Abstract
The Surviving Sepsis Guidelines suggest the use of vasopressin in case of catecholamine-refractory septic shock. Terlipressin (TP) as a V1-selective AVP analogue is a potential alternative, though data regarding the first-line administration in septic shock are scarce. The present study explored and compared the effects of first-line vs. second-line infusion of TP or sole norepinephrine regarding organ function, fluid and norepinephrine requirements and survival in fulminant ovine septic shock. Peritoneal sepsis was induced in 23 ewes after laparotomy and faecal withdrawal from the caecum. After onset of shock, causal and supportive sepsis therapy (antibiotics, peritoneal lavage, fluids and open-label norepinephrine) was performed in all animals. Concurrently, animals were randomized to receive 0.9% sodium chloride (control group) or TP (2 µg∙kg−1∙h−1, first-line group) after shock onset. In the second-line TP group, TP (2 µg∙kg−1∙h−1) was started once norepinephrine requirements exceeded 0.5 µg∙kg−1∙min−1. No significant differences were found between groups regarding survival, haemodynamics as well as fluid- and catecholamine-requirements. Kidney function and electron microscopic kidney injury were comparable between groups. In the present model of fulminant ovine septic shock, first-line TP infusion had no significant effect on fluid and norepinephrine requirements or organ dysfunction as compared to second-line TP infusion or placebo.
Similar content being viewed by others
Introduction
Patients with septic shock commonly require large doses of catecholamines to maintain a sufficient mean arterial pressure (MAP). According to the current sepsis guidelines, norepinephrine is the vasopressor of choice in the treatment of sepsis related vasodilation1. However, there is increasing evidence that high catecholamine doses may have detrimental effects and is associated with increased mortality2,3. Thus, alternative, non-adrenergic vasopressors are desirable as first- or second-line treatment of sepsis-associated vasodilation.
The current sepsis guidelines suggest the vasopressin receptor agonist arginine-vasopressin (AVP) as second-line treatment if MAP cannot be maintained by norepinephrine alone. The second indication for non-adrenergic vasopressors is to reduce to dose of norepinephrine needed. First-line AVP therapy however, is discouraged by the guidelines in fear of ischemic end-organ events. Additionally, the reluctant use of AVP in clinical settings might be based on low experience and the fear of clinicians regarding intestinal or digital ischemia as well as reduced global oxygen delivery and cardiac output4,5. In contrast, evidence suggests that rather sepsis itself is the reason for such complications, and the use of vasopressin analogues does not trigger ischemic events6,7. Notably, administration of AVP in septic patients has been proven safe as supplemental (The Vasopressin in Septic Shock (VASST)-Trial) as well as first-line therapy (Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock (VANISH)- Trial)8,9.
Notably, AVP is not available in several countries. Instead, the vasopressin-receptor agonist terlipressin (TP) is commonly used. TP has a higher selectivity for the V1a-receptor than AVP, and has been demonstrated equally or more effective than AVP in experimental and small clinical trials10,11,12. A single centre randomized controlled trial by Svoboda and colleagues with 30 patients investigated the effects of terlipressin administration in catecholamine-resistant septic shock. The authors concluded that continuous terlipressin infusion was ineffective in reduction of catecholamine requirements and mortality if applied in the late phase of catecholamine-resistant septic shock13. On the other hand, the previous published TERLIVAP-trial, which compared the effects of first-line AVP versus first-line TP in septic shock patients described a reduction in catecholamine requirements and lower rates of new onset tachyarrhythmias within the TP group14. Moreover, experimental data suggest, that V1 agonists may reduce sepsis-associated endothelial injury and capillary leakage, thus favouring early treatment initiation15,16.
Notably, no studies have yet investigated first-line versus second-line treatment with TP as a continuous infusion in septic shock. Therefore, the present study was designed to explore the effects of first-line continuous low-dose administration of TP versus second-line administration (which is the common situation in the clinical setting) regarding fluid and norepinephrine requirements as well as organ function and survival in fulminant ovine septic shock.
Material and Methods
Animal care
After arrival in the research facility, the animals were housed in flocks of 3 to 10 animals under veterinary supervision. Veterinary care attendants visited the sheep twice a day and more often when necessary. A veterinary examination of health status took place on arrival, prior to inclusion in the study and whenever deemed necessary by the veterinary care attendants. All methods were performed in accordance with the National Institutes of Health Guide and as well as the American Physiologic Society’s “Guide for the Care and Use of Laboratory Animals” using established protocols.
Instrumentation
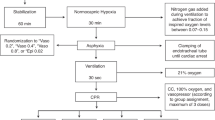
After approval by the Animal Care Committee of the State Government of North-Rhine Westphalia (LANUV NRW, Recklinghausen, Germany) with the approval (ref no. 8.87–50.10.37.09.194), 23 healthy female sheep (median body weight 42.0 kg, 34.0–43.5; 25th− 75th percentile) were anaesthetized by intramuscular injection of S-ketamine (Ketanest® S, 10 mg·kg−1, Parke-Davis, Berlin, Freiburg, Germany) and midazolam (Dormicum®, 0.3 mg·kg−1, Hoffmann-La Roche AG, Grenzach-Wyhlen, Germany). The ewes were held in abstinence from food for 12 hours prior to the instrumentation with free access to water. After endotracheal intubation with a 9.0 tracheal tube (Rüsch, Rüschelit©, Teleflex Medical GmbH, Kernen, Germany), anaesthesia was maintained by inhalational isoflurane with an expiratory fraction of 1.0–1.5% (Forene®; Abbott GmbH & Co. KG, Wiesbaden, Germany). A quadlumen central venous catheter (6 Fr. Quadlumen Catheter Set, PVB Medizintechnik GmbH, Kirchseeon, Germany) was placed using Seldinger’s technique into the right jugular vein through which anaesthesia was supplemented with S-ketamine (1 mg·kg−1·h−1), midazolam (0.3 mg·kg−1·h−1) and lidocain (1.5 mg·kg−1·h−1)17 during the further instrumentation. For continuous hemodynamic surveillance, a pulse contour cardiac output (PiCCO) catheter was placed in the right femoral artery (5 Fr.; Pulsion Medical Systems, Munich, Germany) with connection to a transpulmonary thermodilution and pulse contour cardiac output computer (PiCCO2, Pulsion Medical Systems, München, Germany). A Foley catheter (12 Fr. urinary catheter, Porgès S.A., Le Plessis Robinson-Cedex, France) was inserted to determine urinary output.
Surgical preparation
Following a median laparotomy, the cecum of the animals was detected and incised in order to withdraw 1.5 g·kg−1 faeces. A contamination of the peritoneal cavity was strictly avoided. Two 16 Fr. drains were placed in the mesentery of the small intestine and the abdomen was closed with continuous suture afterwards. After a 2 hours’ phase of recovery, baseline (BL) data were assessed to examine whether the animals fulfil the inclusion criteria.
Inclusion criteria
The following criteria had to be fulfilled at BL before inclusion in the study:
-
Heart rate (HR) < 100 bpm
-
Mean arterial pressure (MAP) 70–120 mmHg
-
Cardiac index (CI) 2.5–6.0 L·min−1·m2
-
Serum lactate ≤1,2 mmol·l−1
-
Temperature 38.0–39.8 °C
-
Arterial pH: 7.30–7.50
-
Arterial carbon dioxide pressure 35–55 mmHg.
The inclusion criteria were based on reference values for healthy sheep18.
Induction of septic shock
After inclusion in the study, autologous faeces were injected into the abdominal cavity via one of the 16 Fr. drain. Onset of septic shock was defined as
-
MAP <60 mm Hg and
-
Serum lactate concentration ≥1.8 mmol·l−1 (i.e. 1.5 times the upper normal limit of sheep18) and
-
Minimum of four hours after instillation of the faeces.
After the onset of septic shock, “shock time” measurements were performed as detailed below.
Randomization
After the “shock time” measurements, the animals were randomly assigned to one of the following study groups:
-
Control (n = 7)
-
[study solution 1: 0.9% saline; study solution 2: 0.9% saline]
-
Terlipressin first-line (n = 8)
-
[study solution 1: TP (2 µg∙kg−1∙h−1); study solution 2: 0.9% saline]
-
Terlipressin second-line (n = 8)
-
[study solution 1: 0.9% saline; study solution 2: TP (2 µg∙kg−1∙h−1)].
The attendant investigators were blinded for study group allocation and content of study drug syringes. Study solution 1 was started immediately after randomisation. The second study solution was initiated when norepinephrine requirements exceeded 0.5 µg∙kg−1∙min−1. Once initiated, both study solutions were administered with a fixed infusion rate until the end of the protocol.
Study protocol
After randomization, study solution 1 was started as specified in the group description and continued throughout the whole experiment. Causal therapy was initiated by intravenous antimicrobial therapy with a bolus infusion of 20 mg·kg−1 meropenem (Meronem©, AstraZeneca GmbH, Wedel, Germany) and followed by continuous intravenous infusion with 2.5 mg·kg−1·h−1. Furthermore, peritoneal lavage was initiated by fractional instillation of four litres of warm (38° Celsius) saline through the abdominal drains until no more faecal contamination was detected.
Supportive fluid therapy was based on dynamic and volumetric hemodynamic parameters. Indications for fluid resuscitation were:
-
Global enddiastolic volume index (GEDI) < 620 mL·m−2 or < BL1 value
-
Stroke volume variation (SVV) > 13%
-
Haematocrit (Hct) > BL1 value.
Contraindications for fluid resuscitation were:
-
Extravascular lung water index (ELWI) ≥ 17 mL·kg−1
-
Horowitz-Index (PaO2/FiO2) <2.
Fluid resuscitation was performed with hydroxyethyl starch (HES) 6% 130/0.4 (Volulyte©, Fresenius Kabi, Bad Homburg, Germany) and balanced crystalloids (Sterofundin© ISO, B. Braun Melsungen, Germany). HES and crystalloids were applied alternately (250 ml HES followed by 500 ml crystalloid) until the maximum dose of HES was reached (50 mL·kg−1). If necessary, further fluid resuscitation was performed with crystalloids only until hemodynamic indicators were met.
Norepinephrine was initiated at the onset of shock and titrated to maintain a MAP ≥65 mmHg up to a maximum dose of 5 µg·kg−1·h−1. If norepinephrine requirements exceeded 0.5 µg∙kg−1∙min−1, the second study solution was initiated as detailed in the group description and continued until the end of the experiment. The maximum dose of norepinephrine was drawn from clinical experience, when no more vasoconstrictive effect of the substance could be expected due to tachyphylactic effects.
Measurements
Hemodynamic parameters, urinary output as well as arterial and central-venous blood gas analyses were documented at BL, shock time and hourly thereafter. Blood and urine samples for laboratory and microbiological analyses were taken at BL, shock time and every four hours afterwards. The samples were immediately centrifuged and stored at −70 °C for later analyses.
Analysed laboratory variables
The following variables were determined from blood and urine samples, respectively:
-
Blood gas analyses (electrolytes, oxygen- and carbon dioxide partial pressure, pH, base excess (BE), haemoglobin, haematocrit, oxygen saturation, lactate, glucose).
-
Parameters of organ (dys-) function (bilirubin, aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), serum creatinine concentration, serum urea concentration, creatinine-clearance).
Aerobic and anaerobic blood cultures were withdrawn under sterile conditions at BL, shock time as well as 8 h, 16 h and 24 h afterwards.
End of protocol and autopsy
At the end of the 24 hours interventional period after shock time the animals were killed in deep propofol anaesthesia (4 mg·kg−1) with a bolus injection of 100 ml of 1-molar potassium chloride solution. All animals were autopsied with removal and weighing of the heart, lungs, kidneys and terminal ileum. Additionally, samples from the kidney were collected for electron microscopic analyses.
Electron microscopy
Transmission electron microscopy (TEM) was performed with a Philips CmlO-Electronic microscope (Philips, Eindhoven, Netherlands) at 80 kV. Cellular damage, cellular oedema and mitochondrial damage was quantified by a pathologist who was blinded for the protocol. Ultrastructural kidney damage was quantified according to the “electron microscopic tubular injury” (EMTI) score19. This score contains the four criteria (1) vacuolar degeneration and swelling of organella, (2) dissociation of epithelium and basal membrane, (3) epithelial cell injury and (4) intratubular precipitation. Each criterion was scored from 0 to 3, thus the total EMTI score (sum of the four criteria) could range from 0 to 12 points19.
Statistical analysis
Statistical analysis was performed with IBM SPSS statistics software version 22 (IBM, Armonk, New York, United States). All data are presented as median and interquartile range (IQR). Comparisons between groups for variables measured only once were made using Kruskal-Wallis H-test. If necessary, post-hoc comparisons were conducted using Dunn’s test. Comparisons between time points were made using Wilcoxon signed-rank test. Comparisons between groups for repeatedly measured variables were conducted by calculation of generalized estimating equations (GEE) with group as factor and time as covariate20. Asymptotic two-sided p-values smaller than 0.05 were assumed as statistically significant.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Results
Features of septic shock (prior to study drug infusion)
All animals developed septic shock between BL and shock time with reductions in MAP, CI and with lactic acidosis (see Supplemental Digital Content 1 Table 1, BL versus shock time data). Renal function decreased during this time and acute kidney injury occurred, which was classified according to the KDIGO guidelines21 using diuresis and creatinine concentration (see Supplemental Digital Content 1 Table 1, BL versus shock time data).
Hemodynamic and oxygen transport variables (during study drug infusion)
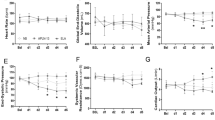
There were no differences between the study groups regarding hemodynamic variables (see Table 1, Supplemental Digital Content 2 Fig. 1 and Supplemental Digital Content 3 Fig. 2, cardiac index and heart rate). Haematocrit was higher within the TP second-line group (p < 0.05) as compared to TP first-line group (see Supplemental Digital Content 4 Fig. 3, Haematocrit concentration). All other measured parameters of oxygen transport were comparable between the study groups (see Table 2).
Cumulative fluid requirements per hour. The figure demonstrated the cumulative fluid requirements of the study animals over time within the 24-hour interventional period. The average initiation-points of the study solutions are highlighted in the figure. (A) Shock time and initiation of the 1st study solution. (B) Average start of the 2nd study solution. Data are presented as mean [standard deviation].
Fluid and norepinephrine requirements
There were no differences between the study groups regarding cumulative fluid and norepinephrine requirements (see Figs 1, 2 and 3) over the 24-hour interventional period, though the catecholamine-requirements in the TP first-line group tended to be lower without statistical significance (median norepinephrine requirements per body weight per hours alive [µg·kg−1·h−1]: control group 57.2 [30.9; 287.9]; TP first-line 30.3 [6.1; 79.3]; TP second-line 66.6 [37.7; 107.3]). The initiation of the second study solution was after 6.0 h [4.0; 11.5] in the control group, 5.0 h [2.0; 6.0] in the TP first-line group and 6.0 h [5.0; 6.0] in the TP second-line group. Mean start of the second study solution over all groups was 6.2 h [±4.1] after shock time. The cumulative fluid requirements of the TP groups were lower as compared to the control group without statistical significance (see Figs 1 and 3).
Organ function and EMTI-Score
All animals developed acute kidney injury at shock time, which persisted despite study therapy (see Table 3). Kidney function and injury were comparable between groups as measured by serum creatinine, creatinine clearance, diuresis and EMTI score (see Fig. 4 and Table 3).
All animals developed an increase of liver enzymes over the interventional period. The animals of the TP first-line group showed significantly elevated ASAT and ALAT as compared to the control group (each p < 0.05, see Supplemental Digital Content 5 Fig. 4 and Supplemental Digital Content 6 Fig. 5, ASAT and ALAT). Serum vasopressin levels were lower in the control group as compared to the TP groups (each p < 0.05, see Table 3).
All other measured variables of organ function as well as organ weights and relative organ weights showed no differences between the study groups (see Table 3 and Supplemental Digital Content 7 Table 2, Organ weights).
Blood cultures
The blood cultures taken at BL were mostly sterile or contained single bacteria of the skin flora, whereas a broad spectrum of intestinal bacteria was detected in the blood cultures taken at shock time. The bacterial load decreased over the interventional period. Enterococcus faecium was the most frequently detected bacterial species at the end of the study (see Supplemental Digital Content 8 Figure 6, results from blood cultures).
Survival
In the control-group and the TP first-line group, each five of eight animals survived the interventional period (62.5%). Six of eight animals survived in the TP second-line group (75%). The mean survival times were 23.6 h (23.1; 24) in the TP second-line group followed by the control-group [20.8 h (17.6; 23.9)] and the TP first-line group [19.6 h, 16.6; 23.6)]. There were no statistically significant differences between the groups regarding 24- h survival (see Fig. 5).
Discussion
The present study compared the effects of a first-line versus second-line therapy with continuous low-dose TP in ovine septic shock on fluid- and catecholamine requirements as well as organ function and survival. All study animals developed septic shock with hyperlactatemia and acidosis as well as organ dysfunction with the onset of septic shock. Haemodynamics were characterized by a hyperdynamic circulation with high-dose norepinephrine requirements. There were no differences regarding amounts of intravenous fluids or catecholamines and survival between the study groups, though the animals of the TP first-line group tended to receive lower amounts of norepinephrine. No other relevant side effects of terlipressin were detected. Furthermore, no differences between the groups regarding kidney function as measured by diuresis, creatinine or ultrastructural kidney damage quantified by electronic microscopic could be observed.
Septic shock induced in the present study matched the definition of the current international consensus definitions of sepsis and septic shock (Sepsis-3), which define sepsis as a life-threatening organ dysfunction caused by a dysregulated host response following infection22. Furthermore, these criteria require not only sepsis with persisting hypotension and the need for vasopressors to maintain a MAP ≥ 65 mmHg but also hyperlactatemia despite adequate volume resuscitation22. Infection was induced successfully with peritonitis and consecutive bacteraemia, which was proven by blood cultures (see Supplemental Digital Content 8 Figure 6, results from blood cultures).
A small pilot-trial investigated the effects of first-line AVP versus TP in human septic shock (TERLIVAP). Within this RCT, first-line continuous low-dose administration of TP reduced catecholamine requirements more effectively as compared to AVP and also reduced the risk of new onset tachyarrhythmia14. There were no significant differences in norepinephrine requirements between the study groups in the present study, which is an unexpected result that contradicts previous data. One possible explanation might be the dosage of the administered study solutions. Although the applied dose in the study animals (2 µg∙kg−1∙h−1) was higher than the dosage in the TERLIVAP trial (1.3 µg∙kg−1∙h−1), which was able to show a significant reduction in catecholamine requirements14, underdosing of TP in the present study must be considered as a possible explanation. This is especially true since the required norepinephrine doses were higher than 1 µg∙kg−1∙min−1 in many animals, suggesting a very severe vasodilatory shock state. The persistent bacteraemia in the present trial might also be interpreted as a sign of severe disease. Since haemodynamics and infection could not be sufficiently stabilized, the present model may be regarded as refractory septic shock. The results from the “Vasopressin and Septic Shock Trial” (VASST) demonstrated beneficial effects of vasopressin administration only in patients with less-severe septic shock8, which is another explanation why the therapeutic strategies used in the present trial were ineffective. Other studies using terlipressin in ovine systemic inflammation demonstrated reduction of catecholamine requirements, however, these trials were performed in endotoxemia and not in animals with fulminant abdominal sepsis11. In the TERLIVAP trial, significant differences regarding catecholamine requirements between the study groups were measured at least 24 hours after study drug initiation. Thus, another explanation for the lack of differences regarding catecholamine requirements might be the length of the present observational period. Maybe significant differences in catecholamine requirements need some time to occur with continuous infusion of TP, whereas bolus infusion shows immediate hemodynamic effects23. It is finally possible, that in severe shock states not only norepinephrine but also non-adrenergic vasopressors need dose adjustment. However, further increase of the terlipressin dose might be associated with increased adverse effects and should therefore be investigated carefully in future trials. There is currently no data available regarding the long-term effects of terlipressin on organ function or adverse effects. Svoboda and colleagues investigated continuous terlipressin administration in catecholamine-refractory septic shock and described no adverse effects13. Yildizdas et al. used terlipressin as a bolus rescue-therapy in children suffering from septic shock and described no adverse effects or detrimental organ affection as well24. Together with the mentioned findings from the TERLIVAP-trial (observational period 48 hours), administration of TP in sepsis seems to be safe in short-term use. However, though the half-life of terlipressin is quite low, one cannot exclude that potential harmful effects on organ function might occur with delay (>48 hours) and were not monitored in the available studies. Accordingly, future trials on terlipressin in sepsis should consider longer observational periods and follow-up of the patients with focus on long-term organ failure.
Furthermore, species related differences in (receptor-)physiology may also play a role in this context25. AVP and its synthetic analogues (especially TP) are potent vasopressors, causing vasoconstriction by activation of vasopressin (V)-receptors. While arginine-vasopressin (AVP) has an identical affinity to the (vascular) V1 receptor as compared to the (renal) V2 receptor (V1/V2-ratio of 1), terlipressin is more V1-selective (V1/V2-ratio of 2.2) in humans. It should be considered, that this receptor affinity may differ in sheep. Other studies demonstrated beneficial effects of terlipressin in ovine endotoxemia11, however, the observed effects did not prove that the V1/V2-ratio is comparable to human beings.
Increases in haemoglobin and haematocrit levels in septic patients are commonly interpreted as a consequence of both hypovolemia and capillary leakage26. The observed increase in haematocrit at shock time indicates that relevant capillary leakage was induced in the present model. Though the cumulative fluid requirements and organ weights were comparable between groups, the haematocrit of the TP second-line group raised significantly over the 24-hour period as compared to the TP first-line group. This might indicate more severe capillary leakage in the TP second-line group, although serum lactate concentrations and catecholamine requirements were comparable between the study groups and offer no hints for a higher severity of septic shock. Additionally, one would expect more fluid requirements in case of higher capillary leakage, however, the cumulative fluid requirements tended to be lower in the TP groups as compared to the control group.
Regarding organ function, there were no differences in acute kidney injury or tubular damage of the animals between the study groups as measured by retention parameters, urinary output and EMTI score. However, there were some differences between the groups in the measured liver enzymes which should be addressed in the following. TP is commonly used in the clinical setting to treat variceal bleeding of the oesophagus. The mechanism behind this is a vasoconstrictive effect of TP on dilated splanchnic blood vessels with consecutive reduction of blood flow and pressure in the portal vein27. The increase of liver enzymes in the TP first-line group of the present investigation could be explained by a reduced liver perfusion due to the described mechanism. On the other hand, it has been shown that the reduction in portal venous blood flow by vasopressin agonists is compensated by an increase in hepatic arterial blood flow (so called hepatic artery buffer response)28,29. Furthermore, no differences regarding bilirubin levels were observed.
In the TERLIVAP-trial, the investigated septic patients who received continuous low-dose TP showed reduced levels of serum bilirubin as compared to the patients who were treated with norepinephrine or vasopressin. There were no differences between the study patients regarding ASAT, ALAT and activated partial thromboplastin time ratio (aPTTr)14. Nevertheless, the observed increase in ASAT and ALAT in the present study appears to be a clear pharmacological effect of TP, since it was most pronounced in the TP first-line group and less pronounced in the second-line group, whereas ASAT and ALAT activities were lowest in the control group. The relevance of this finding should be focussed in future studies.
There are some limitations in the present study which should be addressed:
Since the study was performed in an animal model, results and conclusions should be transferred to clinical settings with caution. Though the hemodynamic pattern of healthy and septic sheep is similar to human beings30,31, the effects of vasoactive substances may be different between species, especially regarding the substructure of the vasopressin receptors and the V1/V2 ratio. Furthermore, though the model is of clinical relevance, one should consider that septic shock was fulminant, and thus any pharmacological intervention may have been futile. It must be noted, that the 24-hour observational period in the present study is quite short for a complex disease like sepsis and for detection of long-term terlipressin effects. Another limitation of the present investigation was the use of HES in septic shock, which was an accepted strategy at the time of initiation of the study with the mentioned maximum dose of 50 ml·kg−1 BW32. Since fluid therapy was identical among group, this should not induce a relevant bias. Furthermore, the antimicrobial therapy with meropenem failed to eliminate Enterococcus faecium (natural resistance against carbapenems) in blood cultures. In future studies using the present model, antimicrobial chemotherapy might include additional gram-positive coverage, e.g. vancomycin.
Conclusion
In the present study, first-line versus second-line administration of continuous low-dose terlipressin in fulminant ovine septic shock had no influence on norepinephrine and fluid requirements, organ injury or 24-h survival. No beneficial effects of terlipressin were observed, most likely due to the fulminant sepsis with refractory vasoplegia or consecutive underdosing of terlipressin in relation to the severity of the shock state.
References
Rhodes, A. et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 18, 017–4683 (2017).
Jenkins, C. R., Gomersall, C. D., Leung, P. & Joynt, G. M. Outcome of patients receiving high dose vasopressor therapy: a retrospective cohort study. Anaesth Intensive Care 37, 286–289 (2009).
Dopp-Zemel, D. & Groeneveld, A. B. High-dose norepinephrine treatment: determinants of mortality and futility in critically ill patients. Am J Crit Care 22, 22–32 (2013).
Dunser, M. W. et al. Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: incidence and risk factors. Crit Care Med 31, 1394–1398 (2003).
Klinzing, S., Simon, M., Reinhart, K., Bredle, D. L. & Meier-Hellmann, A. High-dose vasopressin is not superior to norepinephrine in septic shock. Crit Care Med 31, 2646–2650 (2003).
Luckner, G. et al. Vasopressin as adjunct vasopressor for vasodilatory shock due to non-occlusive mesenteric ischemia. Anaesthesist 55, 283–286, https://doi.org/10.1007/s00101-005-0958-3 (2006).
Luckner, G. et al. Cutaneous vascular reactivity and flow motion response to vasopressin in advanced vasodilatory shock and severe postoperative multiple organ dysfunction syndrome. Crit Care 10, R40 (2006).
Russell, J. A. et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358, 877–887 (2008).
Gordon, A. C. et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. Jama 316, 509–518 (2016).
Rehberg, S. et al. Role of arginine vasopressin and terlipressin as first-line vasopressor agents in fulminant ovine septic shock. Intensive Care Med 35, 1286–1296, https://doi.org/10.1007/s00134-009-1470-z (2009).
Lange, M. et al. Effects of two different dosing regimens of terlipressin on organ functions in ovine endotoxemia. Inflamm Res 60, 429–437, https://doi.org/10.1007/s00011-010-0299-9 (2011).
Lange, M., Ertmer, C. & Westphal, M. Vasopressin vs. terlipressin in the treatment of cardiovascular failure in sepsis. Intensive Care Med 34, 821–832 (2008).
Svoboda, P. et al. Terlipressin in the treatment of late phase catecholamine-resistant septic shock. Hepato-gastroenterology 59, 1043–1047, https://doi.org/10.5754/hge10550 (2012).
Morelli, A. et al. Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): a randomized, controlled pilot study. Crit Care 13, R130 (2009).
He, X. et al. A Selective V(1A) Receptor Agonist, Selepressin, Is Superior to Arginine Vasopressin and to Norepinephrine in Ovine Septic Shock. Crit Care Med 44, 23–31, https://doi.org/10.1097/ccm.0000000000001380 (2016).
Maybauer, M. O. et al. The Selective Vasopressin Type 1a Receptor Agonist Selepressin (FE 202158) Blocks Vascular Leak in Ovine Severe Sepsis. Crit Care Med 26, 26 (2014).
Weibel, S. et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth 116, 770–783 (2016).
Kampmeier, T. et al. Provision of physiological data and reference values in awake and anaesthetized female sheep aged 6-12 months. Vet Anaesth Analg 11, 30027–30022 (2017).
Ertmer, C. et al. Effects of balanced crystalloid vs. 0.9% saline-based vs. balanced 6% tetrastarch infusion on renal function and tubular integrity in ovine endotoxemic shock. Crit Care Med 39, 783–792, https://doi.org/10.1097/CCM.0b013e318206d403 (2011).
Ma, Y., Mazumdar, M. & Memtsoudis, S. G. Beyond repeated-measures analysis of variance: advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med 37, 99–105 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int (2) Suppl, 1–138 (2012).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 315, 801–810 (2016).
Morelli, A. et al. Short-term effects of terlipressin bolus infusion on sublingual microcirculatory blood flow during septic shock. Intensive Care Med 37, 963–969, https://doi.org/10.1007/s00134-011-2148-x (2011).
Yildizdas, D., Yapicioglu, H., Celik, U., Sertdemir, Y. & Alhan, E. Terlipressin as a rescue therapy for catecholamine-resistant septic shock in children. Intensive Care Med 34, 511–517, https://doi.org/10.1007/s00134-007-0971-x (2008).
Kampmeier, T. G., Ertmer, C. & Rehberg, S. Translational research in sepsis - an ultimate challenge? Exp Transl Stroke Med 3, 14, https://doi.org/10.1186/2040-7378-3-14 (2011).
Lundblad, C., Axelberg, H. & Grande, P. O. Treatment with the sphingosine-1-phosphate analogue FTY 720 reduces loss of plasma volume during experimental sepsis in the rat. Acta Anaesthesiol Scand 57, 713–718 (2013).
Dohler, K. D., Walker, S., Mentz, P., Forssmann, K. & Staritz, M. Vasoconstrictive Therapies for Bleeding Esophageal Varices and their Mechanisms of Action. Z Gastroenterol 41, 1001–1016 (2003).
Eipel, C., Abshagen, K. & Vollmar, B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol 16, 6046–6057 (2010).
Krejci, V., Hiltebrand, L. B., Jakob, S. M., Takala, J. & Sigurdsson, G. H. Vasopressin in septic shock: effects on pancreatic, renal, and hepatic blood flow. Crit Care 11 (2007).
Rademaker, M. T., Charles, C. J., Nicholls, M. G. & Richards, A. M. Haemodynamic, endocrine and renal actions of adrenomedullin 5 in an ovine model of heart failure. Clin Sci (Lond) 122, 429–437, https://doi.org/10.1042/CS20110483 (2012).
Ertmer, C. et al. Exogenous adrenomedullin prevents and reverses hypodynamic circulation and pulmonary hypertension in ovine endotoxaemia. Br J Anaesth 99, 830–836, https://doi.org/10.1093/bja/aem295 (2007).
Dellinger, R. P. et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36, 296–327 (2008).
Acknowledgements
We acknowledge support by Open Access Publication Fund of University of Muenster. Support was provided solely from institutional and departmental sources.
Author information
Authors and Affiliations
Contributions
T.G.K. Concepted study design, performed analysis on all samples, interpreted data, wrote manuscript and acts as corresponding author. P.H.A. Made substantial contributions to conception and design and acquisition of data, interpreted data, participated in drafting the article and revising it critically for important intellectual content. M.H. Concepted study design, performed analysis on all samples, interpreted data, wrote manuscript and revised it critically for important intellectual content. L.M.S. Made substantial contributions to data interpretation and manuscript drafting. Gave final approval of the version to be submitted and any revised version. K.B. Made substantial contributions to data interpretation and manuscript drafting. Gave final approval of the version to be submitted and any revised version. A.M. Made substantial contributions to data interpretation and manuscript drafting. Gave final approval of the version to be submitted and any revised version. S.R. Made substantial contributions to conception and design and acquisition of data, participated in drafting the article and revising it critically for important intellectual content. C.E. Concepted study design, interpreted data, participated in drafting the article and revising it critically for important intellectual content.
Corresponding author
Ethics declarations
Competing Interests
Tim-Gerald Kampmeier received travel reimbursements and honoraria as a consultant from Fresenius Kabi Germany. Sebastian Rehberg has received travel fees provided by Orion Pharma and Amomed Pharma and is Medical Advisor for Amomed Pharma and Fresenius Kabi Germany. For the remaining authors no financial and non-financial competing interests were declared.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kampmeier, T.G., Arnemann, P.H., Hessler, M. et al. Comparison of first-line and second-line terlipressin versus sole norepinephrine in fulminant ovine septic shock. Sci Rep 8, 7105 (2018). https://doi.org/10.1038/s41598-018-25570-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25570-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.