Abstract
Growing evidence shows that improper intake of vitamin B6 increases cancer risk and several studies indicate that diabetic patients have a higher risk of developing tumors. We previously demonstrated that in Drosophila the deficiency of Pyridoxal 5′ phosphate (PLP), the active form of vitamin B6, causes chromosome aberrations (CABs), one of cancer prerequisites, and increases hemolymph glucose content. Starting from these data we asked if it was possible to provide a link between the aforementioned studies. Thus, we tested the effect of low PLP levels on DNA integrity in diabetic cells. To this aim we generated two Drosophila models of type 2 diabetes, the first by impairing insulin signaling and the second by rearing flies in high sugar diet. We showed that glucose treatment induced CABs in diabetic individuals but not in controls. More interestingly, PLP deficiency caused high frequencies of CABs in both diabetic models demonstrating that hyperglycemia, combined to reduced PLP level, impairs DNA integrity. PLP-depleted diabetic cells accumulated Advanced Glycation End products (AGEs) that largely contribute to CABs as α-lipoic acid, an AGE inhibitor, rescued not only AGEs but also CABs. These data, extrapolated to humans, indicate that low PLP levels, impacting on DNA integrity, may be considered one of the possible links between diabetes and cancer.
Similar content being viewed by others
Introduction
Growing evidence associates micronutrients as minerals and vitamins to genome integrity maintenance showing that their deficiency can damage DNA analogously to common carcinogens. Micronutrients act as co-factors or substrates for enzymes that counteract genotoxins as well as enzymes working in DNA metabolism (reviewed in1). Vitamins belonging to B group as folates (vitamin B9 and its derivatives) and B12 vitamin act as cofactors of enzymes involved in the conversion of dUMP in dTMP by the transfer of a methyl group. It has been shown that in human cells and rodents2 the impairment of this reaction due to folate and B12 deficiency causes Uracil misincorporation, a mutagenic lesion leading to point mutation, micronuclei formation and chromosome aberrations (CABs) that are known to contribute to the genesis and progression of human cancers3,4,5. Another consequence of impaired dUMP conversion is nucleotide pool imbalance that can compromise crucial cellular processes as DNA synthesis and repair. Impairment of DNA repair has been observed in cultured cells and animal models deprived of folates (reviewed in6). Thus, it is not surprising that low folate levels have been correlated with some cancers as demonstrated by many epidemiological studies7,8,9,10.
Pyridoxal-5′-phosphate (PLP), the active form of vitamin B6, plays a role in protecting cells from DNA damage. Mammals produce PLP from vitamers (pyridoxine, pyridoxamine and pyridoxal), which are introduced by the diet and recycled in a “salvage pathway” through the action of two enzymes, Pyridoxal kinase and Pyrodoxine/pyridoxamine oxidase. PLP serves as a co-enzyme in approximately 160 enzyme reactions that regulate metabolism of glucose, lipids, amino acids, heme, DNA/RNA and many neurotransmitters11,12,13. In addition to work as cofactor, vitamin B6 plays antioxidant roles by counteracting the formation of genotoxic compound, the Advanced Glycation End-products (AGEs)14,15 and by quenching oxygen reactive species16,17. Given its role in numerous metabolic reactions PLP has been associated to many human disorders including diabetes and cancer but underlying mechanisms are not fully elucidated (reviewed in14,18,19,20). High expression level of Pyridoxal kinase (PDXK) has been positively correlated with survival of nonsmall cell lung cancer (NSCLC) patients21. In addition, Vitamin B6 intake and blood PLP levels were inversely correlated with the colorectal cancer risk22.
We recently demonstrated in Drosophila that PLP deficiency caused either by mutations in the pyridoxal kinase-coding gene (dPdxk) or by vitamin B6 antagonists results in chromosome aberrations (CABs) suggesting a role for Pyrodoxal kinase in chromosome integrity maintenance. This function is evolutionarily conserved in human and yeast as PDXK depletion by RNA interference in HeLa cells induces CAB formation23 and mutations in BUD16 gene, encoding Saccharomyces cerevisiae Pyridoxal kinase, result in gross chromosome rearrangements24. More interestingly CAB frequency in Drosophila and human PLP-depleted cells was strongly enhanced by glucose treatment. In addition, PLP deficiency increases glucose content in dPdxk1 mutant hemolymph and causes AGE accumulation in neuroblasts. Treatment of dPdxk1 mutant cells with α-lipoic acid (ALA), a well-known AGE inhibitor, lowers both AGE formation and CAB frequency, suggesting a possible AGE-CAB cause-effect relationship. These findings indicate that a high intracellular glucose level has a dramatic clastogenic effect if combined with PLP deficiency and have prompted us to investigate if in diabetes low PLP levels can impair DNA integrity. Epidemiological studies clearly indicate that diabetes increases the risk of many types of cancer with mechanisms not yet fully understood. Possible links between these two diseases are hyperinsulinemia, hyperglycemia and fat-induced chronic inflammation. Several lines of evidence indicate that hyperglycemia may lead to cancer through DNA damage (reviewed in25). It is well known that high glucose causes DNA damage by increasing the oxygen reactive species production via several mechanisms (namely the polyol pathway, hexosamine pathway, AGE pathway, and protein kinase C)26,27. Interestingly, oxidative damage and DNA strand breaks have been found in both type 1 and type 2 diabetic patients28,29,30.
To investigate if in diabetic cells PLP deficiency can impair genome integrity we used Drosophila as a model system. For the past decade Drosophila turned out to be a promising organism to model diabetes as flies and humans largely share mechanisms underlying the maintenance of balance between stored and circulating glucose (reviewed in31). Insulin pathway signaling, a biochemical pathway well conserved in flies (reviewed in32), plays a crucial role in glucose homeostasis maintenance as it triggers the uptake of glucose into liver, adipose tissue and muscle and promotes the storage of these nutrients in the form of glycogen. In humans and mice insulin pathway impairment, due to mutations, cause severe insulin resistance syndromes and type 2 diabetes (reviewed in33). Also in Drosophila malfunctioning of insulin signaling, induced either by mutations or by feeding flies with a sugar rich diet, produce diabetic hallmarks that mimic type 2 diabetes (reviewed in34).
In this work, we generated two different diabetic fly models, the first by depleting three well conserved proteins involved in insulin pathway as InR, Chico (IRS) and Akt1 and the second by rearing flies in sugar rich diet35. We showed that PLP deficiency in both models of diabetes caused severe chromosome and DNA damage, probably through AGE accumulation in turn caused by high glucose levels.
These results allowed to generalize the concept that PLP deficiency combined with high glucose strongly impairs genome integrity, suggesting that in diabetic patients low PLP levels may represent a cancer risk factor.
Results
Generation of diabetic fly models
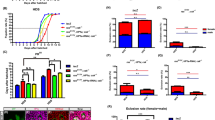
We previously demonstrated that low PLP levels, due to mutations in the encoding Pyridoxal kinase gene (dPdxk), cause CABs and increase glucose content in larval hemolymph23. We provided evidence that these phenotypes are linked because high glucose causes AGE accumulation which in turn is responsible for CAB phenotype23. Starting from these results, we asked whether low vitamin B6 levels could be genotoxic in diabetes. To answer to this question, we generated two fly models of type 2 diabetes (reviewed in31,34) using two strategies. In the first we silenced three evolutionarily conserved genes encoding proteins working in the insulin signaling pathway: Insulin receptor (InR), Insulin receptor substrate (Chico/IRS) and Akt1 (reviewed by32). In the second we reared wild type larvae on a high sugar diet (HSD) according to Musselman et al.35. To deplete InR and Chico we performed in vivo RNA interference (RNAi) in fat body (using the ppl-Gal4 driver), an organ analogue to vertebrate adipose tissue and liver, crucial for nutrient storage and energy metabolism. Targeted gene knockdown was detected by reverse transcriptase-PCR (see Supplementary Fig. S1). The effects of Akt1 depletion were evaluated using the hypomorphic late lethal allele Akt104226 and all experiments reported in this paper were conducted on hemizygous individuals (Akt104226/Df) to detect stronger phenotypes. Although in flies the most abundant circulating sugar is trehalose (a disaccharide formed by two glucose units) we focused on glucose as trehalose is a stable molecule and a non-reducing sugar36. In addition, evidence showed that glucose but not trehalose levels are regulated by mechanisms similar to those of higher organisms37. Glucose content in hemolymph from InRRNAi, chicoRNAi Akt104226/Df and HSD fed larvae was increased with respect to wild type and comparable to that exhibited by dPdxk1 positive control23 (Fig. 1A). In addition, InR and Chico RNAi-induced depletion, as well as HSD feeding, resulted in small size adults, a fly hallmark of insulin signaling impairment38,39,40 (Fig. 1B–D; Supplementary Fig. S2). It was not possible to analyze body size in Akt104226/Df individuals as they die as third instar larvae.
Diabetic hallmarks caused by insulin signaling impairment and high sugar diet (HSD) feeding. (A) Glucose content in hemolymph from wild type (wt), RNAi/mutant and HSD fed larvae. dPdxk1 was used as positive control. Columns are the means of 5 independent sample (20 larvae each) measurements ± SEM. ***Significantly different in the Student’s t test with p < 0.001. (B) Examples of body size reduction observed in InRRNAi, chicoRNAi and HSD fed flies compared to wt control. (C) Examples of wing size reduction observed in InRRNAi, chicoRNAi and HSD fed flies compared to wt control. (D) Quantification of wing area reduction. Columns represent mean values of wing area reduction in InRRNAi, chicoRNAi and HSD fed flies compared to wt control. At least 30 wings per genotype were examined ***significantly different in the Student’s t test with p < 0.001.
Glucose treatment induces CABs in diabetic cells
Evidence shows that glucose induces chromosome damage41,42. Thus, we first asked whether the high glucose content present in InR, Chico and Akt depleted individuals and also in HSD fed larvae affected DNA integrity. DNA damage was evaluated in two different ways. In the first we examined chromosomes from Akt104226/Df, chicoRNAi and HSD fed third instar larvae in colchicine treated brain preparations (Fig. 2A), in the second, because of the low mitotic index induced by InR silencing, we examined γ-H2Av foci in InRRNAi brains fixed and immunostained with the pS137 anti-phospho-histone antibody that specifically recognizes γ-H2Av43 (Fig. 2C). γ-H2AV is the homolog of the mammalian γ-H2AX and represents a suitable marker to study DNA damage since it marks the DSBs44, which are known to be at the basis of CABs45.
Glucose supplement induces DNA damage in brains from chicoRNAi, Akt104226/Df, InRRNAi, and HSD fed larvae. (A) Examples of CABs observed in 1% glucose (G) treated brains from chicoRNAi, Akt104226/Df and HSD fed larvae. Df is Df(3)Exel7328 that uncovers Akt1. (A1) wild type male metaphase; (A2) chromatid deletion of a major autosome, arrow; (A3) isochromatid deletion of a major autosome, arrow; (A4) isochromatid deletion of a major autosome, arrows; (A5) autosome-X chromosome dicentric chromosome (arrowhead) with acentric fragments, arrow; (A6) metaphase with extensive chromosome fragmentation; (A7) autosome-autosome dicentric (arrowhead) chromosome with acentric fragments (arrow) and isochromatid deletion of the X chromosome (arrow); (A8) metaphase with extensive chromosome fragmentation. Scale bar 5 μm. (B) CAB frequencies. Un = untreated. G = 1% glucose. Each bar of the graph is the mean frequency of CABs ± SEM obtained examining at least 800 metaphases in at least 8 brains. * and *** significantly different in the Student’s t test with p < 0.05 and p < 0.001 respectively. (C) Examples and (D) frequencies of γ-H2Av foci observed in wild-type (wt) and InRRNAi brain nuclei untreated (un) and 1% glucose (G) treated. Bars show the mean values of three independent experiments ± SEM obtained by examining at least 500 cells/brain in 4 brains. **Significantly different in the Student’s t test with p < 0.01.
Neuroblasts from chicoRNAi and HSD fed larvae exhibited frequencies of cells with broken chromosomes comparable to that of controls (0.5% and 0.3% respectively vs 0.44%) (Fig. 2B). Akt104226/Df cells displayed a small but significant CAB frequency (1.86%) (Fig. 2B). In this case CABs are not due to the high endogenous glucose content since Akt104226/Df mutants displayed hemolymph glucose levels comparable to those exhibited by chicoRNAi and HSD fed larvae (Fig. 1A).
Since we previously showed that sugars such as glucose, fructose and sucrose strongly increased CAB frequency in dPdxk1 neuroblasts23, we incubated brains from Akt104226/Df, chicoRNAi and HSD fed larvae in 1% glucose and we tested them for CABs. In all examined cases (Fig. 2A,B) we found CABs ranging from 4.6% to 6.6%; in contrast glucose did not affect chromosome integrity in wild type neuroblasts, indicating that only diabetic cells are sensitive to extra sugar. Glucose treatment generated complex rearrangements; besides chromatid and isochromatid deletions (Fig. 2A2–A4), we observed dicentric chromosomes accompanied by acentric fragments (formed when two broken non homologous chromosomes join together end to end) and also cells containing more than one rearrangement (Fig. 2A5–A8). Glucose induced DNA damage also in InR depleted cells. While InRRNAi untreated larvae showed a percentage of γ-H2Av positive nuclei (4.5%) comparable to controls (4%), glucose treatment significantly increased this frequency (11.7% vs 4.3% in controls) (Fig. 2C,D).
Together these data indicate that the addition of extra glucose to brains from chicoRNAi Akt104226/Df, InRRNAi and HSD fed larvae causes chromosome damage differently than wild type, suggesting that diabetic cells are sensitive to glucose for CAB formation.
PLP deficiency induces high levels of DNA damage in diabetic cells
Our primary aim was to verify whether it is possible to generalize the assumption that a simultaneous occurrence of high glucose and low PLP affects genome integrity. Thus, we examined chromosome and DNA damage in our diabetic models deprived of PLP. To deplete PLP we employed two strategies: in the first we fed Akt104226/Df, InRRNAi, chicoRNAi and HSD reared larvae with a strong PLP antagonist, the 4-Deoxypyridoxine (4-DP), and in the second we generated Akt104226dPdxk1 double mutant individuals performing genetic crosses. 4-DP caused a substantial increase in chromosome breakage in chicoRNAi (~83-fold) Akt104226/Df (~55-fold) and HSD larvae (~64-fold) (Fig. 3A,B) clearly indicating that PLP deficiency strongly impacted on chromosome integrity in both diabetes models. A significant, although lower, CAB increase (~24-fold) were also found in wild type 4-DP treated cells in agree with the PLP role in genome integrity maintenance, already demonstrated by our and other studies23,24. 4-DP enhanced CAB complexity more than glucose and produced cells with extensive chromosome fragmentation (Fig. 3A). As exactly quantifying the number of CABs in these cells was difficult, we decided to arbitrarily assign only five CABs to each cell with multifragmented chromosomes. As a consequence, CAB percentage reported in Fig. 3B may represent an underestimation. 4-DP also enhanced the frequency of γ-H2Av positive cells in InRRNAi larvae (24 vs 9.8% in control) (Fig. 3C,D). Interestingly most of 4-DP treated nuclei presented a foci pattern very similar to X rays-treated cells46 with a mean of about 40 foci per cell that is consistent with the complexity of chromosome rearrangements observed in 4-DP treated brains from chicoRNAi, Akt104226/Df and HSD fed individuals.
Effect of PLP deficiency on chromosome and DNA integrity in diabetic larvae. (A) Examples of complex rearrangements and metaphases with multifragmented chromosomes observed in chicoRNAi and Akt104226 /Df neuroblasts from larvae fed with 4-DP 2 mM and from Akt104226 dPdxk1 double mutant larvae (Scale bar 5 μm). (B) CAB frequencies. †Significantly different in the Student’s t test with p < 0.001 compared to untreated counterparts. (C) Examples and (D) frequencies of γ-H2Av positive cells from wild type (wt) and InRRNAi untreated (un) and 4-DP 2 mM fed larvae. Bars show the mean values of three independent experiments ± SEM obtained by examining at least 500 cells/brain in 4 brains. ***Significantly different in the Student’s t test with p < 0.001. (E) CAB frequencies in Akt104226 dPdxk1 double mutant brains. Each column is the mean frequency of CABs ± SEM obtained examining at least 600 metaphases in at least 6 brains. Akt104226 dPdxk1 CAB frequency was significantly higher than the sum of single mutant CAB frequency in Chi square test with p < 0.01. (F) Glucose content in hemolymph from 4-DP treated InRRNAi, chicoRNAi, Akt104226/Df and HSD feed larvae. *,** and *** significantly different in the Student’s t test with p < 0.05, p < 0.01 and p < 0.001 respectively.
The examination of Akt104226dPdxk1 brain cells revealed a CAB frequency (23%) higher than the sum of the frequencies displayed by each single mutant (Akt104226 1.1%; dPdxk1 5%) (Fig. 3E). These data suggest a strong synergistic interaction between dPdxk1 and Akt104226 mutations confirming the hypothesis that CABs originate by a combination of high glucose and low vitamin B6 levels accordingly with results obtained with 4-DP.
Based on the consideration that dPdxk1 mutants contain more glucose than wild type strain we asked if PLP depletion could further increase glucose level in larval hemolymph from Akt104226/Df, InRRNAi, chicoRNAi and HSD fed individuals. As showed in Fig. 3F, 4-DP fed larvae exhibited a small but significant glucose content increase, with respect to untreated individuals.
Taken together these results indicate that in diabetic cells PLP deficiency strongly impairs DNA integrity.
AGEs contribute to DNA damage occurring in diabetic cells deprived of PLP
It is well known that PLP counteracts the accumulation of AGEs, genotoxic compounds abundant in most cells and fluid of diabetic patients14,15,47. AGEs originate from non-enzymatic amino group glycations of proteins, lipids and nucleic acids48,49, that accumulate during senescence and in high glucose conditions and their formation have been associated to reactive oxygen species production26. To investigate the mechanism through which PLP protects from damage in high glucose conditions we immunostained untreated, 1% glucose and 4-DP treated brains from InRRNAi, ChicoRNAi and Akt104226/Df larvae using a human antibody against AGEs. We did not test HSD larvae for AGEs since we expected that there is the same mechanism at the basis of CAB formation in both models of diabetes. InR, Chico or Akt1 depleted neuroblasts showed AGE accumulation (Fig. 4A,B) more pronounced after glucose treatment. 4-DP feeding further increased the frequency of cells positive to the anti-AGE staining in all examined cases (Fig. 4A,C). We next incubated Akt104226/Df, InRRNAi and chicoRNAi brains in 10 mM α-lipoic acid (ALA), an antioxidant compound that counteracts AGE formation and cooperates with PLP in ameliorating insulin resistance in prediabetic rats50,51. In all cases ALA treatment decreased AGE accumulation in both glucose and 4-DP treated cells (Fig. 4B,C). More interestingly ALA also decreased DNA damage. As showed in Fig. 5A, in Akt104226/Df, and chicoRNAicells ALA treatment reduced CAB frequency to control values in both glucose treated and 4-DP fed larvae. Similarly, ALA also lowered the percentage of InRRNAi γ-H2Av positive cells in neuroblasts from glucose treated and 4-DP fed larvae (Fig. 5B). These results suggested a cause effect relationship between AGEs and CABs and indicated that AGEs are largely responsible for chromosome and DNA damage occurring in diabetic PLP depleted cells.
InRRNAi, chicoRNAi and Akt104226/Df larvae accumulate AGEs that are increased by glucose and 4-DP treatment. (A) Examples of cells stained with an anti-human AGE antibody from untreated (un), 1% glucose (G) treated and 4-DP fed larvae. Scale bar, 5 μm. (B) Frequencies of AGE-positive cells in wild type (wt), InRRNAi, chicoRNAi and Akt104226/Df mutant brains untreated and exposed to 1% glucose (G) with or without α-lipoid acid (ALA). ALA treatment reduces drastically AGEs. (C) Frequencies of AGE-positive cells in wild type (wt), InRRNAi, chicoRNAi and Akt104226/Df mutant brains from 4-DP fed larvae untreated (un) and treated with ALA. Bars in B and C graphs represent the mean frequencies of AGE-positive cells (±SEM) obtained by examining at least 300 cells/brain in 4 brains. *,** and *** significantly different in the Student’s t test with p < 0.05, p < 0.01, p < 0.001 respectively.
α-lipoid acid (ALA) reduces DNA damage in InRRNAi, chicoRNAi and Akt104226/Df neuroblasts. (A) Effect of ALA on CAB frequencies in wild type (wt) chicoRNAi and Akt104226/Df neuroblasts 1% glucose (G) and 4-DP treated. Columns represent the mean frequency of CABs (±SEM) obtained by examining at least 600 metaphases from at least 6 brains. * and *** significantly different in the Student’s t test with p < 0.05 and p < 0.001. (B) Effect of ALA treatment on γ-H2Av foci in wt and InRRNAi neuroblasts 1% glucose (G) and 4-DP treated. Bars show the mean values of three independent experiments ± SEM. ** and *** significantly different in the Student’s t test with p < 0.01 and p < 0.001.
PLP rescues glucose-induced DNA damage in diabetic cells
We previously demonstrated that PLP supplementation in both untreated and glucose treated dPdxk1 mutants rescued AGE accumulation confirming the role of vitamin B6 in counteracting these compounds also in Drosophila23. Based on these findings, we asked if PLP supplementation could reduce chromosome and DNA damage in Akt104226/Df, chicoRNAi and InRRNAi glucose treated brains. Thus, we reared mutant/RNAi larvae in food supplemented with 10−2 M PLP and examined them for CABs or γ-H2Av foci. To ascertain the PLP specificity we also tested brains from PLP fed individuals bearing twins (tws) mutation that causes a high frequency of CABs (about 40%) impairing a pathway uncorrelated with glucose metabolism46. PLP rescued glucose induced chromosome breakage in Akt104226/Df and chicoRNAi cells (Fig. 6A) but it did not influence CAB frequency in tws430 mutant brains (Fig. 6B) confirming its specificity. PLP also rescued the percentage of InRRNAi glucose treated γ-H2Av positive cells (Fig. 6C).
PLP rescues CABs and γ-H2Av foci in chicoRNAi, Akt104226/Df and InRRNAi brains. (A) Effect of PLP on CAB frequencies in 1% glucose (G) treated wild type (wt), chicoRNAi and Akt104226/Df larval neuroblasts. Columns represent the mean frequency of CABs (±SEM) obtained by examining at least 600 metaphases from at least 6 brains. * and *** significantly different in the Student’s t test with p < 0.05 and p < 0.001 respectively. (B) Effect of PLP on tws430 CAB frequency. Columns represent the mean frequency of CABs (±SEM) obtained by examining at least 500 metaphases from at least 5 brains. (C) Effect of PLP on γ-H2Av foci in wt and InRRNAi 1% glucose (G) treated neuroblasts. Bars show the mean values of three independent experiments ± SEM. *** significantly different in the Student’s t test with p < 0.001.
Taken together these data indicate that vitamin B6 improves glucose-induced DNA damage and strongly support the hypothesis that PLP is involved in chromosome integrity maintenance in high glucose conditions.
Remarkably PLP also rescued glucose content and body size in InRRNAi and ChicoRNAi individuals suggesting that vitamin B6 plays a role in ameliorating the tissue sensitivity to insulin (Fig. 7A,B). These findings are consistent with our previous data23 indicating that Pdxk1 mutations cause a mild insulin resistance. Mechanisms linking PLP to insulin resistance will be the subject of future studies.
PLP rescues glucose content and small body size in diabetic individuals. (A) Glucose content in InRRNAi and chicoRNAi larval hemolymph after PLP treatment. Columns are the means of 5 independent sample (20 larvae each) measurements ± SEM. *** significantly different in the Student’s t test with p < 0.001. (B) PLP treatment rescues body size in chicoRNAi and InRRNAi individuals. B1 Example of small body size in chicoRNAi (or InRRNAi); B2 rescued body size in chicoRNAi (or InRRNAi) flies treated with PLP.
Discussion
For the past decade, Drosophila has been fast gaining popularity as a useful model system to study diabetes as it shares with humans mechanisms and molecules involved in hemolymph/blood glucose regulation. In Drosophila stable levels of circulating sugars are maintained by eight insulin-like proteins (Ilps) that are released in response to high glucose levels and by the adipokinetic hormone (AKH) that it is similar to glucagon and is released in response to low levels of circulating sugar52,53,54,55. Type 1 and 2 diabetes have been modeled in Drosophila reproducing hallmarks of both diseases35,56,57,58 giving the chance to isolate new genes and identify functional interactions that can be exploited for therapeutic interventions on human patients.
We demonstrated that PLP deficiency due to mutations in dPdxk Drosophila gene causes DNA damage and increases glucose content in larval hemolymph. We showed that these phenotypes are correlated and that high glucose is largely responsible for DNA damage. In addition we showed that also in human HeLa cells the relationship between PDXK, DNA integrity and glucose content has been maintained in the course of evolution23.
In the present work we have provided evidences that PLP deficiency in fly diabetic cells strongly impair DNA integrity. We demonstrated the combined genotoxic effect of low PLP and high glucose levels using two different diabetes models, the first obtained by downregulating the expression of three well conserved insulin pathway genes: InR, chico and Akt1, and the second obtained by feeding flies with a high glucose diet (HSD). Both insulin signal impairment and HSD feeding are considered promising strategies to model type 2 diabetes in flies34. Glucose levels obtained by depleting InR, Chico or Akt1 were similar to those obtained by Ugrankar et al.37, although we used a different fat body driver to silence InR and chico and also a different Akt1 allele.
We showed that high level of endogenous glucose caused by either insulin signaling impairment or HSD feeding is not sufficient to compromise DNA integrity. A possible explanation of this can be that fly metabolism relies mainly on treahalose that represents the more abundant circulating not-reducing sugar with ratio 100:1 with respect to glucose. Thus, to observe glycation effects the system need to be forced by adding more glucose. We showed in fact that glucose treatment in Inr, Chico or Akt1 depleted brains as well as in neuroblast from HSD fed larvae caused CABs or γ-H2Av foci. In contrast glucose did not influence chromosome integrity in wild type cells suggesting that diabetic cells are more sensitive to glucose respect to wild type cells. We found that Akt104226/Df mutants displayed a low frequency of CABs also in untreated cells. However, based on evidence indicating that the human AKT counterpart is involved in DNA integrity maintenance59 we think that CABs in these cells are not related to glucose metabolism, but are due to an improper DNA repair.
More importantly, we demonstrated that in both our hyperglycemic models, DNA damage was exacerbated when PLP levels were decreased by 4-DP, a strong PLP antagonist. 4-DP caused a dramatic CAB increase in chicoRNAi (83.6%), Akt104226/Df (57.4%) and HSD larval brains (64%) and also enhanced about 5 times the percentage of γ-H2AV positive cells in InRRNAi individuals with respect to untreated counterparts. 4-DP further increased glucose content that, combined with an impaired antioxidant defense system, caused by PLP deficiency, may contribute to amplify chromosome and DNA damage. In addition we provided genetic evidence of a strong synergistic interaction between Akt1 and dPdxk1 mutations in CAB formation in double mutant individuals, accordingly with results obtained using 4-DP.
These results demonstrate that it is possible to extend the concept that low PLP levels cause DNA damage in high glucose conditions also to other diabetic contexts different from those caused by Pdxk1 mutation23.
Regarding the mechanism leading to CABs in diabetic PLP deprived cells we identified the pathway that leads to AGE formation as the best candidate that allow to correlate PLP, glucose and DNA damage. We showed that InRRNAi, chicoRNAi or Akt104226/Df diabetic cells accumulate AGEs which in turn are reduced by α-lipoic acid (ALA). More interestingly ALA also rescued both CABs and γ-H2Av foci suggesting a cause effect relationship between AGEs and CABs. As AGEs are known to be genotoxic through oxygen reactive species formation, these findings indicate that in diabetic cells PLP may safeguard genome integrity preventing AGEs from attack DNA. Our hypothesis is based on in vitro15 and in vivo studies showing that PLP is able to counteract AGE formation. It has been reported that PLP inhibits AGE accumulation in streptozotocin-induced diabetic rats, preventing the progression of nephropathy60. In addition, in glomerular cell nuclei of these rats PLP reduced the levels of carboxyethyl-2′deoxyguanosine (CEdG), a DNA glycation product that can cause strand breaks61. The exact mechanism through which PLP counteracts AGEs is not to date fully elucidated. A hypothesis is that PLP may block AGE formation by trapping the 3 deoxyglucosone (3-DG), an intermediate product of AGE metabolism. It has been in fact indicated, by in vitro experiments, that PLP is able to sequestrate 3-DG in a dose dependent manner62. However also other mechanisms are possible. PLP also works as a cofactor of enzymes that convert dUMP in dTMP. dPdxk1 mutants have an unbalanced amount of dUMP respect to dTMP23 but as are not sensitive to replication block induced by HU, this excess of dUMP does not seem to be the principal mechanism that leads to CABs. Anyway, we cannot exclude that nucleotide unbalance caused by PLP depletion can slightly affect chromosome stability in our diabetes models by influencing the rate of DNA repair. Furthermore, to the hypersensitivity of diabetic cells to 4-DP toxicity can contribute an impairment of DNA repair associated to diabetes. It has been demonstrated that diabetic patients showed, besides high levels of basal endogenous and oxidative damage, an increased sensitivity to DNA damaging agents combined to a decreased capability to repair damage induced by these drugs63.
Altogether our data, extrapolated to human, strongly support the hypothesis that DNA damage represents one of the causative factors linking diabetes to cancer. In addition, they highlight the crucial role of PLP in chromosome stability maintenance in diabetic cells suggesting that low PLP levels can represent a critical cancer risk factor for diabetic patients. Low PLP levels in normal physiology represent a rare condition due to excessive consumption of alcohol, drugs commonly used to treat pathologies as tuberculosis or arthritis (e.i. isonyazide, cycloserine, penicillamine) or celiac disease and renal dialysis64. However, interestingly, low PLP levels have been associated to diabetes in murine models and epidemiological studies65,66,67. In particular, a recent study68 based on the examination of three groups of individuals, diabetic, diabetic with nephropathy and healthy subjects, showed that respectively 63% and 58% of diabetic patients with and without nephropathy presented low PLP plasma levels (<30 nM/L). Moreover, altered levels of PLP precursors pyridoxine and pyridoxamine have been found in diabetic patients suggesting an impaired PLP metabolism associated to diabetes. At the light of these considerations our findings suggest that in diabetic individuals PLP levels should be kept under control in order to limit the risk of DNA damage.
Methods
Drosophila strains and crosses
InRv992 and chicov7776 lines were obtained from the Vienna Drosophila Resource Center (VDRC) stock center. dPdxk1 mutation has been described previously23. ppl-Gal-4 driver52, Akt104226 and Df Exel 7328 (89A12-89B6) lines were obtained from the Bloominghton Stock Center. InRRNAi and chicoRNAi flies were generated by crossing females bearing the RNAi construct to males carrying the fat body specific ppl-Gal4 driver. Mutations and deficiencies were kept in stock over the TM6C Sb, Tb balancer; homozygous and hemizygous mutant larvae were recognized for their non-Tubby phenotype. The Oregon-R strain was used as wild-type control.
Double mutants dPdxk1 Akt104226 were generated by recombination by crossing Akt104226/TM6C females to dPdxk1/TM6C males. Akt104226/dPdxk1 females resulting from this cross were mated to ApXa/TM6C males. Single males resulting from this cross were mated to ApXa/TM6C females. From these crosses were established about 60 stocks crossing inter se males and females carrying recombinant chromosomes balanced over TM6 C. The presence of both mutations on the recombinant chromosomes was verified by complementation analysis. The balancers and the genetic markers used in these crosses are described in detail in FlyBase (http://flybase.bio.indiana.edu/).
Fly food recipes
All flies, from embryo stage, were raised on a standard food. To induce insulin resistance wild type larvae were reared on a sugar rich medium which is a standard food with an increased sucrose concentration. 100 ml food contains: agar (0.68 g), yeast (6.52 g), flour (3 g) propionic acid (600 µl) and sucrose (5.13 g = 0.15 M for standard food; 34.2 g = 1.0 M for high sugar diet, HSD).
Chromosome cytology
Colchicine-treated larval brain metaphases for CAB scoring were obtained as previously described69. For immunostaining, brains from third instar larvae were dissected and fixed as described in Bonaccorsi et al.70. Brain preparations were rinsed in PBS 0.1% Triton (PBST), incubated overnight at 4 °C with primary antibodies diluted in PBST, rinsed in PBST, and then incubated for 1 hr at room temperature with the secondary antibody. The primary antibodies used were: rabbit anti-Histone H2AvD pS137 (1:100 in PBST; Rockland code #600-401-914) and rabbit anti-human AGE antibody (1:200 in PBST; ab23722, Abcam, UK). Both antibodies were detected with Alexa-Fluor-555-conjugated anti-rabbit antibody (1:300 in PBST; Molecular Probes, Eugene, OR). All fixed preparations were mounted in Vectashield H-1200 with DAPI (Vector Laboratories, Burlingame, CA) to stain the DNA. Observations were carried out using a Zeiss Axioplan fluorescence microscope equipped with CCD camera (Photometrics CoolSnap HQ).
Drug treatments
To evaluate the effects of PLP inhibitor 4-deoxypyridoxine (4-DP) on CABs and γ-H2Av foci, flies were allowed laying eggs on a fly standard growth medium for 5 days. Then, adults were removed and 4-DP 2 mM was added to medium (containing first and second instar larvae). 5 days later third instar larvae were dissected and brains fixed. For chromosome preparations brains were incubated 90 min in colchicine before fixing. To detect γ-H2Av foci brains were treated according to immunostaining procedure.
To test the effects of glucose, PLP, and α-lipoic acid (ALA) on CABs and γ-H2Av foci, brains were dissected from third instar larvae and incubated in 2 ml of saline supplemented with 10% fetal bovine serum (FBS, Corning) for 4 hours with or without addition of 1% glucose, 1 mM PLP or 10 mM ALA. For chromosome preparations 1 h before fixation brains were treated with colchicine 10−2 M (final concentration). For γ-H2Av foci detection brains were treated according to immunostaining procedure.
Picture acquisition
Pictures of adult flies were taken using a Nikon D5200 digital camera mounted on a stereomicroscope (Nikon SMZ-1). Pictures were taken using a 1/6 second exposure, and 800 iso.
Glucose and wing measurement
Glucose concentration in Drosophila hemolymph was measured using the Infinity Glucose Hexokinase reagent (Thermo scientific). Hemolymph collection and glucose measurement were done as described in Marzio et al.23.
For wing analysis, flies were anesthetized with CO2. Dissected wings were placed in absolute ethanol, and mounted in a 6:5 mixture of lactic acid/ethanol71. Measurements were made directly on digitized images of mounted wings using ImageJ software.
Weight analysis
Body weight of individual male and female flies (n = 20) was measured with a precision weight scale (Gibertini E42; range 0.1 mg-120 g). Flies were reared under the same growth conditions and were age matched (2 days old) before weighing.
Nucleic acid extraction, PCR, and RT- PCR
Preparation of fly RNA, PCR, RT-PCR and agarose gel electrophoresis were performed with standard procedures. RNA extraction was performed with the RNeasy Mini Kit (Qiagen, Hilden, Germany). For RT-PCR we used 1 μg of RNA to synthesize complementary DNAs (cDNAs) using the Quantitect Reverse transcription kit (Quiagen cod # 205311) For cDNA amplification were used the following primers:
chico F CTGACATTCGTGTGCATTGGA
chico R ATGCTTGTTGGTTGAGTGCGG
InR F GTGCTCCTCCGGTCTTATCGA
InR R GTGACGTTCAGCATAGCGGAG
rp49 F TACAGGCCCAAGATCGTGAA
rp49 R ACGTTGTGCACCAGGAACTT
Statistical analysis
Results are expressed as means ± SEM; probability values < 0.05 were considered statistically significant. Statistical analysis of the data was done with the two-tailed Student’s t-test.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fenech, M. F. Dietary reference values of individual micronutrients and nutriomes for genome damage prevention: current status and a road map to the future. Am J Clin Nutr 91, 1438S–1454S, https://doi.org/10.3945/ajcn.2010.28674D (2010).
Fenech, M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat Res 733, 21–33, https://doi.org/10.1016/j.mrfmmm.2011.11.003 (2012).
Mitelman, F., Johansson, B. & Mertens, F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer 7, 233–245, https://doi.org/10.1038/nrc2091 (2007).
Aguilera, A. & Gomez-Gonzalez, B. Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet 9, 204–217, https://doi.org/10.1038/nrg2268 (2008).
Bunting, S. F. & Nussenzweig, A. End-joining, translocations and cancer. Nat Rev Cancer 13, 443–454, https://doi.org/10.1038/nrc3537 (2013).
Duthie, S. J. Folate and cancer: how DNA damage, repair and methylation impact on colon carcinogenesis. J Inherit Metab Dis 34, 101–109, https://doi.org/10.1007/s10545-010-9128-0 (2011).
Shrubsole, M. J. et al. Dietary folate intake and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Res 61, 7136–7141 (2001).
Kim, Y. I. Folate and colorectal cancer: an evidence-based critical review. Mol Nutr Food Res 51, 267–292, https://doi.org/10.1002/mnfr.200600191 (2007).
Mason, J. B. & Tang, S. Y. Folate status and colorectal cancer risk: A 2016 update. Mol Aspects Med 53, 73–79, https://doi.org/10.1016/j.mam.2016.11.010 (2017).
Cantarella, C. D., Ragusa, D., Giammanco, M. & Tosi, S. Folate deficiency as predisposing factor for childhood leukaemia: a review of the literature. Genes Nutr 12, 14, https://doi.org/10.1186/s12263-017-0560-8 (2017).
Percudani, R. & Peracchi, A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep 4, 850–854, https://doi.org/10.1038/sj.embor.embor914 (2003).
Percudani, R. & Peracchi, A. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics 10, 273, https://doi.org/10.1186/1471-2105-10-273 (2009).
di Salvo, M. L., Contestabile, R. & Safo, M. K. Vitamin B(6) salvage enzymes: mechanism, structure and regulation. Biochim Biophys Acta 1814, 1597–1608, https://doi.org/10.1016/j.bbapap.2010.12.006 (2011).
Hellmann, H. & Mooney, S. Vitamin B6: a molecule for human health? Molecules 15, 442–459, https://doi.org/10.3390/molecules15010442 (2010).
Booth, A. A., Khalifah, R. G. & Hudson, B. G. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: comparison with aminoguanidine. Biochem Biophys Res Commun 220, 113–119, https://doi.org/10.1006/bbrc.1996.0366 (1996).
Bilski, P., Li, M. Y., Ehrenshaft, M., Daub, M. E. & Chignell, C. F. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem Photobiol 71, 129–134 (2000).
Denslow, S. A., Walls, A. A. & Daub, M. E. Regulation of biosynthetic genes and antioxidant properties of vitamin B6 vitamers during plant defense responses. Physiological and Molecular Plant Pathology 66, 244–255, https://doi.org/10.1016/j.pmpp.2005.09.004 (2005).
Ames, B. N. & Wakimoto, P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev Cancer 2, 694–704, https://doi.org/10.1038/nrc886 (2002).
Galluzzi, L. et al. Effects of vitamin B6 metabolism on oncogenesis, tumor progression and therapeutic responses. Oncogene 32, 4995–5004, https://doi.org/10.1038/onc.2012.623 (2013).
Mocellin, S., Briarava, M. & Pilati, P. Vitamin B6 and Cancer Risk: A Field Synopsis and Meta-Analysis. J Natl Cancer Inst 109, 1–9, https://doi.org/10.1093/jnci/djw230 (2017).
Galluzzi, L. et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep 2, 257–269, https://doi.org/10.1016/j.celrep.2012.06.017 (2012).
Gylling, B. et al. Vitamin B-6 and colorectal cancer risk: a prospective population-based study using 3 distinct plasma markers of vitamin B-6 status. Am J Clin Nutr 105, 897–904, https://doi.org/10.3945/ajcn.116.139337 (2017).
Marzio, A., Merigliano, C., Gatti, M. & Verni, F. Sugar and chromosome stability: clastogenic effects of sugars in vitamin B6-deficient cells. PLoS Genet 10, e1004199, https://doi.org/10.1371/journal.pgen.1004199 (2014).
Kanellis, P. et al. A screen for suppressors of gross chromosomal rearrangements identifies a conserved role for PLP in preventing DNA lesions. PLoS Genet 3, e134, https://doi.org/10.1371/journal.pgen.0030134 (2007).
Lee, S. C. & Chan, J. C. Evidence for DNA damage as a biological link between diabetes and cancer. Chin Med J (Engl) 128, 1543–1548, https://doi.org/10.4103/0366-6999.157693 (2015).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820, https://doi.org/10.1038/414813a (2001).
Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care 5, 561–568 (2002).
Hinokio, Y. et al. Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia 42, 995–998, https://doi.org/10.1007/s001250051258 (1999).
Goodarzi, M. T., Navidi, A. A., Rezaei, M. & Babahmadi-Rezaei, H. Oxidative damage to DNA and lipids: correlation with protein glycation in patients with type 1 diabetes. J Clin Lab Anal 24, 72–76, https://doi.org/10.1002/jcla.20328 (2010).
Tatsch, E. et al. Association between DNA strand breakage and oxidative, inflammatory and endothelial biomarkers in type 2 diabetes. Mutat Res 732, 16–20, https://doi.org/10.1016/j.mrfmmm.2012.01.004 (2012).
Graham, P. & Pick, L. Drosophila as a Model for Diabetes and Diseases of Insulin Resistance. Curr Top Dev Biol 121, 397–419, https://doi.org/10.1016/bs.ctdb.2016.07.011 (2017).
Garofalo, R. S. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol Metab 13, 156–162 (2002).
Boucher, J., Kleinridders, A. & Kahn, C. R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6, https://doi.org/10.1101/cshperspect.a009191 (2014).
Alfa, R. W. & Kim, S. K. Using Drosophila to discover mechanisms underlying type 2 diabetes. Dis Model Mech 9, 365–376, https://doi.org/10.1242/dmm.023887 (2016).
Musselman, L. P. et al. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech 4, 842–849, https://doi.org/10.1242/dmm.007948 (2011).
Thompson, S. N. Trehalose – The Insect ‘Blood’. Sugar. 31, 205–285, https://doi.org/10.1016/s0065-2806(03)31004-5 (2003).
Ugrankar, R. et al. Drosophila glucome screening identifies Ck1alpha as a regulator of mammalian glucose metabolism. Nat Commun 6, 7102, https://doi.org/10.1038/ncomms8102 (2015).
Bohni, R. et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97, 865–875 (1999).
Tatar, M. et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110, https://doi.org/10.1126/science.1057987 (2001).
Murillo-Maldonado, J. M., Sanchez-Chavez, G., Salgado, L. M., Salceda, R. & Riesgo-Escovar, J. R. Drosophila insulin pathway mutants affect visual physiology and brain function besides growth, lipid, and carbohydrate metabolism. Diabetes 60, 1632–1636, https://doi.org/10.2337/db10-1288 (2011).
Lorenzi, M., Montisano, D. F., Toledo, S. & Barrieux, A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest 77, 322–325, https://doi.org/10.1172/JCI112295 (1986).
Rezabakhsh, A. et al. Endothelial cells’ biophysical, biochemical, and chromosomal aberrancies in high-glucose condition within the diabetic range. Cell Biochem Funct 35, 83–97, https://doi.org/10.1002/cbf.3251 (2017).
Polo, S. E. & Jackson, S. P. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev 25, 409–433, https://doi.org/10.1101/gad.2021311 (2011).
Madigan, J. P., Chotkowski, H. L. & Glaser, R. L. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res 30, 3698–3705 (2002).
Jeggo, P. A., Pearl, L. H. & Carr, A. M. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer 16, 35–42, https://doi.org/10.1038/nrc.2015.4 (2016).
Merigliano, C. et al. A Role for the Twins Protein Phosphatase (PP2A-B55) in the Maintenance of Drosophila Genome Integrity. Genetics 205, 1151–1167, https://doi.org/10.1534/genetics.116.192781 (2017).
Vlassara, H. et al. Identifying advanced glycation end products as a major source of oxidants in aging: implications for the management and/or prevention of reduced renal function in elderly persons. Semin Nephrol 29, 594–603, https://doi.org/10.1016/j.semnephrol.2009.07.013 (2009).
Stopper, H., Schinzel, R., Sebekova, K. & Heidland, A. Genotoxicity of advanced glycation end products in mammalian cells. Cancer Lett 190, 151–156 (2003).
Schupp, N., Schinzel, R. & Heidland, A. & Stopper, H. Genotoxicity of advanced glycation end products: involvement of oxidative stress and of angiotensin II type 1 receptors. Ann N Y Acad Sci 1043, 685–695, https://doi.org/10.1196/annals.1333.079 (2005).
Golbidi, S., Badran, M. & Laher, I. Diabetes and alpha lipoic Acid. Front Pharmacol 2, 69, https://doi.org/10.3389/fphar.2011.00069 (2011).
Muellenbach, E. A. et al. Interactions of the advanced glycation end product inhibitor pyridoxamine and the antioxidant alpha-lipoic acid on insulin resistance in the obese Zucker rat. Metabolism 57, 1465–1472, https://doi.org/10.1016/j.metabol.2008.05.018 (2008).
Colombani, J. et al. A nutrient sensor mechanism controls Drosophila growth. Cell 114, 739–749 (2003).
Haselton, A. T. & Fridell, Y. W. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. Aging (Albany NY) 2, 523–526, https://doi.org/10.18632/aging.100185 (2010).
Kim, S. K. & Rulifson, E. J. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431, 316–320, https://doi.org/10.1038/nature02897 (2004).
Lee, G. & Park, J. H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167, 311–323 (2004).
Rulifson, E. J., Kim, S. K. & Nusse, R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120, https://doi.org/10.1126/science.1070058 (2002).
Broughton, S. J. et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA 102, 3105–3110, https://doi.org/10.1073/pnas.0405775102 (2005).
Ikeya, T., Galic, M., Belawat, P., Nairz, K. & Hafen, E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12, 1293–1300 (2002).
Fraser, M. et al. MRE11 promotes AKT phosphorylation in direct response to DNA double-strand breaks. Cell Cycle 10, 2218–2232, https://doi.org/10.4161/cc.10.13.16305 (2011).
Nakamura, S., Li, H., Adijiang, A., Pischetsrieder, M. & Niwa, T. Pyridoxal phosphate prevents progression of diabetic nephropathy. Nephrol Dial Transplant 22, 2165–2174, https://doi.org/10.1093/ndt/gfm166 (2007).
Pischetsrieder, M., Seidel, W., Munch, G. & Schinzel, R. N(2)-(1-Carboxyethyl)deoxyguanosine, a nonenzymatic glycation adduct of DNA, induces single-strand breaks and increases mutation frequencies. Biochem Biophys Res Commun 264, 544–549, https://doi.org/10.1006/bbrc.1999.1528 (1999).
Nakamura, S. & Niwa, T. Pyridoxal phosphate and hepatocyte growth factor prevent dialysate-induced peritoneal damage. J Am Soc Nephrol 16, 144–150, https://doi.org/10.1681/ASN.2004020120 (2005).
Blasiak, J. et al. DNA damage and repair in type 2 diabetes mellitus. Mutat Res 554, 297–304, https://doi.org/10.1016/j.mrfmmm.2004.05.011 (2004).
Clayton, P. T. B6-responsive disorders: a model of vitamin dependency. J Inherit Metab Dis 29, 317–326, https://doi.org/10.1007/s10545-005-0243-2 (2006).
Okada, M., Shibuya, M., Yamamoto, E. & Murakami, Y. Effect of diabetes on vitamin B6 requirement in experimental animals. Diabetes Obes Metab 1, 221–225 (1999).
Leklem, J. E. & Hollenbeck, C. B. Acute ingestion of glucose decreases plasma pyridoxal 5′-phosphate and total vitamin B-6 concentration. Am J Clin Nutr 51, 832–836 (1990).
Ahn, H. J., Min, K. W. & Cho, Y. O. Assessment of vitamin B(6) status in Korean patients with newly diagnosed type 2 diabetes. Nutr Res Pract 5, 34–39, https://doi.org/10.4162/nrp.2011.5.1.34 (2011).
Nix, W. A. et al. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res Clin Pract 107, 157–165, https://doi.org/10.1016/j.diabres.2014.09.058 (2015).
Gatti, M. & Goldberg, M. L. Mutations affecting cell division in Drosophila. Methods Cell Biol 35, 543–586 (1991).
Bonaccorsi, S., Giansanti, M. G. & Gatti, M. Spindle assembly in Drosophila neuroblasts and ganglion mother cells. Nat Cell Biol 2, 54–56, https://doi.org/10.1038/71378 (2000).
Diaz-Benjumea, F. J. & Hafen, E. The sevenless signalling cassette mediates Drosophila EGF receptor function during epidermal development. Development 120, 569–578 (1994).
Author information
Authors and Affiliations
Contributions
E.M., C.M. performed the experiments. C.M., M.L.T., I.S. contributed to the design of the experiments and to the writing of the manuscript. F.V. designed the experiments and wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Merigliano, C., Mascolo, E., La Torre, M. et al. Protective role of vitamin B6 (PLP) against DNA damage in Drosophila models of type 2 diabetes. Sci Rep 8, 11432 (2018). https://doi.org/10.1038/s41598-018-29801-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29801-z
This article is cited by
-
The expression of four pyridoxal kinase (PDXK) human variants in Drosophila impacts on genome integrity
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.