Abstract
Background
Current neonatal resuscitation guidelines recommend epinephrine for cardiac arrest. Vasopressin might be an alternative during asphyxial cardiac arrest. We aimed to compare vasopressin and epinephrine on incidence and time to return of spontaneous circulation (ROSC) in asphyxiated newborn piglets.
Design/methods
Newborn piglets (n = 8/group) were anesthetized, intubated, instrumented, and exposed to 30 min of normocapnic hypoxia, followed by asphyxia and asystolic cardiac arrest. Piglets were randomized to 0.2, 0.4, or 0.8IU/kg vasopressin, or 0.02 mg/kg epinephrine. Hemodynamic parameters were continuously measured.
Results
Median (IQR) time to ROSC was 172(103–418)s, 157(100–413)s, 122(93–289)s, and 276(117–480)s for 0.2, 0.4, 0.8IU/kg vasopressin, and 0.02 mg/kg epinephrine groups, respectively (p = 0.59). The number of piglets that achieved ROSC was 6(75%), 6(75%), 7(88%), and 5(63%) for 0.2, 0.4, 0.8IU/kg vasopressin, and 0.02 mg/kg epinephrine, respectively (p = 0.94). The epinephrine group had a 60% (3/5) rate of post-ROSC survival compared to 83% (5/6), 83% (5/6), and 57% (4/7) in the 0.2, 0.4, and 0.8IU/kg vasopressin groups, respectively (p = 0.61).
Conclusion
Time to and incidence of ROSC were not different between all vasopressin dosages and epinephrine. However, non-significantly lower time to ROSC and higher post-ROSC survival in vasopressin groups warrant further investigation.
Impact
-
Time to and incidence of ROSC were not statistically different between all vasopressin dosages and epinephrine.
-
Non-significantly lower time to ROSC and higher post-ROSC survival in vasopressin-treated piglets.
-
Overall poorer hemodynamic recovery following ROSC in epinephrine piglets compared to vasopressin groups.
-
Human neonatal clinical trials examining the efficacy of vasopressin during asphyxial cardiac arrest will begin recruitment soon.
Similar content being viewed by others
Introduction
The International Liaison Committee on Resuscitation recommends epinephrine when a newborn’s heart rate remains <60 beats/minute despite chest compressions (CCs) and positive pressure ventilation (PPV) with 100% oxygen.1 Epinephrine possesses chronotropic, dromotropic, inotropic, and lusitropic effects and is an effective systemic vasoconstrictor; however, it also increases myocardial oxygen demand with reduced contractile response during respiratory and metabolic acidosis.2,3,4 Furthermore, its infrequent use and the inability to predict which newborns will require cardiopulmonary resuscitation (CPR), has led to a lack of clinical data on epinephrine.5,6
Vasopressin may be an alternative as it is a systematic vasoconstrictor and pulmonary vasodilator whose mechanism of action is unaffected by metabolic or respiratory acidosis.4,7 Vasopressin mediates smooth muscle contraction, thereby directly increasing systemic vascular resistance and, consequently, enhancing coronary artery perfusion.8 In adults with asystolic out-of-hospital cardiac arrest, vasopressin resulted in significantly higher rates of survival to hospital admission (29% vs. 20%, p = 0.02) and discharge (5% vs. 2%, p = 0.04) compared to epinephrine.9 Asphyxia resulting in asystole is one of the main cause of cardiac arrest in newborns, therefore vasopressin might be an alternative during neonatal CPR. Contradicting results about the effectiveness of vasopressin during neonatal CPR have been reported. McNamara et al., randomized 69 newborn piglets to 0.01 or 0.03 mg/kg epinephrine, 0.2 or 0.4IU/kg vasopressin, or saline (control) during CPR.10 Vasopressin with 0.4IU/kg (n = 9/10 (90%)) resulted in higher survival rate compared to 0.01 mg/kg epinephrine (n = 5/13 (36%); p = 0.006) and control (n = 5/12 (43%); p = 0.03), while there was no difference in survival between 0.2IU/kg vasopressin and 0.03 mg/kg epinephrine.10 Rawat et al., randomized 27 near-term lambs to either 0.03 mg/kg epinephrine or 0.4IU/kg vasopressin and reported no difference in time or number of lambs achieving ROSC [70% (7/10) with epinephrine by 8(2) minutes and 33% (3/9) vasopressin lambs by 13(6) minutes, no p value reported].11
We aimed to compare different dosages of vasopressin with epinephrine during neonatal CPR in our post-transitional model of neonatal asphyxia. Our hypothesis was that vasopressin compared to epinephrine would decrease the time to return of spontaneous circulation (ROSC) in asphyxiated neonatal piglets.
Methods
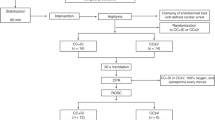
Thirty-two newborn mixed breed piglets were obtained on the day of experimentation from the University Swine Research Technology Center. Piglets were clinically normal and free of diseases that could affect results. All experiments were conducted in accordance with the guidelines and approval of the Animal Care and Use Committee (Health Sciences), University of Alberta [AUP00002920], presented according to the ARRIVE guidelines,12 and registered at preclinicaltrials.eu (PCTE0000378). A graphical display of the study protocol is presented in Fig. 1.
Randomization
Piglets were randomly allocated to intravenous administration of vasopressin 0.2IU/kg, 0.4IU/kg, 0.8IU/kg, or epinephrine 0.02 mg/kg. Allocation was block randomized with variable sized blocks using a computer-generated randomization program (http://www.randomizer.org). Sequentially numbered, sealed, brown envelopes containing the allocation were opened during the experiment (Fig. 1).
Blinding
GMS assessed cardiac arrest and was blinded to group allocation until confirmed cardiac arrest. Following stabilization, TFL opened the randomization envelope and was solely responsible for preparing and administering vasopressin/epinephrine. As TFL was not blinded to treatment allocation, he had no role in asphyxiation, cardiac arrest assessment, or administration of PPV. The content of the drug syringe was only known to TFL, the remaining team was blinded to the type of drug treatments.
Animal preparation
Piglets were instrumented as previously described with modifications.13,14,15 Following the induction of anesthesia using isoflurane, piglets were intubated via a tracheostomy, and pressure-controlled ventilation (Sechrist Infant Ventilator Model IV-100; Sechrist Industries, Anaheim, California) was commenced at a respiratory rate of 16–20 breaths/min and pressure of 20/5cmH2O. Oxygen saturation was kept within 90–100%, glucose level and hydration was maintained with an intravenous infusion of 5% dextrose at 10 mL/kg/hr. During the experiment anesthesia was maintained with intravenous propofol 5–10 mg/kg/hr and morphine 0.1 mg/kg/hr. Additional doses of propofol (1–2 mg/kg) and morphine (0.05–0.1 mg/kg) were also given as needed. The piglet’s normothermic body temperature was maintained at 38.5–39.5 °C using an overhead warmer and a heating pad.
Hemodynamic parameters
A 5-French Argyle® (Klein-Baker Medical Inc. San Antonio, TX) double-lumen catheter was inserted via the right femoral vein for administration of fluids and medications. A 5-French Argyle® single-lumen catheter was inserted above the right renal artery via the femoral artery for continuous arterial blood pressure monitoring in addition to arterial blood gas measurements. The right common carotid artery was also exposed and encircled with a real-time ultrasonic flow probe (2 mm; Transonic Systems Inc., Ithica, NY) to measure cerebral blood flow.
Piglets were placed in supine position and allowed to recover from surgical instrumentation until baseline hemodynamic measures were stable (minimum one hour). Ventilator rate was adjusted to keep the partial arterial CO2 between 35 and 45 mmHg as determined by periodic arterial blood gas analysis. Mean systemic arterial pressure (MAP), systemic systolic and diastolic arterial pressure, heart rate, and percutaneous oxygen saturation were continuously measured and recorded throughout the experiment with a Hewlett Packard 78833B monitor (Hewlett Packard Co., Palo Alto, CA).
Cerebral perfusion
Cerebral regional oxygenation (crSO2) was measured using the InvosTM Cerebral/Somatic Oximeter Monitor (Invos 5100, Somanetics Corp., Troy, MI). The sensors were placed on the right forehead of the piglet and secured with wrap and tape. Light shielding was achieved with a slim cap. The InvosTM Cerebral/Somatic Oximeter Monitor calculates crSO2, which is expressed as the percentage of oxygenated hemoglobin (oxygenated hemoglobin/total hemoglobin). Values of regional oxygen saturation are stored every second with a sample rate of 0.13 Hz.16
Experimental protocol
Following surgical instrumentation and stabilization procedure, a subsequently numbered, sealed brown envelope containing the assigned intervention was opened (Fig. 1). Piglets were randomized to receive an intravenous bolus of 0.1 mL/kg of one of the following four groups: 0.2IU/kg, 0.4IU/kg, 0.8IU/kg vasopressin, or 0.02 mg/kg epinephrine, followed with a 3 mL saline bolus. Piglets were exposed to 30 min of normocapnic hypoxia, followed by asphyxia. Asphyxia was achieved by disconnecting the ventilator and clamping the endotracheal tube until asystole. Asystole was defined as no heart rate audible during auscultation with a MAP ~ 0 mmHg, and the absence of carotid blood flow and discernable ECG activity. Fifteen seconds after diagnosed asystole, PPV was performed for 30 s with a Neopuff T-Piece (Fisher & Paykel, Auckland, New Zealand). The default settings of the experiment were a peak inflating pressure of 30cmH2O, a positive end expiratory pressure of 5cmH2O, and a gas flow of 8 L/min.
After 30 s of PPV, chest compressions (CCs) with sustained inflations were started. 100% oxygen was commenced 30 s after start of CCs. CCs were performed mechanically using an automated machine, specifically designed in our laboratory,17,18,19,20,21 at a rate of 90/min, acceleration of compression of 500 cm/s2, recoil speed of 50 cm/s, and with an anterior-posterior chest diameter of 33%. Vasopressin or epinephrine was administered intravenously 1 min after the start of CCs and administered every 3 min as needed if no ROSC was observed, to a maximum of three doses. ROSC was defined as an unassisted heart rate ≥100 bpm for 15 s. Successful ROSC was assessed by ECG activity and confirmed through auscultation by GMS. After ROSC, piglets recovered for four hours before the piglets were euthanized with an intravenous overdose of sodium pentobarbital (120 mg/kg). During the four hour post-ROSC period, piglets with a combined heart rate <50 bpm and MAP < 15 mmHg were defined as dead.
Sample size and power estimates
Our primary outcome measure was incidence and time to achieve ROSC as well as hemodynamic changes. We hypothesized that the use of 0.8IU/kg vasopressin during CPR would reduce time to achieve ROSC. A sample size of 32 piglets (8 per group) was sufficient to detect a clinically important (20%) reduction in time to achieve ROSC (i.e., 180 s vs. 140 s), with 80% power and a 2-tailed alpha error of 0.05. We used 0.02 mg/kg of epinephrine and 0.2 IU/kg, 0.4 IU/kg, and 0.8 IU/kg of vasopressin as these doses were currently or previously recommended.22,23
Data collection and analysis
Demographics of study piglets were recorded. Transonic flow probes, heart rate and pressure transducer outputs were digitized and recorded with LabChart® programming software (ADInstruments, Houston, TX). Airway pressures, gas flow, tidal volume, and end tidal-CO2 were measured and analyzed using Flow Tool Physiologic Waveform Viewer (Philips Healthcare, Wallingford, CT).
The data are presented as mean (standard deviation–SD) for normally distributed continuous variables and median (interquartile range–IQR) when the distribution was skewed. For all respiratory parameters, continuous values during CPR were analyzed. The data was tested for normality (Shapiro-Wilk and Kolmogorov-Smirnov test) and compared using ANOVA for repeated measures using Tukey post-test. Fisher’s exact test was used for categorical variables. P values are 2-sided and p < 0.05 was considered statistically significant. Statistical analyses were performed with SigmaPlot (Systat Software Inc, San Jose).
Results
Thirty-two newborn mixed breed piglets (0–3 days old, weight 1.8–2.4 kg) were obtained on the day of the experiment and there were no differences in the baseline parameters between the groups (Table 1). Respiratory parameters during chest compressions were not different between the groups (Table 2).
Resuscitation
Resuscitation characteristics are presented in Table 3. Median (IQR) time to ROSC appeared shorter in vasopressin piglets compared to the epinephrine group, but this did not reach significance (p = 0.59). ROSC was achieved in 6 (75%), 6 (75%), 7 (88%), and 5 (63%) piglets in the 0.2 IU/kg, 0.4 IU/kg, 0.8 IU/kg vasopressin, and 0.02 mg/kg epinephrine groups, respectively (p = 0.94). Rates of post-ROSC survival, defined as survival four hours after ROSC, at which point piglets were euthanized, were 83% (5/6), 83% (5/6), 57% (4/6), and 60% (3/5) in vasopressin 0.2, 0.4, 0.8 IU/kg, and epinephrine groups, respectively (p = 0.61).
Changes in Hemodynamic Parameters
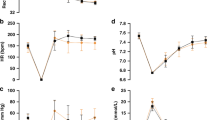
Baseline hemodynamic parameters were not different between groups (Figs. 2, 3). Highest heart rates were observed in vasopressin 0.2IU/kg and 0.4IU/kg groups, while highest MAP was observed with vasopressin 0.4 IU/kg 240 min post-ROSC (p < 0.05). All hemodynamic changes of the epinephrine group, except heart rate, declined gradually throughout the recovery period and were significantly lower than baseline values by the end of the experimental period. Differences between vasopressin and 0.02 mg/kg epinephrine were not significantly different except for 240 min after resuscitation, in which heart rate, MAP, systolic and diastolic pressure, carotid blood flow, and cerebral regional oxygen saturation were all significantly higher with vasopressin 0.4IU/kg (p < 0.05). Changes in arterial blood gas are summarized in Table 4. Except for 0.8IU/kg, all vasopressin-treated piglets recovered back to their baseline values. In contrast, pH, base excess, and lactate were significantly lower than baseline in the epinephrine group 240 min after ROSC (p < 0.05).
Hemodynamic changes in (a) heart rate (bpm, beats per minute), (b) carotid blood flow (mL/min), and (c) brain oxygen saturation (%) in piglets administered vasopressin 0.2IU/kg (closed circle), 0.4IU/kg (closed triangle), 0.8IU/kg (closed square), and 0.02 mg/kg epinephrine (open circle). Data are presented as mean (SD). # Significantly different from baseline; * Significantly different from the epinephrine 0.02 mg/kg group at the concurrent time point (p < 0.05).
Hemodynamic changes in (a) MAP, mean arterial pressure (mmHg), (b) systolic pressure (mmHg), and (c) diastolic pressure (mmHg) in piglets administered vasopressin 0.2IU/kg (closed circle), 0.4IU/kg (closed triangle), 0.8IU/kg (closed square), and 0.02 mg/kg epinephrine (open circle). Data are presented as mean (SD). # Significantly different from baseline; *Significantly different from the epinephrine 0.02 mg/kg group at the concurrent time point (p < 0.05).
Discussion
Current neonatal resuscitation guidelines recommend 0.01–0.03 mg/kg intravenous epinephrine during CPR if the heart rate has not increased to ≥60 beats/minute following PPV with 100% oxygen and CCs.1,24 However, epinephrine increases myocardial oxygen demand, potentially causing brain damage with persisting hypoxia, and can result in necrotizing enterocolitis or renal failure at high doses.25,26 Vasopressin may be an alternative to epinephrine; pediatric and adult studies reported that vasopressin is more effective during asystolic cardiac arrest, one of the main cardiac arrest rhythm in newborn infants.9,27,28 The current study compared three different vasopressin dosages with 0.02 mg/kg epinephrine in a neonatal post-transitional piglet model of asphyxial cardiac arrest. The results can be summarized as follows: (1) time to ROSC and survival was not statistically significant different between groups (Table 3); (2) rates of post-ROSC survival observed in vasopressin 0.2 and 0.4 IU/kg was 83% ((5/6) in both groups), 57% (4/7) in vasopressin 0.8 IU/kg, and 60% (3/5) in the epinephrine group (p = 0.61); and (3) overall hemodynamic and blood gas recovery of 0.4IU/kg vasopressin piglets was significantly better to that of the epinephrine group.
McNamara et al. demonstrated that treating piglets with vasopressin resulted in higher rates of survival compared to epinephrine (p < 0.05).10 However, Rawat et al., reported no difference in time to ROSC or the number of near-term lambs that achieved ROSC following treatment with either vasopressin or epinephrine.11 In the current study, rates of post-ROSC survival was 83% (5/6) in both 0.2 and 0.4IU/kg vasopressin groups and 60% (3/5) in the epinephrine group (Table 3; p = 0.61). Relatively improved post-ROSC survival in vasopressin groups may result from the ability of vasopressin to maintain its vasoconstricting efficacy during severe acidosis (pH < 7.2; effect on ɑ-adrenergic receptors but not vasopressin receptors), commonly present during asystole, whereas epinephrine does not.4,29
A rat model of asphyxial cardiac arrest compared 0.4, 0.8, and 2.4IU/kg intravenous vasopressin and saline control and reported all vasopressin-resuscitated rats had an 80% (8/10) rate of ROSC (p < 0.01), with mean(SD) times to ROSC of 60(17)s, 73(25) s, 84(44)s, and 205 s(no SD reported) in vasopressin 0.4, 0.8, 2.4 IU/kg, and saline groups, respectively (p > 0.05).30 Higher rates of ROSC reported by McNamara et al., and Chen et al., with vasopressin compared to epinephrine may be a result of improved systematic vasoconstriction with vasopressin.4,31 In the current study, median(IQR) time to ROSC was 122(93–289)s in the 0.8IU/kg vasopressin group, 157(100–413)s in the 0.4IU/kg vasopressin group, and 276(117–480)s in the epinephrine group (p = 0.59). As observed in the current study, Chen et al., reported heart rate was most improved in vasopressin 0.4IU/kg resuscitated rats following ROSC compared to all other groups (p < 0.05).30
Diastolic, systolic, and MAP with 0.4IU/kg vasopressin was significantly higher 240 min post-ROSC than the epinephrine group, which had significantly decreased values compared to baseline (Fig. 3; p < 0.05). Increased diastolic pressure 240 min after ROSC suggests improved coronary artery perfusion. Vasopressin and epinephrine half-lives are 10–35 and ~5 min, respectively.32,33 Therefore, hemodynamic differences 240 min post-ROSC are not a result of circulating vasopressor concentrations, and instead reflect patterns of recovery that persist following ROSC.
All hemodynamic changes, except heart rate, of the epinephrine group declined gradually throughout the recovery period and were significantly lower than baseline values by the end of the experimental period. Differences between vasopressin and 0.02 mg/kg epinephrine were not significantly different except 240 min after resuscitation, in which heart rate, MAP, systolic and diastolic pressure, carotid blood flow, and cerebral regional oxygen saturation were all significantly higher with vasopressin 0.4IU/kg (p < 0.05).
The vasopressin 0.4IU/kg group had significantly greater blood flow at 180- and 240-minutes post-ROSC compared to the epinephrine group (Fig. 2b); (p < 0.05), which had significantly lower blood flow than its baseline values at 120-, 180-, and 240-minutes following ROSC. Brain oxygen saturation values of vasopressin groups returned to baseline following ROSC at all time points, while the epinephrine group had significantly decreased saturation values at 180- and 240-minutes post-ROSC compared to baseline (Fig. 2c); (p < 0.05). Brain oxygen saturation was significantly higher in the 0.4IU/kg vasopressin group at 180- and 240-minutes post-ROSC compared to the epinephrine group (Fig. 2c); (p < 0.05). Decreased carotid blood flow and brain oxygen saturation in the epinephrine group following ROSC are likely a result of its ɑ1-agonist action, which decreases cerebral cortical microcirculatory blood flow and increases cerebral ischemia and hypercarbia following ROSC.34 Comparatively, vasopressin increases carotid blood flow, cerebral perfusion, and brain oxygen saturation.35,36 We speculate increased MAP following ROSC in vasopressin groups does not result in hyperperfusion injuries, as values returned to baseline.
Rawat et al., did not report a significant difference in carotid blood flow between epinephrine and vasopressin groups; however, stroke carotid flow volume, defined as carotid flow volume per heartbeat, was significantly higher in vasopressin lambs (0.175(0.08)mL/kg) compared to the epinephrine group (0.109(0.02)mL/kg; p < 0.001).11 The same study euthanized lambs approximately 20 min after ROSC, therefore potentially significant differences between vasopressin and epinephrine groups that may exist beyond the immediate post-ROSC period were not reported.11 Rawat et al., reported increased pulmonary blood flow in vasopressin-resuscitated lambs compared to the epinephrine group (p = 0.0001), while McNamara et al., reported a decreased inverse ratio of pulmonary artery acceleration time to right ventricular ejection in the vasopressin groups, indicating lower pulmonary vascular resistance in the vasopressin groups.10,11 Rapid restoration of cerebral blood flow and brain oxygen saturation to baseline values with 0.4IU/kg vasopressin in the current study may translate to favorable neurological outcomes following ROSC. Clinical trials comparing vasopressin with epinephrine during CPR including neurological outcomes are warranted. Few adverse effects of vasopressin have been documented. Vasopressin decreased cardiac output in adult dogs with pulmonary hypertension with an infusion of high dose vasopressin (1.16IU/kg/hour; p < 0.05).37 Additionally, cases of intestinal ischemia have been reported in critically ill patients following high single doses (~4–16IU) of vasopressin.38 Increased digital ischemia and severe diarrhea has been reported in septic shock patients treated with high-dose terlipressin (4 mg/day), a synthetic analog of vasopressin.39
Limitations
The use of an automated CC machine reduces variations that may arise from human-administered CCs. However, several limitations should be considered before implementing vasopressin in neonatal resuscitation. Our asphyxia model uses piglets that have already undergone the fetal-to-neonatal transition, and piglets were sedated/anesthetized. Furthermore, our model requires piglets to be intubated with a tightly sealed endotracheal tube to prevent any endotracheal tube leak; this may not occur in the delivery room as mask ventilation is frequently used. Nevertheless, our findings are still clinically relevant as the distribution of cardiac output to vital organs (i.e. brain and heart) in the fetus and post-transitional neonate during asphyxia episodes are quantitatively similar.40,41 Our resuscitation model is slightly different from the currently recommended resuscitation guidelines, as we administered epinephrine 90 s after PPV initiation. This may have influenced our results. Additionally, piglets were euthanized four hours after ROSC; therefore, no comparisons could be made on long-term outcomes between groups, which is a limitation of this study.
Conclusion
Time to and incidence of ROSC were not different between all vasopressin dosages and epinephrine. However, better hemodynamic and blood gas recoveries following asphyxia-induced asystolic cardiac arrest in the 0.4IU/kg vasopressin group suggests this may be the optimal dosage during neonatal resuscitation. Improved hemodynamic parameters following ROSC with 0.4IU/kg vasopressin compared to epinephrine warrants further investigation.
Data sharing
All data generated or analyzed during this study are included in this published article. Data used to generate the results reported in this study will be made available following publication to researchers who provide a methodologically sound proposal.
References
Wyckoff, M. H. et al. Neonatal Life Support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 142, S185–S221 (2020).
Motiejunaite, J., Amar, L. & Vidal-Petiot, E. Adrenergic receptors and cardiovascular effects of catecholamines. Annales d’Endocrinologie 82, 193–197 (2021).
Turner, D. W., Attridge, R. L. & Hughes, D. W. Vasopressin associated with an increase in return of spontaneous circulation in acidotic cardiopulmonary arrest patients. Ann. Pharmacother. 48, 986–991 (2014).
Fox, A. W., May, R. E. & Mitch, W. E. Comparison of peptide and nonpeptide receptor-mediated responses in rat tail artery. J. Cardiovasc Pharm. 20, 282–289 (1992).
Pinto, M. et al. Evidence on adrenaline use in resuscitation and its relevance to newborn infants: A non-systematic review. Neonatology 111, 37–44 (2017).
Perlman, J. M. & Risser, R. Cardiopulmonary resuscitation in the delivery room. Associated clinical events. Arch. Pediatr. Adolesc. Med 149, 20–25 (1995).
Pelletier, J.-S., Dicken, B., Bigam, D. & Cheung, P.-Y. Cardiac effects of vasopressin. J. Cardiovascular Pharmacol. 64, 100 (2014).
Prengel, A. W., Lindner, K. H., Keller, A. & Lurie, K. G. Cardiovascular function during the postresuscitation phase after cardiac arrest in pigs: a comparison of epinephrine versus vasopressin. Crit. Care Med. 24, 2014–2019 (1996).
Wenzel, V. et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N. Engl. J. Med. 350, 105–113 (2004).
McNamara, P. J., Engelberts, D., Finelli, M., Adeli, K. & Kavanagh, B. P. Vasopressin improves survival compared with epinephrine in a neonatal piglet model of asphyxial cardiac arrest. Pediatr. Res. 75, 738–748 (2014).
Rawat, M. et al. Masked randomized trial of epinephrine versus vasopressin in an ovine model of perinatal cardiac arrest. Child. (Basel) 10, 349 (2023).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Schmölzer, G. M. et al. Cardiopulmonary resuscitation with chest compressions during sustained inflations: a new technique of neonatal resuscitation that improves recovery and survival in a neonatal porcine model. Circulation 128, 2495–2503 (2013).
Schmölzer, G. M. et al. 3:1 compression to ventilation ratio versus continuous chest compression with asynchronous ventilation in a porcine model of neonatal resuscitation. Resuscitation 85, 270–275 (2014).
Cheung, P.-Y., Gill, R. S. & Bigam, D. L. A swine model of neonatal asphyxia. J. Vis. Exp. 3166 (2011) https://doi.org/10.3791/3166.
Pichler, G. et al. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J. Pediatr. 163, 1558–1563 (2013).
Bruckner, M. et al. Assessment of optimal chest compression depth during neonatal cardiopulmonary resuscitation: a randomised controlled animal trial. Arch. Dis. Child Fetal Neonatal Ed. 107, 262–268 (2022).
Bruckner, M. et al. Effects of varying chest compression depths on carotid blood flow and blood pressure in asphyxiated piglets. Arch. Dis. Child Fetal Neonatal Ed. 106, 553–556 (2021).
Bruckner, M. et al. Haemodynamic changes with varying chest compression rates in asphyxiated piglets. Arch. Dis. Child Fetal Neonatal Ed. 108, 200–203 (2023).
Bruckner, M. et al. Chest compression rates of 90/min versus 180/min during neonatal cardiopulmonary resuscitation: A randomized controlled animal trial. Child. (Basel) 9, 1838 (2022).
Bruckner, M. et al.,. L,Varying Chest Compression Rates During Neonatal Cardiopulmonary Resuscitation: A Randomized Controlled Animal Trial. https://www.researchsquare.com/article/rs-1153413/v1 (2021) https://doi.org/10.21203/rs.3.rs-1153413/v1.
M Weiner, G. Textbook of neonatal resuscitation. in (American Academy, 2016).
Link, M. S. et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 132, S444–S464 (2015).
Aziz, K. et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 142, S524–S550 (2020).
Antonucci, R., Antonucci, L., Locci, C., Porcella, A. & Cuzzolin, L. Current challenges in neonatal resuscitation: What is the role of adrenaline? Pediatr. Drugs 20, 417–428 (2018).
Cheung, P. Y. et al. Systemic, pulmonary and mesenteric perfusion and oxygenation effects of dopamine and epinephrine. Am. J. Respir. Crit. Care Med 155, 32–37 (1997).
Mann, K., Berg, R. & Nadkarni, V. Beneficial effects of vasopressin in prolonged pediatric cardiac arrest: a case series. Resuscitation 52, 149–56 (2002).
Matok, I. et al. Beneficial effects of terlipressin in prolonged pediatric cardiopulmonary resuscitation: a case series. Crit. Care Med. 35, 1161–1164 (2007).
Schaer, H. Influence of respiratory and metabolic acidosis on epinephrine-inotropic effect in isolated guinea pig atria. Pflug. Arch. 347, 297–307 (1974).
Chen, M.-H. et al. Dose-response of vasopressin in a rat model of asphyxial cardiac arrest. Am. J. Emerg. Med. 27, 935–941 (2009).
Ornato, J. P. Optimal vasopressor drug therapy during resuscitation. Crit. Care 12, 123 (2008).
Mitra, J. K., Roy, J. & Sengupta, S. Vasopressin: Its current role in anesthetic practice. Indian J. Crit. Care Med. 15, 71–77 (2011).
Dalal, R. & Grujic, D. Epinephrine. in StatPearls (StatPearls Publishing, 2023).
Ristagno, G. et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation*. Crit. Care Med. 37, 1408 (2009).
Ristagno, G., Sun, S., Tang, W., Castillo, C. & Weil, M. H. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation*. Crit. Care Med. 35, 2145–2149 (2007).
Wenzel, V., Lindner, K. H., Augenstein, S., Prengel, A. W. & Strohmenger, H. U. Vasopressin combined with epinephrine decreases cerebral perfusion compared with vasopressin alone during cardiopulmonary resuscitation in pigs. Stroke 29, 1462–1468 (1998).
Leather, H. A., Segers, P., Berends, N., Vandermeersch, E. & Wouters, P. F. Effects of vasopressin on right ventricular function in an experimental model of acute pulmonary hypertension*. Crit. Care Med. 30, 2548 (2002).
Shelly, M. P., Greatorex, R., Calne, R. Y. & Park, G. R. The physiological effects of vasopressin when used to control intra-abdominal bleeding. Intensive Care Med. 14, 526–531 (1988).
Liu, Z.-M. et al. Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med. 44, 1816–1825 (2018).
Cohn, H. E., Sacks, E. J., Heymann, M. A. & Rudolph, A. M. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am. J. Obstet. Gynecol. 120, 817–824 (1974).
Rudolph, A. M. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ. Res. 57, 811–821 (1985).
Funding
We would like to thank the public for donating money to our funding agencies: MR is a recipient of the University of Alberta Graduate Recruitment Scholarship, Canadian Institutes of Health Research Canada Graduate Scholarships-Master’s program, and Walter H Johns Graduate Fellowship. The study was supported by a Project Grant from the Canadian Institute of Health Research, Institute of Human Development, Child, and Youth Health.
Author information
Authors and Affiliations
Contributions
Conception and design: G.M.S., P.Y.C., M.O.R., T.F.L. Collection and assembly of data: G.M.S., P.Y.C., M.O.R., T.F.L., M.R. Analysis and interpretation of the data: G.M.S., P.Y.C., M.R., M.O.R., T.F.L., M.R. Drafting of the 1st draft: M.R. Critical revision of the article for important intellectual content: G.M.S., P.Y.C., M.R., M.O.R., T.F.L., M.R. Final approval of the article: G.M.S., P.Y.C., M.R., M.O.R., T.F.L., M.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramsie, M., Cheung, PY., Lee, TF. et al. Comparison of various vasopressin doses to epinephrine during cardiopulmonary resuscitation in asphyxiated neonatal piglets. Pediatr Res 95, 1265–1272 (2024). https://doi.org/10.1038/s41390-023-02858-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02858-x