Abstract
Children with brain tumors (CBT) are at higher risk of cardiovascular disease and type 2 diabetes compared to the general population, in which birth weight is a risk factor for these diseases. However, this is not known in CBT. The primary aim of this study was to explore the association between birth weight and body mass measures in CBT, compared to non-cancer controls. This is a secondary data analysis using cross-sectional data from the CanDECIDE study (n = 78 CBT and n = 133 non-cancer controls). Age, sex, and birth weight (grams) were self-reported, and confirmed through examination of the medical records. Body mass index (BMI) was calculated from height and weight measures and reported as kg/m2. BMI z-scores were obtained for subjects under the age of 20 years. Multivariable linear regression was used to evaluate the relationship between birth weight and BMI and BMI z-score, adjusted for age, sex, puberty, and fat mass percentage. Higher birth weight was associated with higher BMI and BMI z-score among CBT and controls. In conclusion, birth weight is a risk factor for higher body mass during childhood in CBT, and this may help the identification of children at risk of future obesity and cardiometabolic risk.
Similar content being viewed by others
Introduction
Brain tumors are the most common cause of cancer-related deaths in children1,2. While brain tumors are a heterogeneous group with some fairly aggressive subtypes, advancements in imaging and therapeutic breakthroughs have increased the number of children surviving these tumors1,2. This important milestone has been offset by the emergence of co-morbidities and premature mortality in survivors3,4,5,6,7. While traditionally reported outcome determinants include tumor recurrence and secondary tumors, recent evidence suggests that children with brain tumors (CBT) are at higher risk of premature cardiovascular diseases including hypertension, cerebrovascular events and type 2 diabetes compared to non-cancer controls8,9,10. While the mechanisms leading to these cardiometabolic disorders are not well understood, the combined burden of the tumor and treatment with these emerging chronic disorders will increasingly contribute to adverse outcomes in CBT as these survivors live longer and get older.
The global obesity epidemic is the main catalyst of cardiometabolic disorders in the general population11,12,13,14, and the association of obesity with future type 2 diabetes and cardiovascular diseases has been tracked to childhood15. Defining the determinants of obesity, cardiovascular disease and diabetes in CBT will permit the prioritization of children who need early intervention to improve survivors’ quality of life and lifespan.
Over the past two decades, evidence has validated the role of birth weight as a risk factor for adult obesity and cardiometabolic disorders16,17; however, it is unclear if birth weight in CBT is a risk factor for obesity during childhood. Given that CBT have excess adiposity in comparison to non-cancer controls18, it may be a potential early predictor of further adverse cardiometabolic outcomes. In addition, while CBT have similar rates of overweight/obesity when compared to non-cancer controls, this still leaves one-in-four CBT in the overweight/obese category based on body mass measures19. Body mass index (BMI) is the most used screening tool to assess obesity risk, and may still be a predictor of future cardiometabolic risk, as these children get older. Therefore, the primary aim of this paper was to explore if birth weight is a risk factor for higher body mass in CBT, compared to non-cancer controls.
Results
Population characteristics
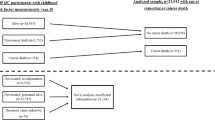
We included 78 CBT (n = 33 females [42.3%]) and 133 non-cancer controls (n = 60 females, [45.1%]) in this study. The characteristics of the study population are shown in Table 1. The two groups have similar age, sex, ethnicity, BMI, BMI z-score, and birth weight distribution. However, the control group was taller and weighed more than CBT. On the other hand, CBT had higher fat mass percentage (%FM) (CBT 25.80 ± 9.60% versus controls 22.40 ± 9.80%, p = 0.01).
Participants born at full-term comprised the largest groups (CBT n = 45 [57.70%]; controls n = 65 [48.90%]). More controls were born pre-term (CBT n = 3 [3.80%]; controls n = 21 [15.80%], p = 0.008) while both have similar distribution for early-term (CBT n = 10 [12.80%]; controls n = 24 [18.00%], p = 0.32) and late-term (CBT n = 20 [25.60%]; controls n = 23 [17.30%], p = 0.15).
The majority of both CBT and controls were born appropriate for gestational age (AGA) (CBT n = 60 [76.90%]; controls n = 90 [67.70%]). Both groups had a similar distribution for those who were born small for gestational age (SGA) (CBT n = 11 [14.10%]; controls n = 12 [9.00%], p = 0.25) while large for gestational age (LGA) was more common in controls (CBT n = 7 [9.00%]; controls n = 31 [23.30%], p = 0.009). Maternal gestational diabetes was reported in two CBT (2.60%) and in one control (0.80%), while preeclampsia was reported in five CBT (6.40%) and 10 controls (7.50%).
The majority of the subjects had normal birth weight (CBT n = 62 [79.50%]; controls n = 104 [78.20%]). Six CBT (7.70%) and six controls (4.50%) were born with a low birth weight (<2500 grams). High birth weight (>4000 grams) was reported in ten CBT (12.80%) and 23 controls (17.30%).
Tumor characteristics and treatments
Brain tumor characteristics and therapeutic modalities are reported in Table 2. The most common tumors in this study population were low-grade gliomas (n = 45 [57.70%]). Brain tumors were equally distributed between supratentorial and infratentorial regions. The treatments used in participants were surgery alone (n = 25 [32.00%]), and a combination of surgery, radiotherapy, and chemotherapy (n = 24 [30.80%]). Other single treatment options included radiotherapy alone (n = 2 [2.60%]), and chemotherapy alone (n = 7 [9.00%]). Other combinations of treatment modalities were surgery and radiotherapy (n = 6 [7.70%]), surgery and chemotherapy (n = 5 [6.40%]), and radiotherapy and chemotherapy (n = 1 [1.30%]). Eight CBT (10.20%) were managed conservatively at the time of inclusion in the study.
The association of birth weight and body mass
To explore the association of birth weight with body mass, we performed multivariable regression analyses in CBT, adjusted for age, sex, puberty, and %FM. For every unit increase in birth weight, BMI increased by 0.18 units (95%CI 0.03,0.33; p = 0.02) in CBT and by 0.17 units (95%CI 0.07,0.27; p = 0.001) in controls. Similarly, for every unit increase in birth weight, BMI z-score also increased by 3.69 units (95%CI 1.12,6.25; p = 0.006) in CBT and by 2.15 units (95%CI 0.75,3.55; p = 0.003) in controls.
To determine if the association between birth weight and body mass differs between CBT and controls, an interaction term (birth weight*brain tumor status) was introduced (Table 3). Birth weight is associated with body mass, and the effect of birth weight on body mass was similar between CBT and controls (BMI β = 0.02; 95% CI −0.15, 0.20; p = 0.80; BMI z-score β = 2.02; 95% CI −0.62,4.67; p = 0.13).
Discussion
The emergence of cardiovascular diseases and type 2 diabetes in survivors of childhood brain tumors are likely to contribute to adverse prognoses, and there is an urgent need to identify the drivers of these outcomes to mitigate their effects on the life span and quality of life. In this study, we demonstrate that birth weight is a risk factor for higher body mass in CBT during childhood, and this relationship was similar to that noted in non-cancer controls.
The influence of the in- and ex-utero environments on the risk of obesity and cardiometabolic risk is an important determinant of health outcomes, based on evidence from studies in the general population20,21,22. One of the potential early and feasible measures that forecast these outcomes is birth weight.
Birth weight is driven by several factors, including genes that determine body size and growth, and the intrauterine environment23. It is estimated that 10–40% of birth weight is driven by genetic factors, with several loci identified to suggest genetic links with body weight and mass23,24. In addition, fetal metabolic programing in utero in response to the intrauterine environment contributes to cardiometabolic health postnatally through epigenetic and other mechanisms25.
The exposure of an embryo to an adverse intrauterine environment and excess metabolic stress leads to the re-programming of the metabolic pathways, to adapt to in-utero scarcity or excess of nutrients22,26. Clinically, this manifests with infants being born small or large for gestational age27. However, it is likely that at intermediate stages of metabolic stress, some babies may have a birth weight within the normal range, but have been exposed to an environment that can alter their metabolic trajectory28.
The evidence for the association of certain categories of birth weight with adult BMI and cardiometabolic disorders was highlighted in studies from David Barker and the Dutch famine cohort and others, and showed that birth weight and maternal-fetal undernutrition was linked to low birth weight that was associated with adult obesity and adverse cardiometabolic outcomes29,30,31,32,33,34.
In addition, the link between birth weight and obesity was highlighted in previous reports showing that those born SGA or LGA to be at risk of adult obesity22. However, recent studies report that in those with a birth weight that is sometimes within the normal range, or who have high birth weight (>4000 grams), are at risk of adult obesity28,35,36. Contrary to previous evidence37,38,39, some studies did not show linear, J-shaped or U-shaped associations of birth weight with adult obesity35,36.
Our data show a positive relationship between birth weight and body mass measures in CBT and controls in childhood. This is congruent with recent large-scale studies that have provided further evidence of similar results in the general pediatric population27,40,41. Birth weight may help identify those CBT who are at risk of adult obesity. Detailed study of growth paths and longer follow-up period are needed to determine if birth weight is a risk factor for obesity in CBT as they reach adulthood.
While available evidence suggest that obese children are at risk of becoming obese adults42,43,44, the association between birth weight, childhood BMI and future cardiometabolic risk is more complex45. It has been reported that birth weight below 3.4 kg, which is still considered appropriate for gestational age, and high BMI during childhood were independently associated with increased risk of coronary heart disease20. The association between low birth weight and type 2 diabetes was also reported46,47. The evidence indicates that both birth weight and BMI need to be scrutinized in CBT and controls to identify subjects who are at an increased risk of cardiometabolic disorders, as they appear to be independently linked to these outcomes.
While the majority of evidence has focused on high (>4000 g) and low (<2500 g) birth weight and its association with obesity risk28,35, it is less clear how a normal birth weight affect this trajectory of adult obesity and cardiometabolic outcomes in CBT. Our data suggest that as weight trends higher while still within the normal range, this represents a risk factor for higher body mass. However, more prospective data sets are required to validate this observation and its association with cardiometabolic outcomes in CBT.
One of the strengths of our study is the inclusion of non-cancer controls to provide a comparison group. It is a strength to have this control group because when all is equal for birth weight between cases and controls, we can test the effect of having cancer and therapy on the association between birth weight and BMI.
It has been shown that CBT have increased adiposity early in life post completion of therapy, and are at higher risk of cardiovascular diseases and diabetes compared to the general population, despite having similar BMI to controls9,18,48. CBT can have disproportionate effects of their tumor and its treatment on cardiometabolic outcomes at a similar obesity rate, and birth weight may be a potential risk factor for these outcomes18,49,50.
There are several limitations in this study. We did not have sufficient power to determine the association of birth weight with young adult BMI, as the number of young adult subjects in our study was small. While we demonstrate that birth weight is positively associated with body mass measures in adolescence, our data does not distinguish whether this is a result of the expansion of lean body mass or fat mass, as BMI is a measure of total body mass. A recent study showed that birth weight was associated with fat-free mass, but not with fat mass among children and adolescents51. This will require further clarification in future studies.
This analysis is cross-sectional and therefore it is not clear if subjects were overweight or obese before their brain tumor diagnoses. In addition, the data used in the study were collected as a part of the Canadian Study of Determinants of Endometabolic Health in Children (CanDECIDE study)52, and this paper is not related to the primary study question. Therefore, the study was not designed to answer this question specifically. Prospective collection of data from diagnosis onwards and correlating growth data with those from earlier time points may help define growth patterns of those survivors at risk of obesity.
In conclusion, cardiometabolic disorders are occurring at a relatively young age in CBT, and are emerging as significant morbidities and as potential determinants of longevity5,9,48. Our results suggest that birth weight is a risk factor for higher body mass in CBT in the early years post treatment. Future studies need to focus on determining the early origins of obesity and cardiometabolic risk in survivors. This will help identify survivors who are at particular risk of these complications, and birth weight may be one of the risk markers used to stratify cardiometabolic risk in CBT.
Methods
Participants
This secondary data analysis used data that were collected as a part of the CanDECIDE Study. This is a cohort study conducted at McMaster Children’s Hospital in Hamilton, Ontario, Canada52,53.
Briefly, we recruited participants who were 5 years and older, with no history of autoimmune diseases or infection, and have not received immunosuppressive therapy for at least 15 days prior to enrollment. Participants were consecutively recruited and the study recruitment took place between November 2012-March 2017. The Hamilton Integrated Research Ethics Board approved the study, and participants provided written informed consent. The study procedures were carried out in accordance with relevant guidelines and legal regulations.
Clinical Data and Anthropometric measures
The collected data included age, sex, ethnicity, puberty, pregnancy gestation, maternal gestational diabetes and preeclampsia, and reported birth weight using standardized questionnaires52,53. While reported birth weight correlates with measured birth weight54, the reported birth weight was verified from the medical records. In CBT, we also collected data regarding tumor type, location, sidedness and treatment modalities.
Gestation was defined for those born at less than 37 weeks as preterm, between 37–38 + 6/40 weeks as early term, 39–40 + 6/40 weeks as full term, and 41–41 + 6/40 weeks as late term gestations55. Normal birth weight was defined to be between 2500–4000 grams56. Infants born SGA were defined as those with a birth weight below the10th percentile, AGA as 10th-90th percentile, and LGA as above 90th percentile using Canadian reference ranges57.
Anthropometric measurements performed included height measured to 0.1 cm using a stadiometer, and weight measured using an electronic weighing scale (Seca, USA) and measured to the closest 0.1 kg. BMI was calculated in kg/m2 for all subjects. For those under 20 years of age (CBT n = 62, controls n = 133), BMI percentile and BMI z-scores were also obtained based on the Children’s BMI Tool for Schools58 and the Centers for Disease Control and Prevention (CDC) growth chart59, respectively. Subjects with BMI ≥ 85th –<95th percentile were classified as overweight, and those above 95th percentile were classified as obese60.
Adiposity was determined by measuring fat mass percentage (%FM) using the Tanita body fat monitor (Tanita Corporation, Illinois, USA) for those under 18 years of age, and with the InBody520 body composition analyzer (Biospace Co., Ltd, Korea) for those 18 years and older as previously reported18.
Statistical Analysis
All analyses were performed using PASW version 18 statistical package61. Kolmogorov-Smirnov test was used to assess the normality of data distribution, and data were log-transformed if they were non-normally distributed. Outliers were examined with box plot and visual inspection of extreme values. Multiple imputations was done for missing data62. Mean and standard deviation (SD) were reported for continuous variables, while the categorical variables were reported as counts with percentages. Independent sample t-tests were used to compare continuous variables between CBT and non-cancer controls while chi-square tests were performed to compare categorical variables.
To explore the association between birth weight and body mass measures in CBT, multivariable linear regression analysis was performed in this group. The dependent variables included BMI and BMI z-scores in separate models. The independent variables included birth weight, age, sex, puberty, and %FM.
The relationship between birth weight and body mass in CBT and non-cancer controls was explored by adding an interaction term (birth weight*brain tumor status). Both CBT and non-cancer controls were included in the regression analysis.
Results were presented as estimated β coefficients, 95% confidence intervals (CI), and associated p-value. The criterion for statistical significance was set at alpha = 0.05.
Data availability
The dataset used for statistical analysis for the current study is available from the corresponding author.
References
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66, 271–289, https://doi.org/10.3322/caac.21349 (2016).
Woehrer, A. et al. Relative survival of patients with non-malignant central nervous system tumours: a descriptive study by the Austrian Brain Tumour Registry. Br J Cancer 110, 286–296, https://doi.org/10.1038/bjc.2013.714 (2014).
Mertens, A. C. et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol 19, 3163–3172 (2001).
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355, 1572–1582, https://doi.org/10.1056/NEJMsa060185 (2006).
Pietilä, S. et al. Obesity and metabolic changes are common in young childhood brain tumor survivors. Pediatr Blood Cancer 52, 853–859, https://doi.org/10.1002/pbc.21936 (2009).
Prasad, P. K., Signorello, L. B., Friedman, D. L., Boice, J. D. Jr & Pukkala, E. Long-term non-cancer mortality in pediatric and young adult cancer survivors in Finland. Pediatr Blood Cancer 58, 421–427, https://doi.org/10.1002/pbc.23296 (2012).
Samaan, M. C. & Akhtar-Danesh, N. The impact of age and race on longevity in pediatric astrocytic tumors: A population-based study. Pediatr Blood Cancer 62, 1567–1571, https://doi.org/10.1002/pbc.25522 (2015).
Gurney, J. G. et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 97, 663–673, https://doi.org/10.1002/cncr.11095 (2003).
Heikens, J. et al. Long term survivors of childhood brain cancer have an increased risk for cardiovascular disease. Cancer 88, 2116–2121 (2000).
Meacham, L. R. et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med 169, 1381–1388, https://doi.org/10.1001/archinternmed.2009.209 (2009).
Mathers, C. D. & Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine 3, e442, https://doi.org/10.1371/journal.pmed.0030442 (2006).
Mathers, C. D., Lopez, A. D. & Murray, C. J. L. in Global Burden of Disease and Risk Factors (eds A. D. Lopez et al.) (World Bank The International Bank for Reconstruction and Development/The World Bank Group., 2006).
Murray, C. J. & Lopez, A. D. Measuring the global burden of disease. N Engl J Med 369, 448–457, https://doi.org/10.1056/NEJMra1201534 (2013).
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384, 766–781, https://doi.org/10.1016/S0140-6736(14)60460-8 (2014).
Reilly, J. J. & Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 35, 891–898, https://doi.org/10.1038/ijo.2010.222 (2011).
Jornayvaz, F. R. et al. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: the CoLaus study. Cardiovascular Diabetology 15, 73, https://doi.org/10.1186/s12933-016-0389-2 (2016).
Rooney, B. L., Mathiason, M. A. & Schauberger, C. W. Predictors of Obesity in Childhood, Adolescence, and Adulthood in a Birth Cohort. Maternal and Child Health Journal 15, 1166–1175, https://doi.org/10.1007/s10995-010-0689-1 (2011).
Wang, K. W. et al. Adiposity in childhood brain tumors: A report from the Canadian Study of Determinants of Endometabolic Health in Children (CanDECIDE Study). Scientific reports 7, 45078, https://doi.org/10.1038/srep45078 (2017).
Wang, K. W. et al. Overweight, obesity and adiposity in survivors of childhood brain tumours: a systematic review and meta-analysis. Clinical obesity, https://doi.org/10.1111/cob.12224 (2017).
Andersen, L. G. et al. Birth weight, childhood body mass index and risk of coronary heart disease in adults: combined historical cohort studies. PLoS One 5, e14126, https://doi.org/10.1371/journal.pone.0014126 (2010).
Boney, C. M., Verma, A., Tucker, R. & Vohr, B. R. Metabolic Syndrome in Childhood: Association With Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 115, e290–e296, https://doi.org/10.1542/peds.2004-1808 (2005).
Ornoy, A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reproductive toxicology (Elmsford, N.Y.) 32, 205–212, https://doi.org/10.1016/j.reprotox.2011.05.002 (2011).
Li, A. et al. Parental and child genetic contributions to obesity traits in early life based on 83 loci validated in adults: the FAMILY study. Pediatr Obes, https://doi.org/10.1111/ijpo.12205 (2016).
Lunde, A., Melve, K. K., Gjessing, H. K., Skjaerven, R. & Irgens, L. M. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. American journal of epidemiology 165, 734–741, https://doi.org/10.1093/aje/kwk107 (2007).
Wadhwa, P. D., Buss, C., Entringer, S. & Swanson, J. M. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Seminars in reproductive medicine 27, 358–368, https://doi.org/10.1055/s-0029-1237424 (2009).
Nistala, R. et al. Prenatal Programming and Epigenetics in the Genesis of the Cardiorenal Syndrome. Cardiorenal Medicine 1, 243–254, https://doi.org/10.1159/000332756 (2011).
Kuhle, S., Maguire, B., Ata, N., MacInnis, N. & Dodds, L. Birth Weight for Gestational Age, Anthropometric Measures, and Cardiovascular Disease Markers in Children. J Pediatr 182, 99–106, https://doi.org/10.1016/j.jpeds.2016.11.067 (2016).
Schellong, K., Schulz, S. & Harder, T. & Plagemann, A. Birth Weight and Long-Term Overweight Risk: Systematic Review and a Meta-Analysis Including 643,902 Persons from 66 Studies and 26 Countries Globally. PLoS ONE 7, e47776, https://doi.org/10.1371/journal.pone.0047776 (2012).
Barker, D. J. The fetal origins of type 2 diabetes mellitus. Annals of internal medicine 130, 322–324 (1999).
Barker, D. J. Fetal origins of cardiovascular disease. Ann Med 31(Suppl 1), 3–6 (1999).
Lithell, H. O. et al. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. Bmj 312, 406–410 (1996).
Painter, R. C. et al. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr 84, 322–327 (2006).
Ravelli, A. C. et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177 (1998).
Roseboom, T. J. et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart (British Cardiac Society) 84, 595–598 (2000).
Yu, Z. B. et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev 12, 525–542, https://doi.org/10.1111/j.1467-789X.2011.00867.x (2011).
Zhao, Y., Wang, S. F., Mu, M. & Sheng, J. Birth weight and overweight/obesity in adults: a meta-analysis. Eur J Pediatr 171, 1737–1746, https://doi.org/10.1007/s00431-012-1701-0 (2012).
Curhan, G. C. et al. Birth weight and adult hypertension and obesity in women. Circulation 94, 1310–1315 (1996).
Fall, C. H. et al. Fetal and infant growth and cardiovascular risk factors in women. BMJ 310, 428–432 (1995).
Parsons, T. J., Power, C. & Manor, O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ 323, 1331–1335 (2001).
Mitchell, E. A. et al. Birth weight and subsequent body mass index in children: an international cross-sectional study. Pediatr Obes. https://doi.org/10.1111/ijpo.12138 (2016).
Qiao, Y. et al. Birth weight and childhood obesity: a 12-country study. International Journal of Obesity Supplements 5, S74–S79, https://doi.org/10.1038/ijosup.2015.23 (2015).
Bray, G. A. Predicting obesity in adults from childhood and adolescent weight. Am J Clin Nutr 76, 497–498 (2002).
Guo, S. S., Wu, W., Chumlea, W. C. & Roche, A. F. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr 76, 653–658 (2002).
Simmonds, M., Llewellyn, A., Owen, C. G. & Woolacott, N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev 17, 95–107, https://doi.org/10.1111/obr.12334 (2016).
Lloyd, L. J., Langley-Evans, S. C. & McMullen, S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes (Lond) 36, 1–11, https://doi.org/10.1038/ijo.2011.186 (2012).
Eriksson, J. G., Kajantie, E., Lampl, M. & Osmond, C. Trajectories of body mass index amongst children who develop type 2 diabetes as adults. Journal of internal medicine 278, 219–226, https://doi.org/10.1111/joim.12354 (2015).
Forsen, T. et al. The fetal and childhood growth of persons who develop type 2 diabetes. Annals of internal medicine 133, 176–182 (2000).
Holmqvist, A. S. et al. Adult life after childhood cancer in Scandinavia: diabetes mellitus following treatment for cancer in childhood. Eur J Cancer 50, 1169–1175, https://doi.org/10.1016/j.ejca.2014.01.014 (2014).
Green, D. M. et al. Risk factors for obesity in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 30, 246–255, https://doi.org/10.1200/JCO.2010.34.4267 (2012).
Lustig, R. H. et al. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab 88, 611–616, https://doi.org/10.1210/jc.2002-021180 (2003).
Singhal, A., Wells, J., Cole, T. J., Fewtrell, M. & Lucas, A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr 77, 726–730 (2003).
Samaan, M. C., Thabane, L., Burrow, S., Dillenburg, R. F. & Scheinemann, K. Canadian Study of Determinants of Endometabolic Health in ChIlDrEn (CanDECIDE study): a cohort study protocol examining the mechanisms of obesity in survivors of childhood brain tumours. BMJ Open 3, https://doi.org/10.1136/bmjopen-2013-002869 (2013).
Samaan, M. C. et al. Recruitment feasibility to a cohort study of endocrine and metabolic health among survivors of childhood brain tumours: a report from the Canadian study of Determinants of Endometabolic Health in ChIlDrEn (CanDECIDE). BMJ Open 4, e005295, https://doi.org/10.1136/bmjopen-2014-005295 (2014).
Shenkin, S. D. et al. Validity of recalled v. recorded birth weight: a systematic review and meta-analysis. Journal of Developmental Origins of Health and Disease, 1–12, https://doi.org/10.1017/S2040174416000581 (2016).
Spong, C. Y. D. “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA 309, 2445–2446, https://doi.org/10.1001/jama.2013.6235 (2013).
CDC/Massachusetts WIC Nutritional Program. 2008 Pregnancy Data Report. Massachusetts Department of Public Health http://www.mass.gov/eohhs/docs/dph/wic/pnss-report.pdf (2009).
Kramer, M. S. et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 108, E35 (2001).
Nihiser, A. J. et al. Body mass index measurement in schools. J Sch Health 77, 651–671; quiz 722–654, https://doi.org/10.1111/j.1746-1561.2007.00249.x (2007).
Kuczmarski, R. J. et al. 2000 CDC Growth Charts for the UnitedStates: methods and development. Vital Health Stat 11, 1–190 (2002).
Barlow, S. E. & Expert, C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120(Suppl 4), S164–192, https://doi.org/10.1542/peds.2007-2329C (2007).
SPSS Inc. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc. (2009).
Sterne, J. A. et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338, b2393, https://doi.org/10.1136/bmj.b2393 (2009).
Acknowledgements
We thank the participants and their families for taking part in the study, and the staff who helped with recruitment. Dr. Samaan was funded by the Hamilton Health Sciences and Foundation New Investigator Fund, and the Pediatric Oncology Group of Ontario (POGO) Research Unit. Ms. Kuan-Wen Wang was funded by the Ontario Graduate Scholarship Program, and the Canadian Institutes of Health Research (CIHR) Canada Graduate Scholarship-Masters.
Author information
Authors and Affiliations
Contributions
M.C.S. is the guarantor. Research question and study design were defined by K.W.W., R.J.d.S., A.F., D.L.J., S.M.Z., S.R.R., S.B., L.T. and M.C.S. K.W.W. performed subject recruitment and data collection, supported by M.C.S., A.F. and S.B. R.J.d.S. and L.T. provided supports to research methods and statistical analyses. Data interpretation was completed by K.W.W., R.J.d.S., A.F., D.L.J., S.M.Z., S.R.R., S.B., L.T. and M.C.S. The manuscript was drafted by K.W.W. and M.C.S. and reviewed by all authors, who agreed with its content.
Corresponding author
Ethics declarations
Competing Interests
Dr. de Souza has been involved with the World Health Organization’s Nutrition Guidelines Advisory Group and was paid for travel and accommodation to attend meetings. He also received grants from the Canadian Foundation for Dietetic Research and the Canadian Institutes of Health Research. The other authors declare no conflicts of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, KW., de Souza, R.J., Fleming, A. et al. Birth weight and body mass index z-score in childhood brain tumors: A cross-sectional study. Sci Rep 8, 1642 (2018). https://doi.org/10.1038/s41598-018-19924-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19924-8
This article is cited by
-
Body mass index at diagnosis of a childhood brain tumor; a reflection of hypothalamic-pituitary dysfunction or lifestyle?
Supportive Care in Cancer (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.