, self-reported hypertension history, or current treatment with antihypertension drugs. Diabetes mellitus was defined as a fasting glucose level ≥ 7.0 mmol/l, glycated hemoglobin ≥ 6.5%, self-reported diabetic history, or current treatment with antidiabetic drugs.
Outcomes
CKD progression was defined as ESRD (the initiation of replacement treatment therapy) or 40% eGFR decline, which ever occurred first. We selected a 40% eGFR decline over follow-up because this outcome was clinically significant and proposed by the National Kidney Foundation and the US Food and Drug Administration as a broadly accepted measure for kidney function decline40. CKD progression and all-cause death were composite outcomes. CKD progression and all-cause death were ascertained through contact with patients or reports from next of kin, review of medical records and death certifications. Individuals should visit the clinics every 6 months. The allowance of the early and delayed visits would be 1 month from the scheduled visit date. Patients who were not able to show up in hospitals on time were followed up by telephone call or online contact until ESRD, death, withdrawal from the study, loss to follow-up, or January 2020. Greater than 90% of the patients kept in touch during this period. All methods were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
We summarized baseline participant characteristics across tertiles of baseline the lowest nocturnal systolic blood pressure with mean ± (SD) for normal distribution variables, median (interquartile range) for nonnormal distribution variables, number (%) for category variables. We categorized proteinuria as a binary variable [nonheavy proteinuria (< 3.5 g/24 h) part and heavy proteinuria (≥ 3.5 g/24 h)] because the data did not meet the requirement for normal distribution of linear regression. Multiple logistic regression analysis was used to investigate the association between ABPM parameters and heavy proteinuria. Kaplan–Meier survival curves were used to estimate the proportion of participants in different tertiles of LNSBP surviving without a composite outcome for specified durations. Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for time to renal outcomes with adjustment for traditional risk factors, including age, sex, smoking status, medical history of hypertension and diabetes, eGFR, 24-h proteinuria, and etiology of CKD in either death as an outcome or censored data models. The assumption of proportionality was tested using Schoenfeld residuals. No substantial deviations from proportionality were observed. Statistical significance was set at the level of P = 0.05. Analyses were performed with IBM SPSS 25.0 software (IBM Corp., Armonk, NY, USA).
References
Jha, V. et al. Chronic kidney disease: Global dimension and perspectives. Lancet (Lond., Engl.) 382, 260–272. https://doi.org/10.1016/s0140-6736(13)60687-x (2013).
Chen, T. K., Knicely, D. H. & Grams, M. E. Chronic kidney disease diagnosis and management: A review. JAMA 322, 1294–1304. https://doi.org/10.1001/jama.2019.14745 (2019).
Navaneethan, S. D. et al. Blood pressure parameters are associated with all-cause and cause-specific mortality in chronic kidney disease. Kidney Int. 92, 1272–1281. https://doi.org/10.1016/j.kint.2017.04.030 (2017).
Wan, E. Y. F. et al. Association of blood pressure and risk of cardiovascular and chronic kidney disease in Hong Kong hypertensive patients. Hypertension (Dallas, Tex: 1979) 74, 331–340. https://doi.org/10.1161/hypertensionaha.119.13123 (2019).
Chang, A. R., Lóser, M., Malhotra, R. & Appel, L. J. Blood pressure goals in patients with CKD: A review of evidence and guidelines. Clin. J. Am. Soc. Nephrol. 14, 161–169. https://doi.org/10.2215/cjn.07440618 (2019).
Bangash, F. & Agarwal, R. Masked hypertension and white-coat hypertension in chronic kidney disease: A meta-analysis. Clin. J. Am. Soc. Nephrol. 4, 656–664. https://doi.org/10.2215/cjn.05391008 (2009).
Iimuro, S. et al. Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin. J. Am. Soc. Nephrol. 8, 721–730. https://doi.org/10.2215/cjn.06470612 (2013).
Gorostidi, M. et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: A 5,693-patient cross-sectional analysis from Spain. Am. J. Kidney Dis. 62, 285–294. https://doi.org/10.1053/j.ajkd.2013.03.025 (2013).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138, e426–e483. https://doi.org/10.1161/cir.0000000000000597 (2018).
Mancia, G. & Verdecchia, P. Clinical value of ambulatory blood pressure: Evidence and limits. Circ. Res. 116, 1034–1045. https://doi.org/10.1161/circresaha.116.303755 (2015).
Orth, S. R. & Ritz, E. The nephrotic syndrome. N. Engl. J. Med. 338, 1202–1211. https://doi.org/10.1056/nejm199804233381707 (1998).
Hirschberg, R. Bioactivity of glomerular ultrafiltrate during heavy proteinuria may contribute to renal tubulo-interstitial lesions: Evidence for a role for insulin-like growth factor I. J. Clin. Investig. 98, 116–124. https://doi.org/10.1172/jci118755 (1996).
Inker, L. A., Coresh, J., Levey, A. S., Tonelli, M. & Muntner, P. Estimated GFR, albuminuria, and complications of chronic kidney disease. J. Am. Soc. Nephrol. 22, 2322–2331. https://doi.org/10.1681/asn.2010111181 (2011).
Xie, X. et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. Lancet (Lond., Engl.) 387, 435–443. https://doi.org/10.1016/s0140-6736(15)00805-3 (2016).
Marcovecchio, M. L. et al. Ambulatory blood pressure measurements are related to albumin excretion and are predictive for risk of microalbuminuria in young people with type 1 diabetes. Diabetologia 52, 1173–1181. https://doi.org/10.1007/s00125-009-1327-6 (2009).
Oliveras, A., Armario, P., Martell-Clarós, N., Ruilope, L. M. & de la Sierra, A. Urinary albumin excretion is associated with nocturnal systolic blood pressure in resistant hypertensives. Hypertension (Dallas, Tex.: 1979) 57, 556–560. https://doi.org/10.1161/hypertensionaha.110.165563 (2011).
Moran, A. et al. Office and ambulatory blood pressure are independently associated with albuminuria in older subjects with type 2 diabetes. Hypertension (Dallas, Tex.: 1979) 47, 955–961. https://doi.org/10.1161/01.HYP.0000216634.73504.7d (2006).
Ruiz-Hurtado, G. et al. Association between high and very high albuminuria and nighttime blood pressure: Influence of diabetes and chronic kidney disease. Diabetes Care 39, 1729–1737. https://doi.org/10.2337/dc16-0748 (2016).
Lurbe, E. et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N. Engl. J. Med. 347, 797–805. https://doi.org/10.1056/NEJMoa013410 (2002).
Palmas, W. et al. Telemedicine home blood pressure measurements and progression of albuminuria in elderly people with diabetes. Hypertension (Dallas, Tex.: 1979) 51, 1282–1288. https://doi.org/10.1161/hypertensionaha.107.108589 (2008).
Romagnani, P. et al. Chronic kidney disease. Nature reviews. Dis. Primers 3, 17088. https://doi.org/10.1038/nrdp.2017.88 (2017).
Xu, H. et al. Urinary albumin excretion, blood pressure changes and hypertension incidence in the community: Effect modification by kidney function. Nephrol. Dialysis Transplant. 29, 1538–1545. https://doi.org/10.1093/ndt/gfu057 (2014).
Gansevoort, R. T. & Snieder, H. Albuminuria as a cause of hypertension. Nat. Rev. Nephrol. 15, 6–8. https://doi.org/10.1038/s41581-018-0073-8 (2019).
Brantsma, A. H., Bakker, S. J., de Zeeuw, D., de Jong, P. E. & Gansevoort, R. T. Urinary albumin excretion as a predictor of the development of hypertension in the general population. J. Am. Soc. Nephrol. 17, 331–335. https://doi.org/10.1681/asn.2005111153 (2006).
Haas, M. E. et al. Genetic association of albuminuria with cardiometabolic disease and blood pressure. Am. J. Hum. Genet. 103, 461–473. https://doi.org/10.1016/j.ajhg.2018.08.004 (2018).
Coffman, T. M. The inextricable role of the kidney in hypertension. J. Clin. Investig. 124, 2341–2347. https://doi.org/10.1172/jci72274 (2014).
Zoja, C., Abbate, M. & Remuzzi, G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol. Dialysis Transplant. 30, 706–712. https://doi.org/10.1093/ndt/gfu261 (2015).
Salmon, A. H. et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 23, 1339–1350. https://doi.org/10.1681/asn.2012010017 (2012).
Butler, M. J. et al. Aldosterone induces albuminuria via matrix metalloproteinase-dependent damage of the endothelial glycocalyx. Kidney Int. 95, 94–107. https://doi.org/10.1016/j.kint.2018.08.024 (2019).
Artunc, F., Wörn, M., Schork, A. & Bohnert, B. N. Proteasuria—the impact of active urinary proteases on sodium retention in nephrotic syndrome. Acta Physiol. 225, e13249. https://doi.org/10.1111/apha.13249 (2019).
Andreadis, E. A., Geladari, C. V., Angelopoulos, E. T., Kolyvas, G. N. & Papademetriou, V. Morning surge and peak morning ambulatory blood pressure versus automated office blood pressure in predicting cardiovascular disease. High Blood Pressure Cardiovasc. Prev. 26, 209–215. https://doi.org/10.1007/s40292-019-00315-7 (2019).
Cheng, H. M. et al. Prognostic utility of morning blood pressure surge for 20-year all-cause and cardiovascular mortalities: Results of a community-based study. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.117.007667 (2017).
Cuspidi, C. et al. Nighttime blood pressure and new-onset left ventricular hypertrophy: Findings from the Pamela population. Hypertension (Dallas, Tex.: 1979) 62, 78–84. https://doi.org/10.1161/hypertensionaha.111.00682 (2013).
Mateo-Gavira, I. et al. Nocturnal blood pressure is associated with the progression of microvascular complications and hypertension in patients with type 1 diabetes mellitus. J Diabetes Compl. 30, 1326–1332. https://doi.org/10.1016/j.jdiacomp.2016.05.021 (2016).
Cheng, D., Tang, Y., Li, H., Li, Y. & Sang, H. Nighttime blood pressure decline as a predictor of renal injury in patients with hypertension: A population-based cohort study. Aging 11, 4310–4322. https://doi.org/10.18632/aging.101873 (2019).
Yano, Y. et al. Association of daytime and nighttime blood pressure with cardiovascular disease events among African American Individuals. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2019.2845 (2019).
Stevens, P. E. & Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158, 825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007 (2013).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Levey, A. S. et al. GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 64, 821–835. https://doi.org/10.1053/j.ajkd.2014.07.030 (2014).
Acknowledgements
The authors thank all physicians and professors of the participating centers for their excellent cooperation and help. The authors received research support from professors including Xiao-qiang Ding, Hong-li Lin, Ying Li, Li-ning Miao, Guo-hua Ding, Fu-you Liu, Ya-ni He, Jing-ai Fang, Meng-hua Chen, Xin-ling Liang, Hong-guang Zheng, Ai-hua Zhang, Cai-li Wang, Yong-ze Zhuang, Yue-yi Deng, Nian-song Wang, Qiang He, Xiao-ping Yang, Yi-bin Yang.
Funding
This study was supported by the Science and Technology Project of Beijing (D18110700010000, D181100000118002, D181100000118004), the National Key Technology R&D Program (2018YFA0108803).
Author information
Authors and Affiliations
Contributions
X.G.: substantial contributions to the conception, design, analysis, drafting of the work, and revising it critically for important intellectual content. S.L.: contributions to collecting data for the work and revising it critically for important intellectual content. W.W.: contributions to collecting data and analysis for the work. Y.Z.: contributions to collecting data and analysis for the work. C.Z.: contributions to collecting data and analysis for the work. X.C.: contributions to revising it critically for important intellectual content. G.C.: substantial contributions to the conception, design, revising it critically for important intellectual content and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, X., Liang, S., Wang, W. et al. Lowest nocturnal systolic blood pressure is related to heavy proteinuria and outcomes in elderly patients with chronic kidney disease. Sci Rep 11, 5846 (2021). https://doi.org/10.1038/s41598-021-85071-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85071-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.