Abstract
Thirty new title compounds along with five known analogues were prepared from commercially available 2-arylhydrazin-1-ium chlorides and α-ketoglutaric acid. The mycelium growth rate method was used to evaluate inhibition activity against six strains of plant pathogenic fungi. Most of the compounds displayed the activity for each the fungi at 150 μΜ, higher than azoxystrobin, a positive drug. Compound 6-2 showed the lowest average IC50 value of 4.58 μg/mL for all the fungi where F. solani exhibited the highest susceptibility to most of the compounds. For F. solani, some compounds were more active with IC50 values of 2.67–8.48 μM than thiabendazole (IC50 = 9.30 μM) and/or carbendazim (IC50 = 3.36 μM). The SAR showed that the activity is significantly affected by substituents on the A-ring and/or D-ring along with the degree of unsaturation of the C-ring. Thus, a series of new β-carboline compounds with potent antifungal potential were found.
Similar content being viewed by others
Introduction
The most common plant disease is a variety of mycoses caused by plant pathogenic fungi, which only influence the output and quality of agricultural products but also lead to a food safety problem due to their mycotoxins harmful to animal and human health1. Therefore, plant mycosis is an important problem of agricultural production worldwide2. In current agriculture, the prevention and control of plant mycoses mainly depends on widespread application of various fungicides. However, due to the persistent and incorrect use of some commercial fungicides, drug resistance or environmental pollution has become an increasing concern3. Thus, it is necessary to develop new fungicides with novel molecular skeletons and environmentally friendly property. In this respect, natural product-based drug discovery has showed great potential due to the high environment compatibility of natural products in the last twenty years4.
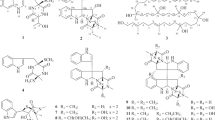
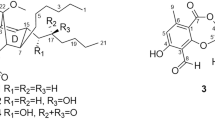
In recent years, our interests have focused on sanguinarine (SA) and chelerythrine (CH) (Fig. 1), two quaternary benzo[c]phenanthridine alkaloids (QBAs) with a variety of pharmacological activities, and their simple structural analogues. Our previous investigations demonstrated that the iminium moiety (C=N+) in SA and CH is a determinant factor for their various bioactivities5,6,7,8 including antifungal5. This result prompted us to design a class of 2-aryl-3,4-dihydroisoquinoliums (ADHIQs) as simple structural analogues of SA or CH (Fig. 1) with the aim of developing QBAs-like drugs. Compared with SA or CH, these analogues generally showed the higher anti-phytopathogenic9,10,11,12,13, anti-cancer8,14 and acaricidal activities15,16. Additionally, the analogues also showed high safety to plant growth17,18. Based on the results above and the antifungal activity of β-carbolines, we further designed a class of hybrids of ADHIQs and β-carbolines, i.e., 2-aryl-3,4-dihydro-β-carbolin-2-iums (ADHBCs) (Fig. 1) in order to find more potent antifungal compounds. Satisfyingly, some of the ADHBCs indeed showed the stronger activity than the corresponding ADHIQs19. Preliminary structure-activity relationship (SAR) showed that the substituents on the D-ring are able to significantly impact the activity of ADHBCs. As our continuing study, herein we reported a range of new ADHBCs with a structural feature of 6-methyl on the A-ring and their inhibition activity against phytopathogenic fungi. Furthermore, the SAR was also discussed. This investigation is aimed at finding more potent antifungal ADHBCs and further knowing the effect of the substituents on the A-ring the activity.
Results and Discussion
Design of compounds
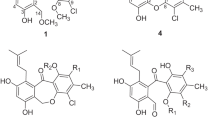
We initially designed the primary parent compound, 9-methyl-2-phenyl-3,4-dihydro-β-carbolin-2-ium bromide (6–34) and its three derivatives only with one 6-methyl (6–1), 6-methoxy (6–32) or 7-fluoro (6–33) in order to inspect the effects of substituents on the A-ring on the antifungal activity. Meanwhile, compound 7 (Fig. 1) as the full aromatization derivative of 6–32 was also designed with the aim of inspecting the degree of unsaturation of the C-ring on the activity. Thereafter, we selected compound 6-1 with the higher activity as a secondary parent compound and designed a series of its derivatives (6–2–6–31) with various substituents on the D-ring to investigate the impact of the substituents of the D-ring on the activity as well as the interaction effect between substituents on the A-ring and the D-ring on the activity. The substitution patterns of target compounds are depicted in Table 1.
Chemistry
Compounds 6-n (n = 1–34) and 7 were synthesized according to our previous method19 outlined in Fig. 2. The key intermediates 5a–5d were synthesized via 6 steps from commercially available 2-arylhydrazin-1-ium chlorides and α-ketoglutaric acid20. Target compounds 6-n were obtained by reaction of 5a–5d and primary aromatic amines in ethanol by using p-toluenesulfonic acid as catalyst in 35‒99% yields. Generally, anilines with electron-donating groups such as methyl, methoxy or hydroxyl gave the higher yield while electron-withdrawing groups such as nitro, trifluoromethyl or acetyl led to the lower yield. Unexpectedly, the method above did not afford some of the desired 6 with 2′-CF3, 2′- or 4′-NO2 or 2′- or 4′-acetyl, due to the lower reactivity of the corresponding aromatic amines. Compound 7 was obtained by treatment of 6–32 with Pd/C in 93% yield.
Synthesis of target compounds 6-1−6-34 and 7. Reagents and conditions: (a) α-ketoglutaric acid, H2O, 24 h at r.t.; (b) EtOH, con. H2SO4, reflux for 12 h; (c) CH3I, NaH, dry DMF, 0 °C; (d) LiAlH4, dry THF, 0 °C to r.t.; (e) active MnO2, CHCl3, r.t.; (f) MsCl, Et3N, LiBr, dry THF, 0 °C to r.t.; (g) R2-PhNH2, TsOH·H2O, EtOH, r.t.; (h) Pd/C, CH3CN, 80 °C.
Except 6–3419,20 and 721, all the target compounds are new compounds and their structures were structurally characterized by spectrometric methods including 1H NMR, 13C NMR, and MS. All compounds 6 displayed some similar spectroscopic features because of the same molecular skeleton. In 1H spectra, all the compounds revealed one singlet signals of H-1 in the range of δH 9.38 to 9.72 ppm, one singlet signal of (N)CH3 at δH ca. 3.97 (3H, s) and two triplet signals of one CH2CH2 moiety at δH ca. 3.5 (2 H, t, J = ca. 8.7 Hz) and ca. 4.5 (2 H, t, J = ca. 8.7 Hz); In 13C NMR, each the compound showed the signals of C-1 in the range of δC 153.2 to 163.6 ppm, (N)CH3 at δC ca. 30.9 and one CH2CH2 moiety at δC ca. 52.0 (C-3) and ca. 20.4 (C-4); Additionally, compounds 6-1‒6-31 also gave signals of 6-Me at δH ca. 2.43 (3H, s) and δC ca. 21.5. In positive HR-MS spectra, each compounds 6-n showed a characteristic ion peak at m/z [M–Br]+. The presence of bromide ion had been confirmed by negative ESI-MS in our previous similar study11,19,21. Due to the similarity of the synthetic method and structures, we did not assay the bromide ion in the present compounds.
Bioactivity
The antifungal activity in vitro of compounds was assayed according to the mycelium linear growth rate method11. Plant pathogenic fungi Colletotrichum gloeosporioides, Fusarium oxysporum f. sp. cucumerinum, Fusarium oxysporum f. sp. vasinfectum, Fusarium oxysporum sp. niveum, Fusarium.solani and Physalospora piricola were used as test fungi. The preliminary activities of compounds 6-n and 7 were determined at 150 μM (ca. 50 μg/mL). Commercial fungicide standards thiabendazole (TBZ), azoxystrobin (ASB) and carbendazim (CBZ) were used as the reference. The results are shown in Table 1.
Satisfyingly, most of the compounds revealed the good to excellent activity with average inhibition rates of 72‒96% against all the fungi, superior to that of ASB (64.5%). According to the average inhibition rates, compounds 6-n may be divided into four groups. The first group consisting of 6-2, 6-4 and 6-14–6-16 showed the highest activity with average inhibition rates of 92–96%. The 2nd group including 18 compounds (6-1, 6-3, 6-6, 6–7, 6-9, 6–10, 6–13, 6–17–6–22, 6–26, 6–27, 6–32 and 6–33) showed the higher activity (71–89%). The 3rd group containing 5 compounds (6–5, 6–8, 6–12, 6–23 and 6–24) displayed the moderate activity (46–66%). The last group consisting of the other (6–11, 6–25 and 6-28–6-31) gave very low or less activity (≤11%). For compounds 6, almost half of all the test items (100/204) revealed >80% of inhibition rate.
In order to explore the antifungal potential and SAR, the more active compounds in Table 1 were subjected to determination of median inhibition concentrations (IC50). Thiabendazole (TBZ) and carbendazim (CBZ) were used as positive controls. Compound 6–34 (R1 = R2 = H) were used as a reference control. The results are shown in Table 2.
Seventeen compounds showed average IC50 values of 12.3–45 μM against all the fungi. Among them, compounds 6–2 and 6-6 gave average IC50 values of <20 μM, eleven compounds (6-1, 6-3, 6-4, 6-7, 6-9, 6-10, 6-13−6-16, 6-18) 20–29 μM and four compounds (6-19–6-21, 6-34) 32–45 μM. Additionally, six compounds (6-12, 6-17, 6-22, 6-23, 6-26, 6-27) exhibited the higher activity with average IC50 values of 18−41 μM against four or five strains out of the fungi. Among all the compounds, 6-2 showed the lowest average IC50 value of 12.3 μM and the highest activity against F. oxysporum f. sp. cucumerinum (IC50 = 11.7 μM), C. gloeosporioides (IC50 = 14.8 μM), F. oxysporum f. sp. vasinfectum (IC50 = 10.3 μM) and P. piricola (IC50 = 10.4 μM), while 6-6 and 6-9 gave the second highest average activity (IC50 = 19.7, 21.4 μM). 6-17 and 6-18 were most active against F. oxysporum sp. niveum (IC50 = 17.4, 17.3 μM) followed by 6-2 (IC50 = 19.2 μM) while 6-7 and 6-15 were most active against F. solani (IC50 = 2.68, 2.67 μM). Among all the test fungi, F. solani showed the highest susceptibility to most of the compounds, followed by P. piricola. For F. solani, 12 compounds (IC50 = 2.7−8.5 μM) were more active than TBZ (IC50 = 9.30 μM) and two compounds (6-7, 6-15) (IC50 ≈ 2.7 μM) were superior to CBZ (IC50 = 3.36 μM). For F. oxysporum f. sp. cucumerinum, the activity of 6-2 (IC50 = 11.7 μM) was comparable with TBZ (IC50 = 12.7 μM).
Discussions
Structure-activity relationship
Based on the results in Tables 1 and 2, we found that the presence of substituents on the A-ring and/or D-ring can significantly impact the activity (Fig. 3). But the influence varies with both substitution patterns of the substituents and the species of the fungi. For the A-ring, the presence of 6–Me can increase the activity against F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. vasinfectum and F. oxysporum sp. niveum but decrease the activity against the other fungi (6-1 vs 6-34) (P < 0.05) (Table 2). However, 6-OMe only significantly increases the activity against F. oxysporum sp. niveum (6-1 vs 6-33) (Table 1). By contrast, 7-F enhances the activity against C. gloeosporioides, F. oxysporum sp. niveum and P. piricola but reduces the activity against the other fungi (6-1 vs 6-32) (Table 1). Interestingly, 6-Me, 6-OMe and 7-F improve the activity against F. oxysporum sp. niveum (Table 1).
A similar case was also observed for substituents on the D-ring. For the halogenated compounds, compared with 6-1 (R2 = H), 2′-F (6-2), 4′-F (6-4), 3′-Cl (6-6), 4′-Cl (6-7) and 3′-Br (6-9) dramatically improve the activity against most or half of the fungi while 3′-F (6-3), 2′-Cl (6-5), 2′-Br (6-8), 4′-Br (6-10), 2′-I (6-11), 3′-I (6-12) and 4′-I (6-13) reduce the activity against most or all of the fungi. It is worth noting that all the mono-halogenated compounds except 6-8 (2′-Br) and 6-11 (2′-I) show enhancement effect of the activity against both C. gloeosporioides and P. piricola (6-2−6-7, 6-9, 6-12, 6-13 vs 6-1). By contrast, only the minority of the mono-halogenated compounds display improvement of the activity on the other fungi (6-2−6-13 vs 6-1). Unexpectedly, dihalogenation of the D-ring causes dramatic decrease or loss of the activity in all the cases (6-28–6-31 vs 6-1) except 2′,6′-diF (6-27) increasing the activity against part of the fungi.
In addition, 2′-, 3′- or 4′-Me can also improve the activity against half or most of the fungi (6-14−6-16 vs 6-1), where three methylated compounds increase the activity on both F. solani and P. piricola. In contrast to methyl (a weak electron-donating group) or halogen atoms (a weak electron-withdrawing group), OH, OMe, CF3, NO2 or acetyl as strong electron-donating or electron-withdrawing groups decreases the activity against most or all of the fungi (6-17–6-26 vs 6-1). Interestingly, three methoxy-substituted isomers (6-17–6-19) and two out of three hydroxylation isomers (6-20–6-22) show improvement of the activity against both F. oxysporum sp. niveum and P. piricola compared with 6-1.
The above SARs are similar but not the same as that of 2-aryl-3,4-dihydro-β-carbin-2-niums without substituents on the A-ring19, where the activity order of the three methylated compounds against F. solani is 2′-Me isomer ≈4′-Me isomer >3′-Me isomer. However, for the present compounds with 6-Me, three methylated compounds show the different activity order for F. solani: 3′-Me isomer (6-15) > 2′-Me isomer (6-14) > 4′-Me isomer (6-16). A similar trend of the activity was also found for hydroxyl-substituted isomers (6-20−6-22). Undoubtedly, the difference is caused by the introduction of 6-methyl to the A-ring, suggesting that substituents on the A-ring influence the effect of substituents of the D-ring on the activity. In other word, there exists an interaction effect between substituents of both the A-ring and the D-ring on the activity.
Comparison of the activity of the 3,4-dihydro compound 6-32 and its full aromatic derivative 7 found that the C-ring aromatization leads to dramatic decrease of the activity. This case is agreement with that of 2-aryl-3,4-dihydro-β-carbon-2-niums without substituents on the A-ring19. This result in combination with the aforementioned substituent effect again suggests that the activity of the compounds is affected by the electron density distribution on its molecular framwork, especially on the C=N+ bond as a structural determinant of the bioactivities including antimicrobial, acaricidal, anticancer and anti-acetylcholinesterase activity6,7,8,9,10,11,12,13,14,15,16,19,21.
Interestingly, 6-2 gave almost the same EC50 values (≈10.4 μM) for F. oxysporum f. sp. vasinfectum and P. piricola, but showed two obvious different concentration-effect curves for the two fungus species (Fig. 4). Similarly, compounds 6-7 and 6-15 having almost the same EC50 values (≈2.7 μM) for F. solani also showed the different concentration-effect curves (Fig. 5). The trend of two curves in Fig. 4 indicates that 6-2 is obviously more active against F. oxysporum f. sp. vasinfectum than P. piricola while Fig. 5 shows that 6-15 is more active than 6-7 against F. solani. The results above suggest that there are very likely more than one bio-target in the same fungal cell for this class of compounds. The speculation above may be supported to some extent by the fact that SA or CH, as the model compound of 6-n, can affect the activities of various enzymes in cells22. For the fungus having multi-acceptors of the compounds, an IC50 value actually reflects a combined consequence of action of the compound and all the corresponding acceptors in the fungal cell. Although the same class of compounds may have different degree of biological effect for one specific bio-target in the same fungal cell, it is still possible for these compounds to have the same or similar overall biologic effect such as IC50 values for the fungus containing multi-acceptors. Obviously, for the fungi having multiple drug targets, IC50 values don’t fully or exactly reflect the difference of the activity of the different drug. However, to date, no representation methods of activity suitable for the situation above has been reported. In our opinion, using [IC50 × (IC90 − IC50)]1/2 instead of IC50 should be more reasonable to represent the strength of activity.
Compared with the isoquinoline analogues (ADHIQs) (Fig. 1) with the same 2-aryl, the present compounds are more active in most cases. For example, for F. oxysporum f. sp. vasinfectum, compound 6-2 (R2 = 2′-F) with an IC50 value of 10.3 μM was more active than the corresponding 2′-F-subtituted isoquinoline compound (R1 = H) (IC50 = 72.2 μM)12. A similar case was also observed in our previous study on antifungal activity of the other 9-methyl-3,4-dihydro-β-carboline compounds without substituents on the A-ring (Fig. 1, ADHBCs)19. The results above further indicate that 2-phenyl-3,4-dihydro-β-carboline is a more promising lead structure than 2-phenyl-3,4-dihydroisoquinoline for development of new antifungal agents.
In conclusion, we synthesized a range of new 2-aryl-3,4-dihydro-β-carboline bromides and evaluated for their antifungal activity in vitro against phytopathogenic fungi. The most of the compounds were found to be more active than azoxystrobin, a positive fungicide, in most cases. Among all the fungi, F. solani showed the highest susceptibility to most of compounds and some of the compounds were more active than positive drugs thiabendazole and/or carbendazim. Compound 6-2 showed the greatest potential with IC50 values of 7.18−19.2 μM for development of new antifungal agents. SAR analysis showed that substituents on both the A-ring and that the D-ring significantly impact the activity in an independent or combined manner, and the activity should be closely related with the electron density distribution of the molecular skeleton. Therefore, it is necessary to explore the in vivo activity of the compounds and conduct more extensive structure optimization.
Methods
Chemicals
Azoxystrobin (ASB, \(\geqslant \)98%), thiabendazole (TBZ, \(\geqslant \)99%) and carbendazim (CBZ, \(\geqslant \)99%), three commercial fungicide standards, were purchased from Sigma-Aldrich Trading Co. Ltd. (Shanghai, China). p-Tolyhydrazine hydrochloride, p-methoxyphenylhydrazine hydrochloride, p-fluorophenylhydrazine hydrochloride and 2-oxopentanedioic acid were bought from Aladdin Industrial Inc. (Shanghai, China). Other reagents and solvents were obtained locally and of analytical grade. The water used was ion-free.
Instruments
Melting points of the compounds were determined on an XT-4 micro-melting point apparatus (Beijing Tech Instrument Co., Ltd. China) and uncorrected. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance III instrument (1H, 500 MHz; 13C, 125 MHz). Chemical shift values (δ) and coupling constant values (J) were given in parts per million (ppm) and Hz, respectively. Mass spectroscopy were performed using a micrOTOF-Q II instrument (Bruker, Karlsruhe, Germany).
Synthesis
General procedure for the synthesis of compounds 6-1−6-34
According to our previous method19, intermediates 5a (R1 = 5-Me), 5b (R1 = 5-OMe), 5c (R1 = H) and 5d (R1 = 5-F) were prepared via 6 steps starting from 2-oxopentanedioic acid and p-tolyhydrazine hydrochloride, p-methoxyphenylhydrazine hydrochloride, phenylhydrazine hydrochloride and p-fluorophenylhydrazine hydrochloride, respectively. Compound 5a, 5b, 5c or 5d (200 mg) reacted with aniline or substituted anilines in ethanol (20 mL) containing p-toluenesulfonic acid monohydrate (7.6 mg) at room temperature under stirring for 4 h to 3 d. After the end of reaction, the solvent was removed under reduced pressure. The resulting residue was suspended in ethyl acetate. After intensely stirring for about 10 min at room temperature, the solids were collected by filtration and repeatedly washed with ethyl acetate to provide the desired compounds 6. The 1 H and/or 13C NMR data of the compounds above were showed in Supporting Information and consistent with those in literature.
6,9-Dimethyl-2-phenyl-3,4-dihydro-β-carbolin-2-ium bromide (6-1)
Yield, 71%; orange powders; mp 235.2–236.2 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.50 (s, 1H), 7.87 (d, J = 7.7 Hz, 2H), 7.67 (t, J = 7.8 Hz, 2H), 7.63-7.60 (m, 3H), 7.44 (d, J = 8.8 Hz, 1H), 4.60 (t, J = 8.7 Hz, 2H), 3.97 (s, 3H, CH3), 3.50 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 153.8, 143.9, 142.4, 132.9, 131.7, 130.3, 130.3, 129.1, 128.5, 126.0, 124.5, 123.9, 123.2, 121.7, 112.2, 51.9, 30.9, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H19N2+, 275.1543; found, 275.1538.
6,9-Dimethyl-2-(2-fluorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-2)
Yield, 54%; orange powders; mp 327.5–328.6 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.57 (s, 1H), 7.98 (t, J = 7.5 Hz, 1H), 7.69–7.59 (m, 4 H), 7.50 (t, J = 7.5 Hz, 1H), 7.47 (d, J = 8.7 Hz, 1H), 4.49 (t, J = 8.4 Hz, 2H), 3.96 (s, 3H, CH3), 3.50 (t, J = 8.4 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 156.6, 155.3 (d, J = 251.5 Hz), 142.9, 133.6, 132.7 (d, J = 8.6 Hz), 132.0, 131.5 (d, J = 10.3 Hz), 129.0, 127.5, 126.2, 125.7, 124.1, 121.9, 117.7 (d, J = 19.1 Hz), 112.3, 52.8, 31.0, 21.4, 20.4; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18FN2+, 293.1449; found, 293.1445.
6,9-Dimethyl-2-(3-fluorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-3)
Yield, 62%; orange powders; mp 268.3–269.5 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.54 (s, 1H), 7.90 (dt, J = 10.2, 2.1 Hz, 1H), 7.77-7.69 (m, 2H), 7.64 (s, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.49-7.43 (m, 2H), 4.59 (t, J = 8.7 Hz, 2H), 3.98 (s, 3H, CH3), 3.50 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 162.6 (d, J = 245.9 Hz), 154.2, 145.0 (d, J = 10.1 Hz), 142.8, 133.3, 132.1 (d, J = 9.1 Hz), 131.9, 129.1, 125.3, 124.0, 121.8, 119.4 (d, J = 2.8 Hz), 117.1 (d, J = 20.9 Hz),112.3, 111.1 (d, J = 26.1 Hz), 51.8, 31.0, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18FN2+, 293.1449; found, 293.1446.
6,9-Dimethyl-2-(4-fluorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-4)
Yield, 76%; yellow powders; mp 257.4–258.2 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.50 (s, 1H), 7.97-7.93 (m, 2H), 7.63 (s, 1H), 7.60 (d, J = 8.8 Hz, 1H), 7.55 (d, J = 8.8 Hz, 2H), 7.44 (dd, J = 8.8, 1.3 Hz, 1H), 4.57 (t, J = 8.7 Hz, 2H), 3.97 (s, 3H, CH3), 3.49 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 162.7 (d, J = 247.8 Hz), 154.0, 142.4, 140.3(d, J = 2.8 Hz), 132.9, 131.8, 129.0, 125.8 (d, J = 9.1 Hz), 124.5, 124.0, 121.7, 117.2(d, J = 23.5 Hz), 112.2, 52.1, 30.9, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18FN2+, 293.1449; found, 293.1446.
6,9-Dimethyl-2-(2-chlorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-5)
Yield, 62%; orange powders; mp 292.3–293.1 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.58 (s, 1H), 8.00-7.96 (m, 1H), 7.85−7.82 (m, 1H), 7.70−7.66 (m, 2H), 7.65 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.47 (dd, J = 8.9, 1.3 Hz, 1H), 4.42 (t, J = 8.7 Hz, 2H), 3.94 (s, 3H, CH3), 3.54 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 157.3, 142.9, 141.1, 133.6, 132.6, 132.1, 131.3, 129.4, 128.7, 128.6, 125.6, 124.1, 123.3, 121.9, 112.4, 53.0, 30.9, 21.5, 20.5; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18ClN2+, 309.1153, 309.1124; found, 309.1148, 309.1120.
6,9-Dimethyl-2-(3-chlorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-6)
Yield, 55%; orange powders; mp 191.0–192.1 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.53 (s, 1H), 8.07 (s, 1H), 7.85 (d, J = 7.6 Hz, 1H), 7.73-7.65 (m, 2H), 7.63 (s, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.45 (d, J = 8.8 Hz, 1H), 4.58 (t, J = 8.7 Hz, 2H), 3.97 (s, 3H, CH3), 3.50 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 154.3, 144.9, 142.7, 134.5, 133.4, 132.0, 131.9, 130.0, 129.1, 125.3, 124.0, 123.5, 122.0, 121.8, 112.3, 51.8, 30.9, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18ClN2+, 309.1154, 309.1124; found, 309.1137, 309.1126.
6,9-Dimethyl-2-(4-chlorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-7)
Yield, 73%; red powders; mp 199.9–201.7 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.53 (s, 1H), 7.94 (d, J = 8.9 Hz, 2H), 7.76 (d, J = 8.9 Hz, 2H), 7.63 (s, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.44 (d, J = 7.6 Hz, 1H), 4.57 (t, J = 8.7 Hz, 2H), 3.98 (s, 3H, CH3), 3.54 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 153.9, 142.6, 142.5, 134.6, 133.1, 131.8, 130.2, 129.2, 125.2, 124.9, 124.0, 121.8, 112.2, 51.9, 31.0, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18ClN2+, 309.1153, 309.1124; found, 309.1151, 309.1121.
6,9-Dimethyl-2-(2-bromophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-8)
Yield, 80%; yellow powders; mp 310.1–312.0 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.59 (s, 1H), 8.00-7.95 (m, 2H), 7.71 (td, J = 7.7, 1.2Hz, 1H), 7.65 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.61-7.57 (m, 1H), 7.47 (dd, J = 8.8, 1.3 Hz, 1H), 4.41 (t, J = 8.7 Hz, 2H), 3.94 (s, 3H, CH3), 3.56 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 157.4, 142.9, 142.7, 134.3, 133.6, 132.7, 132.1, 130.0, 128.7, 128.6, 125.4, 124.2, 121.8, 118.8, 112.4, 53.2, 31.0, 21.5, 20.6; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18BrN2+, 353.0648, 355.0628; found, 353.0649, 355.0628.
6,9-Dimethyl-2-(3-bromophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-9)
Yield, 58%; orange powders; mp 184.3–185.6 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.53 (s, 1H), 8.19 (t, J = 1.9 Hz, 1H), 7.89 (dd, J = 8.1, 1.7 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.63 (dd, J = 7.9, 2.8 Hz, 2H) 7.60 (d, J = 3.4 Hz, 1H), 7.45 (dd, J = 8.8, 1.2Hz, 1H), 4.57 (t, J = 8.7 Hz, 2H), 3.97 (s, 3H, CH3), 3.49 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 154.2, 145.0, 142.7, 133.3, 133.0, 132.1, 132.0, 129.1, 126.3, 125.3, 124.0, 122.8, 122.4, 121.8, 112.3, 51.9, 31.0, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18BrN2+, 353.0648, 355.0628; found, 353.0610, 355.0632.
6,9-Dimethyl-2-(4-bromophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-10)
Yield, 90%; orange powders; mp 201.0–202.1 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.54 (s, 1H), 7.88 (m, 4 H), 7.62 (s, 1H), 7.60 (d, J = 8.8 Hz, 1H), 7.43 (d, J = 8.6 Hz, 1H), 4.57 (t, J = 8.7 Hz, 2H), 3.98 (s, 3H, CH3), 3.49 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 153.8, 153.7, 143.0, 142.6, 133.1, 131.8, 129.2, 128.5, 125.9, 125.4, 124.9, 124.0, 123.1, 121.8, 112.2, 51.8, 31.1, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18BrN2+, 353.0648, 355.0628; found, 353.0628, 355.0615.
6,9-Dimethyl-2-(2-iodophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-11)
Yield, 78%; orange powders; mp 330.4–331.8 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.59 (s, 1H), 8.15 (dd, J = 7.9, 0.9 Hz, 1H), 7.93 (dd, J = 7.9, 1.2 Hz, 1H), 7.70 (td, J = 7.8, 1.0 Hz, 1H), 7.66 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.47 (dd, J = 8.8, 1.0 Hz, 1H), 7.40 (td, J = 7.8, 1.3 Hz, 1H), 4.38 (t, J = 8.7 Hz, 2H), 3.95 (s, 3H, CH3), 3.61 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 157.3, 146.2, 142.8, 140.4, 133.5, 132.5, 132.1, 130.5, 128.6, 127.8, 125.0, 124.2, 121.8, 112.4, 96.4, 53.4, 31.0, 21.5, 20.8; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18IN2+, 401.0509; found, 401.0511.
6,9-Dimethyl-2-(3-iodophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-12)
Yield, 62%; orange powders; mp 276.7–277.5 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.51 (s, 1H), 8.31-8.27 (m, 1H), 7.95 (d, J = 7.9 Hz, 1H), 7.88 (dd, J = 8.1, 1.9 Hz, 1H), 7.62 (d, J = 5.2 Hz, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.47-7.42 (m, 2H), 4.56 (t, J = 8.7 Hz, 2H), 3.97 (s, 3H, CH3), 3.48 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 154.1, 144.8, 142.6, 138.8, 133.2, 132.0, 131.9, 131.6, 129.1, 125.1, 124.0, 122.7, 121.8, 112.3, 95.9, 51.5, 31.0, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18IN2+, 401.0509; found, 401.0515.
6,9-Dimethyl-2-(4-iodophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-13)
Yield, 51.6%; orange powder; mp 294.1–295.4 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.51 (s, 1H), 8.04 (d, J = 8.7 Hz, 2H), 7.69 (d, J = 8.7 Hz, 2H), 7.63 (s, 1H), 7.60 (d, J = 8.8 Hz, 1H), 7.44 (d, J = 8.8 Hz, 1H), 4.56 (t, J = 8.7 Hz, 2H), 3.97 (s, 3H, CH3), 3.48 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 153.6, 143.5, 142.6, 139.0, 133.1, 131.8, 129.2, 125.2, 124.9, 124.0, 121.8, 112.2, 96.7, 51.7, 31.0, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18IN2+, 401.0509; found, 401.0512.
6,9-Dimethyl-2-(2-methylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-14)
Yield, 91%; orange powders; mp 168.4–169.5 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.45 (s, 1H), 7.70 (d, J = 7.7 Hz, 1H), 7.65 (s, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.53 (d, J = 4.3 Hz, 2H), 7.48 (dd, J = 7.9, 3.9 Hz, 1H), 7.44 (dd, J = 8.8, 1.3 Hz, 1H), 4.39 (t, J = 8.7 Hz, 2H), 3.93 (s, 3H, CH3), 3.51 (t, J = 8.7 Hz, 2H), 2.45 (s, 3H, CH3), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 156.6, 143.3, 142.3, 133.2, 132.8, 132.2, 131.8, 130.8, 128.7, 127.9, 126.4, 124.4, 124.1, 121.7, 112.2, 52.9, 30.9, 21.5, 20.3, 17.8; HR-ESI-MS (m/z): [M–Br]+ calcd. for C20H21N2+, 289.1699; found, 289.1689.
6,9-Dimethyl-2-(3-methylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-15)
Yield, 90%; orange powders; mp 174.0–175.4 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.49 (s, 1H), 7.73 (s, 1H), 7.69-7.65 (m, 1H), 7.63 (s, 1H), 7.60 (d, J = 8.8 Hz, 1H), 7.55 (t, J = 7.8 Hz, 1H), 7.42 (t, J = 8.8 Hz, 2H), 4.58 (t, J = 8.7 Hz, 2H), 3.98 (s, 3H, CH3), 3.49 (t, J = 8.7 Hz, 2H), 2.45 (3H, s, CH3), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 153.5, 143.8, 142.4, 140.1, 132.9, 131.8, 130.9, 130.1, 129.1, 124.5, 124.0, 123.5, 121.7, 120.2, 112.2, 51.9, 30.9, 21.5, 21.4, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C20H21N2+, 289.1699; found, 289.1703.
6,9-Dimethyl-2-(4-methylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-16)
Yield, 82%; orange powders; mp 135.8–136.5 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.45 (s, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.62 (s, 1H), 7.60 (d, J = 8.8 Hz, 1H), 7.47 (d, J = 8.3 Hz, 2H), 7.43 (d, J = 8.8 Hz, 1H), 4.57 (t, J = 8.7 Hz, 2H), 3.96 (s, 3H, CH3), 3.48 (t, J = 8.7 Hz, 2H), 2.42 (d, 6 H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 153.2, 142.3, 141.5, 140.3, 132.7, 131.7, 130.6, 129.1, 128.5, 126.0, 124.2, 124.0, 122.9, 121.7, 112.2, 51.9, 30.9, 21.5, 21.2, 20.2; HR-ESI-MS (m/z): [M–Br]+ calcd. for C20H21N2+, 289.1699; found, 289.1694.
6,9-Dimethyl-2-(2-methoxylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-17)
Yield, 70%; orange powders; mp 279.0–280.1 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.46 (s, 1H), 7.78 (dd, J = 7.8, 1.5 Hz, 1H), 7.65-7.57 (m, 3H), 7.44 (d, J = 9.9 Hz, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 4.37 (t, J = 8.6 Hz, 2H), 3.95 (s, 3H, CH3), 3.94 (s, 3H, CH3), 3.46 (t, J = 8.6 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 156.3, 152.9, 142.3, 132.9, 132.5, 132.3, 131.8, 128.8, 126.9, 124.7, 124.1, 121.7, 121.5, 113.7, 112.2, 57.0, 52.8, 30.9, 21.5, 20.4; HR-ESI-MS (m/z): [M–Br]+ calcd. for C20H21N2O+, 305.1648; found, 305.1654.
6,9-Dimethyl-2-(3-methoxylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-18)
Yield, 70%; orange powders; mp 241.9−242.9 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.50 (1H, s), 7.63 (s, 1H), 7.60 (t, J = 6.6 Hz, 1H), 7.57 (t, J = 8.2Hz, 1H,), 7.52 (t, J = 2.2Hz, 1H), 7.45-7.41 (m, 2H), 7.17 (dd, J = 8.3, 2.2 Hz, 1H), 4.59 (t, J = 8.6 Hz, 2H), 3.98 (s, 3H, CH3), 3.89 (s, 3H, CH3), 3.49 (t, J = 8.6 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 160.6, 153.8, 145.0, 142.4, 132.9, 131.8, 131.2, 129.1, 124.7, 124.0, 121.8, 115.9, 115.1, 112.2, 109.3, 56.4, 52.0, 31.0, 21.5, 20.3; HR-ESI-MS [M–Br]+: Calcd for C20H21N2O+, 305.1648; found, 305.1646.
6,9-Dimethyl-2-(4-methoxylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-19)
Yield, 83%; orange powders; mp 204.5−205.8 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.42 (s, 1H), 7.82 (d, J = 8.9 Hz, 2H), 7.62 (s, 1H), 7.59 (d, J = 8.8 Hz, 1H), 7.42 (d, J = 8.7 Hz, 1H), 7.20 (d, J = 8.9 Hz, 2H), 4.56 (t, J = 8.6 Hz, 2H), 3.96 (s, 3H, CH3), 3.86 (s, 3H, CH3), 3.47 (t, J = 8.6 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 160.6, 152.8, 142.1, 136.9, 132.5, 131.6, 129.1, 128.5, 126.0, 124.7, 124.0, 123.7, 121.6, 115.3, 112.1, 56.3, 52.2, 30.9, 21.5, 20.2; HR-ESI-MS (m/z): [M–Br]+ calcd. for C20H21N2O+, 305.1648; found, 305.1643.
6,9-Dimethyl-2-(2-hydroxyphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-20)
Yield, 82.0%; yellow powder; mp 259.1–261.3 °C; 1H NMR (500 MHz, DMSO-d6): δ 10.87 (s, 1H, OH), 9.45 (s, 1H), 7.71 (dd, J = 7.9, 1.3 Hz, 1H, Ar-H), 7.62 (s 1H), 7.60 (d, J = 8.9 Hz, 1H), 7.46-7.40 (m, 2H), 7.17 (d, J = 8.2 Hz, 1H), 7.05 (t, J = 7.6 Hz, 1H), 4.39 (t, J = 8.7 Hz, 2H), 3.94 (s, 3H, CH3), 3.45 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6): δ 155.9, 151.3, 142.2, 132.7, 132.0, 131.7, 131.5, 128.8, 126.8, 124.3, 124.1, 121.6, 120.2, 117.6, 112.2, 52.6, 30.9, 21.5, 20.4; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H19N2O+, 291.1492; found, 291.1497.
6,9-Dimethyl-2-(3-hydroxyphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-21)
Yield, 99%; orange powders; mp 296.7–298.3 °C; 1H NMR (500 MHz, DMSO-d6): δ 10.18 (s, 1H, OH), 9.44 (s, 1H), 7.62 (s, 1H), 7.60 (d, J = 8.8 Hz, 1H), 7.47-7.40 (m, 2H), 7.27 (dd, J = 7.9, 1.8 Hz, 1 H), 7.22 (t, J = 2.1 Hz, 1H), 7.00 (dd, J = 8.2, 1.8 Hz, 1H), 4.55 (t, J = 8.7 Hz, 2H), 3.96 (s, 3H, CH3), 3.47 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 158.9, 153.5, 145.0, 142.4, 132.9, 131.7, 131.1, 129.1, 124.5, 124.0, 121.7, 117.3, 113.5, 112.2, 110.1, 51.9, 30.9, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H19N2O+, 291.1492; found, 291.1490.
6,9-Dimethyl-2-(4-hydroxyphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-22)
Yield, 82%; yellow powders; mp 315.4–316.5 °C; 1H NMR (500 MHz, DMSO-d6): δ 10.21 (s, 1H, OH), 9.38 (s, 1H), 7.72-7.68 (m, 2H), 7.61 (s, 1H), 7.58 (d, J = 8.8 Hz, 1H), 7.40 (dd, J = 8.8, 1.3 Hz, 1H), 7.01-6.97 (m, 2H), 4.53 (t, J = 8.7 Hz, 2H), 3.96 (s, 3H, CH3), 3.46 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 159.3, 152.2, 142.0, 135.6, 132.3, 131.6, 129.1, 124.7, 124.0, 123.4, 121.6, 116.5, 112.1, 52.2, 30.9, 21.5, 20.2; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H19N2O+, 291.1492; found, 291.1487.
6,9-Dimethyl-2-(3-trifluoromethylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-23)
Yield, 63%; yellow powders; mp 295.8–296.3 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.62 (s, 1H), 8.35 (s, 1H), 8.22 (d, J = 8.7 Hz, 1H), 7.97 (d, J = 7.9 Hz, 1H), 7.91 (t, J = 7.9 Hz, 1H), 7.65 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.45 (dd, J = 8.8, 1.3 Hz, 1H), 4.63 (t, J = 8.7 Hz, 2H), 3.99 (s, 3H, CH3), 3.52 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 154.6, 144.4, 142.7, 133.3, 131.9, 131.6, 130.8 (d, J = 32.7 Hz), 129.2, 127.6, 126.7, 125.4, 125.2, 124.0, 121.9, 120.7 (d, J = 3.9 Hz), 112.3, 52.0, 31.1, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C20H18F3N2+, 343.1417; found, 343.1427.
6,9-Dimethyl-2-(4-trifluoromethylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-24)
Yield, 37%; yellow powders; mp 255.4–256.6 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.59 (s, 1H), 8.09 (q, J = 8.8 Hz, 4 H), 7.65 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.49-7.44 (m, 1H), 4.63 (t, J = 8.7 Hz, 2H), 3.98 (s, 3H, CH3), 3.52 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 154.5, 146.8, 142.9, 133.6, 132.0,130.0, 129.3, 127.5, 127.4, 125.5, 124.2, 124.0, 121.9, 112.3, 51.7, 31.0, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C20H18F3N2+, 343.1417; found, 343.1432.
6,9-Dimethyl-2-(3-nitrophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-25)
Yield, 68%; yellow powders; mp 254.1–255.8 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.69 (s, 1H), 8.81 (t, J = 2.0 Hz, 1H), 8.42 (dd, J = 8.2, 1.6 Hz, 1H), 8.38 (dd, J = 8.1, 1.7 Hz, 1H), 7.97 (t, J = 8.2 Hz, 1H). 7.65 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.46 (d, J = 8.8 Hz, 1H), 4.64 (t, J = 8.7 Hz), 4.00 (s, 3H, CH3), 3.54 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 154.8, 148.7, 144.7, 142.9, 133.5, 132.0, 131.8, 130.0, 129.2, 125.7, 124.6, 124.1, 121.9, 119.0, 112.3, 52.0, 31.1, 21.5, 20.4; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H18N3O2+, 320.1394; found, 320.1395.
6,9-Dimethyl-2-(3-acetylphenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-26)
Yield, 57%; orange powders; mp 242.2–243.3 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.62 (s, 1H, H-1), 8.45-8.41 (m, 1H), 8.17-8.13 (m, 2H), 7.83 (t, J = 7.9 Hz, 1H), 7.64 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.45 (dd, J = 8.8, 1.2 Hz, 1H,), 4.63 (t, J = 8.7 Hz, 2H), 4.00 (s, 3H, CH3), 3.53 (t, J = 8.7 Hz, 2H), 2.71 (s, 3H, CH3), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 197.6, 154.3, 144.2, 142.6, 138.6, 133.1, 131.8, 130.8, 129.7, 129.2, 127.8, 125.0, 124.0, 123.1, 121.8, 112.3, 52.0, 31.1, 27.6, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C21H21N2O+, 317.1648; found, 317.1634.
6,9-Dimethyl-2-(2,6-difluorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-27)
Yield, 48%; red powders; mp 288.6–289.9 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.72 (s, 1H), 7.78-7.70 (m, 1H), 7.65 (s, 1H), 7.63 (d, J = 8.9 Hz, 1H), 7.51 (dd, J = 16.6, 8.2 Hz, 3H), 4.47 (t, J = 8.6 Hz, 2H), 3.94 (s, 3H, CH3), 3.52 (t, J = 8.6 Hz, 2H), 2.42 (s, 3H,CH3); 13C NMR (126 MHz, DMSO-d6): δ 158.3, 156.7 (d, J = 252.8 Hz), 143.7, 134.5, 132.9, 132.4, 128.9, 127.3, 124.3, 122.1, 113.7, 113.6, 112.6, 52.7, 31.0, 21.4, 20.6; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H17F2N2+, 311.1354; found, 311.1360.
6,9-Dimethyl-2-(2,4-dichlorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-28)
Yield, 45%; orange powders; mp 282.1–283.3 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.58 (s, 1H), 8.09-8.03 (m, 2H,), 7.80 (dd, J = 8.6, 2.3 Hz, 1H), 7.64 (s, 1H), 7.62 (d, J = 8.9 Hz, 1H), 7.47 (dd, J = 8.8, 1.1 Hz, 1H), 4.40 (t, J = 8.7 Hz, 2H), 3.94 (s, 3H, CH3), 3.52 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 157.4, 143.0, 140.0, 136.2, 133.8, 132.1, 130.7, 130.3, 130.0, 129.5, 128.7, 125.9, 124.2, 121.9, 112.4, 52.9, 31.0, 21.5, 20.5; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H17Cl2N2+, 343.0764, 345.0734, found 343.0769, 345.0736.
6,9-Dimethyl-2-(3,5-dichlorophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-29)
Yield, 53%; yellow powders; mp 279.0–280.1 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.58 (s, 1H), 8.10 (d, J = 1.5 Hz, 2H), 7.87 (s, 1H), 7.63 (s, 1H), 7.61 (d, J = 8.9 Hz, 1H), 7.46 (d, J = 8.7 Hz, 1H), 4.56 (t, J = 8.7 Hz, 2H), 3.98 (s, 3H, CH3), 3.49 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 154.5, 145.5, 143.0, 135.4, 133.7, 132.0, 129.5, 129.1, 126.0, 124.1, 122.6, 121.9, 112.4, 51.8, 31.0, 21.5, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H17Cl2N2+, 343.0764, 345.0734; found, 343.0662, 345.0687.
6,9-Dimethyl-2-(2,4-dibromophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-30)
Yield, 35%; yellow powders; mp 269.1–270.8 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.59 (s, 1H), 8.28 (d, J = 1.8 Hz, 1H1H), 7.99-7.95 (m, 2H), 7.64 (s, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.47 (dd, J = 8.8, 1.2 Hz, 1H), 4.38 (t, J = 8.7 Hz, 2H), 3.94 (s, 3H, CH3), 3.56 (t, J = 8.7 Hz, 2H), 2.43 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 157.3, 143.0, 142.0, 136.2, 133.7, 132.9, 132.1, 130.2, 128.6, 125.6, 124.7, 124.2, 121.8, 120.5, 112.4, 53.0, 31.0, 21.5, 20.6; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H17Br2N2+, 432.9733, 430.9753, 434.9712; found, 432.9716, 430.9753, 434.9692.
6,9-Dimethyl-2-(2-fluoro-4-bromophenyl)-3,4-dihydro-β-carbolin-2-ium bromide (6-31)
Yield, 63%; orange powders; mp 237.0–238.1 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.56 (s, 1H), 8.02 (dd, J = 10.5, 2.0 Hz, 1H,Ar-H), 7.94 (t, J = 8.5 Hz, 1H), 7.76 (dd, J = 8.6, 1.2 Hz, 1H), 7.63 (s, 1H), 7.62 (d, J = 9.0 Hz, 1H), 7.47 (dd, J = 8.8, 1.1 Hz, 1 H), 4.46 (t, J = 8.7 Hz, 2H), 3.95 (s, 3H, CH3), 3.48 (t, J = 8.7 Hz, 2H), 2.42 (s, 3H, CH3); 13C NMR (126 MHz, DMSO-d6): δ 156.5, 155.4 (d, J = 256.1 Hz), 143.0, 133.7, 132.1, 131.0 (d, J = 10.0 Hz), 129.3 (d, J = 3.5 Hz), 129.03, 129.01, 126.0, 124.1, 124.0 (d, J = 9.0 Hz), 121.9, 121.1 (d, J = 22.7 Hz), 112.4, 52.6, 31.0, 21.4, 20.4; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H17BrFN2+, 371.0554, 373.0534; found, 371.0534, 373.0528.
7-Fluoro-9-methyl-2-phenyl-3,4-dihydro-β-carbolin-2-ium bromide (6-32)
Yield, 68%; orange powders; mp 173.4–174.5; 1H NMR (500 MHz, DMSO-d6): δ 9.53 (s, 1H, H-1), 7.96 (dd, J = 9.0, 5.6 Hz, 1H, Ar-H), 7.89 (d, J = 7.9 Hz, 2H, Ar-H), 7.67 (t, J = 7.8 Hz, 2H), 7.61 (d, J = 7.0 Hz, 1H), 7.45 (dt, J = 7.7, 3.7 Hz, 1H) 7.18 (td, J = 9.3, 2.1 Hz), 4.61 (t, J = 8.8 Hz, 2H), 3.97 (s, 3H, CH3), 3.54 (t, J = 8.8 Hz, 2H); 13C NMR (126 MHz, DMSO-d6): δ 164.2 (d, J = 247.7 Hz), 153.7, 143.8, 130.4, 130.3, 130.2 (d, J = 3.5 Hz), 128.5, 126.1, 126.0, 125.6, 125.5, 123.2, 120.9, 112.7 (d, J = 26.3 Hz), 98.3 (d, J = 27.2 Hz), 51.8, 31.2, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C18H16FN2+, 279.1292; found, 279.1292.
6-Methoxy-9-methyl-2-phenyl-3,4-dihydro-β-carbolin-2-ium bromide (6-33)
Yield, 88%; orange powders; mp 208.9–209.4 °C; 1H NMR (500 MHz, DMSO-d6): δ 9.48 (s, 1H), 7.87 (d, J = 8.1 Hz, 2H), 7.67 (t, J = 7.8 Hz, 2H), 7.63 (d, J = 9.2 Hz, 1H), 7.59 (t, J = 7.4 Hz, 1H), 7.29-7.24 (m, 2H), 4.60 (t, J = 8.7 Hz, 2H), 3.97 (s, 3H, CH3), 3.84 (s, 3H, CH3), 3.49 (t, J = 8.7 Hz, 2H); 13C NMR (126 MHz, DMSO-d6): δ 155.6, 153.3, 143.9, 139.9, 130.3, 130.2, 130.1, 129.3, 128.5, 124.2, 124.0, 123.7, 123.1, 113.7, 101.2, 56.1, 52.0, 31.0, 20.3; HR-ESI-MS (m/z): [M–Br]+ calcd. for C19H19N2O+, 291.1492; found, 291.1509.
9-methyl-2-phenyl-3,4-dihydro-β-carbolin-2-ium bromide (6-34)
Yield, 94%; orange powders; The NMR data and melting point were consistent with those previously reported by us19.
Synthesis of 7-fluoro-9-methyl-2-phenyl-β-carbolin-2-ium bromide (7)
To a solution of compound 6-32 (0.2 mmol) in acetonitrile (40 mL) was added 5% Pd/C (wetted with ca. 55% water) (0.17 mmol, 80 mg). The mixture was refluxed at 80 °C for about 3 days to complete the reaction. The Pd/C powders in the reaction solution was filtered off through a sand core funnel and completely washed with methanol. The combined solution was evaporated under vacuum to yield the desired compound 7. The 1H and 13C NMR data matched those previously reported by us21.
Antifungal Assay
Antifungal activity was determined according to the method previously reported by us11. Briefly, the solution of 10 mL of the compound in 4% DMSO aqueous solution (v/v) was fully mixed with 150 mL of melted PDA agar (50 °C) to provide a medium containing 150 μM of the compounds and 0.25% DMSO (v/v). The mixture was poured into a sterilized Petri dish (ca. 20 mL each plate). Thiabendazole, azoxystrobin and carbendazim (150 μM) were used as positive controls. The medium only containing 0.25% DMSO served as a blank control and no observable effect on the growth of tested fungi was found. A fungus disc (d = 5 mm) cut from beforehand subcultured Petri dishes was placed at the center of the partially solidified medium. The dishes were kept in an incubator at 25 °C for 72 h. Each experiment was conducted in triplicate. The growth inhibition rates were calculated according our previously reported11.
A set of stork solutions with different concentrations of the compound in 5% DMSO aqueous solution was prepared by double-fold dilution method and used to determine median effective concentrations (IC50). According to the same procedure described above, each the stock solution (10 mL) was determined in triplicate for the growth inhibition rates against the fungi. Antifungal toxicity regression equations and the corresponding IC50 values were obtained by using PRISM software ver. 7.00 (GraphPad Software Inc., San Diego, CA, USA)11. Significant difference among IC50 values of various compounds against the same fungus species was analyzed by Duncan’s multiple comparisons using PRISM software ver. 7.0.
Data Availability
Data will be made available upon request to the corresponding authors.
References
Bräse, S., Encinas, A., Keck, J. & Nising, C. F. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 109, 3903–3990 (2009).
Savary, S., Teng, P. S., Willocquet, L. & Nutter, F. W. J. Quantification and modeling of crop losses: A review of purposes. Annu. Rev. Phytopathol. 44, 89–112 (2006).
Delp, C. J. Coping with resistance to plant disease. Plant Dis. 64, 652–657 (1980).
Wedge, D. E. & Camper, N. D. Biologically active natural products. In Agrochemicals and Pharmaceuticals; Cutler, H. G., Cutler, S. J., Eds; CRC Press: Boca Raton, FL, USA, pp 1–15 (2000).
Yang, X. et al. In vitro antifungal activity of sanguinarine and chelerythrine derivatives against phytopathogenic fungi. Molecules. 17, 13026–13035 (2012).
Miao, F. et al. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 25, 863–875 (2011).
Miao, F. et al. Structural modification of sanguinarine and chelerythrine and their in vitro acaricidal activity against Psoroptes cuniculi. Chem. Pharm. Bull. 60, 1508–1513 (2012).
Cao, F. et al. Pseudocyanides of sanguinarine and chelerythrine and their series of structurally simple analogues as new anticancer lead compounds: cytotoxic activity, structure-activity relationship and apoptosis induction. Eur. J. Pharm. Sci. 67, 45–54 (2015).
Zhu, L. et al. New 2-aryl-7,8-dimethoxy-3,4-dihydroisoquinolin-2-ium salts as potential antifungal agents: synthesis, bioactivity and structure-activity relationships. Sci. Rep. 7, 7537 (2017).
Zhu, L. et al. Synthesis, bioactivity and structure−activity relationships of new 2-aryl-8-OR-3,4-dihydroisoquinolin-2-iums salts as potential antifungal agents. Bioorg. Med. Chem. Lett. 26, 2413–2417 (2016).
Yang, R. et al. New class of 2-aryl-6-chloro-3,4-dihydroisoquinolinium salts as potential antifungal agents for plant protection: synthesis, bioactivity and structure-activity relationships. J. Agric. Food Chem. 63, 1906–1914 (2015).
Hou, Z. et al. 2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as novel antifungal lead compounds: biological evaluation and structure-activity relationships. Molecules. 18, 10413–10424 (2013).
Yang, X. et al. Synthesis and in vitro antifungal activities of new 2-aryl-6,7-methylenedioxy-3,4-dihydroisoquinolin-2-ium bromides. Chem. Pharm. Bull. 61, 731–739 (2013).
Cao, F. et al. Cytotoxic activity, apoptosis induction and structure–activity relationship of 8-OR-2-aryl-3,4-dihydroisoquinolin-2-ium salts as promising anticancer agents. Bioorg. Med. Chem. Lett. 27, 55–60 (2017).
Ma, Y. et al. Synthesis of 2-aryl-3,4-dihydroisoquinolin-2-ium bromides and their in vitro acaricidal activity against Psoroptes cuniculi. Chem. Pharm. Bull. 61, 204–211 (2013).
Yang, R. et al. A class of promising acaricidal tetrahydroisoquinoline derivatives: synthesis, biological evaluation and structure-activity relationships. Molecules 19, 8051–8066 (2014).
Zhang, B. et al. Effects of 2-aryl-3,4-dihydroisoquinolin-2-iums on seed germination and seedling growth. J. Northwest A&F University (Nat. Sci. Ed.). 42, 169–174 (2013).
Zheng, Z.-L., Miao, F., Yang, X.-J. & Zhou, L. Effects of 2-aryl-3,4-dihydroisoquinolin-2-iums as antifungal agents on seed germination and seedling growth. J. Northwest A&F University (Nat. Sci. Ed.). 43, 116–122 (2015).
Hou, Z. et al. Design, synthesis, and structure-activity relationship of new 2-aryl-3,4-dihydro-β-carbolin-2-ium salts as antifungal agents. J. Agric. Food Chem. 64, 2847–2854 (2016).
Yasui, E., Wada, M. & Takamura, N. Novel method for synthesis of aryl hydrazones from α-diazo esters: scope and limitations of nucleophiles. Tetrahedron 65, 461–468 (2009).
Zhou, B.-H. et al. New 2-aryl-9-methyl-β-carbolinium salts as potential acetylcholinesterase inhibitor agents: synthesis, bioactivity and structure–activity relationship. Sci. Rep. 8, 1559 (2018).
Slaninova, I., Pencikova, K., Urbanova, J., Slanina, J. & Taborska, E. Antitumour activities of sanguinarine and related alkaloids. Phytochem. Rev. 13, 51–68 (2014).
Acknowledgements
We are very grateful for the financial support from the National Natural Science Foundation of China (No. 31872008) and the Agricultural Science and Technology Innovation Project of Shaanxi Province (2016NY-171, 2016NY-130).
Author information
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Zhang, B., Zhao, W. et al. New 2-aryl-6-methyl-3,4-dihydro-β-carbolin-2-iums as potential antifungal agents: Synthesis, bioactivity and structure-activity relationship. Sci Rep 9, 1941 (2019). https://doi.org/10.1038/s41598-018-38222-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38222-x
This article is cited by
-
Design, synthesis and mechanism study of novel natural-derived isoquinoline derivatives as antifungal agents
Molecular Diversity (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.