Abstract
The differential response of marine populations to climate change remains poorly understood. Here, we combine common garden thermotolerance experiments in aquaria and population genetics to disentangle the factors driving the population response to thermal stress in a temperate habitat-forming species: the octocoral Paramuricea clavata. Using eight populations separated from tens of meters to hundreds of kilometers, which were differentially impacted by recent mortality events, we identify 25 °C as a critical thermal threshold. After one week of exposure at this temperature, seven of the eight populations were affected by tissue necrosis and after 30 days of exposure at this temperature, the mean % of affected colonies increased gradually from 3 to 97%. We then demonstrate the weak relation between the observed differential phenotypic responses and the local temperature regimes experienced by each population. A significant correlation was observed between these responses and the extent of genetic drift impacting each population. Local adaptation may thus be hindered by genetic drift, which seems to be the main driver of the differential response. Accordingly, conservation measures should promote connectivity and control density erosion in order to limit the impact of genetic drift on marine populations facing climate change.
Similar content being viewed by others
Introduction
The current global warming trend is affecting marine ecosystems in several ways, such as by decreasing ocean productivity, altering food web dynamics, shifting species distributions and reducing abundance1,2,3. Among these impacts, the increase in mass mortality events and disease outbreaks in marine species is particularly worrying because it severely threatens the structure and functioning of ecosystems and may significantly alter the provision of ecosystem services4. Generally, positive anomalies in seawater temperature have been concomitant with mass mortalities in affected regions5,6,7,8. These mortalities differentially affected species, populations and individuals at multiple spatial scales7, 9,10,11,12. For instance, field surveys conducted during mass mortality events in 1999 and 2003 in the Northwestern Mediterranean (NWM) revealed that, within the impacted species (mainly anthozoans and sponges), individuals separated by less than one meter showed varying responses ranging from severe to a complete absence of injuries while populations separated by a few to hundreds of kilometers displayed either similar or highly contrasting degrees of impact7. These differential responses might be explained by the complex interactions between populations and their local environments11, 13,14,15. In particular, environmental conditions, such as past and present thermal contexts, and biological processes, such as acclimatization or genetic adaptation, may be important drivers of the response of organisms to thermal stress16, 17. Accordingly, improving our understanding of the influences of environmental and biological factors on the response of populations to thermal stress is necessary to anticipate the consequences of climate change.
Acclimatization and local adaptation are two of the main biological processes modulating population responses to environmental variation, and they may drive the divergence in the phenotypic responses to thermal stress observed among populations18,19,20,21. Acclimatization implies physiological changes that allow an organism to maintain a functional phenotype when facing an environmental stressor19, 21, while local adaptation entails genetic changes driven by divergent selection19, 20, 22, which corresponds to spatial variations in natural selection resulting from local environmental conditions. Consequently, divergent selection may drive local populations to evolve traits that are advantageous in their local environments18, 20. Nevertheless, neutral evolutionary forces such as migration and genetic drift (i.e. the stochastic variations in alleles frequencies, caused by random sampling of genotypes and which is common in small and isolated populations)23 can also affect phenotypic divergence and thus influence differential responses. Migration can counteract divergent selection and limit local adaptation, while genetic drift may hinder local adaptation18.
The role of acclimatization and/or local adaptation to warming has been tested in various marine species through common garden thermotolerance experiments24,25,26 (see Materials and Methods section for a definition). For instance, differential acclimatization to thermal stress was demonstrated in crabs27, whereas local adaptation to thermal conditions has been suggested in corals15 and seagrasses28. Focusing on temperate octocorals, studies conducted on the Mediterranean red coral, Corallium rubrum, demonstrated the existence of differential adaptive abilities of populations depending on the depth of origin of the individuals and of their thermal history29. Local adaptation or acclimatization were considered to explain these results (see also refs 30, 31 and ref. 32 for other examples). However, these studies also suggested that genetic drift and acclimatization might play an important role in the phenotypic divergence between red coral populations by putatively modifying their abilities to adapt to local thermal environments.
In this study, we characterized the response to thermal stress of a temperate coral, the red gorgonian Paramuricea clavata (Risso, 1826) (Cnidaria, Anthozoa, Octocorallia). P. clavata is a key species of Mediterranean coralligenous assemblages and is one of the species most affected by recent mass mortality events7, 33,34,35. We carried out common garden experiments in aquaria using eight populations separated by tens of meters to hundreds of kilometers within the NWM (Fig. 1) and inhabiting contrasting temperature regimes at regional and local scales (ref. 36 and 37, subsection Temperature regimes of the study localities, Fig. 2). The aim of this study was twofold: 1) to acquire basic information about the thermotolerance features of P. clavata by monitoring colonies tissue necrosis at different temperatures (25, 26, 27 and 28 °C) in common garden experiments performed in aquaria; and 2) to evaluate the role of environmental conditions (particularly local thermal regimes) and biological processes (with a focus on local adaptation, genetic drift and acclimatization) in the differential responses of populations to thermal stress by combining colonies tissue necrosis data and population genetic analyses.
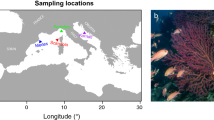
The study localities. The northwestern Mediterranean region with study localities (a). The study localities Medes (b), Calanques (c) and Scandola (d) and their populations: Medes shallow (M-sh, b) and Medes deep (M-d, b), Calanques 1, 2 and 3 shallow (C1-sh, C2-sh, C3-sh, c) and Calanques 1 deep (C1-d, c), Scandola shallow (S-sh, d) and Scandola deep (S-d, d). Figure 1a–d were performed with marmap R package88 (https://epante.wordpress.com/marmap/).
Temperature regimes of the study localities. Average annual temperature cycle (±SD) at 20 and 40 m depth for Medes (a), Calanques (Calanques 1) (b) and Scandola. Boxplots of hourly temperature records (of all available years) considering the July to October period and boxplots considering only the 10% of the data with the highest temperature are presented in (d) and (e) respectively. M-sh and M-d: Medes shallow and deep respectively, C-sh and C-d: Calanques 1 shallow and Calanques 1 deep respectively, S-sh and S-d: Scandola shallow and deep respectively.
Results
Thermal regimes of the study localities and extreme hot conditions
The thermal regimes of the studied localities have been previously characterized36, 37. Important differences in the summer thermal regimes dynamics36 and in the characteristics of temperature anomalies associated with mass mortality events37 were revealed. Moreover, during these events, differences in temperature conditions were found as the main factor explaining the observed inter-regional variability in mortality impacts37.
In this study, local thermal regimes at depths at which common garden experiments colonies were sampled were documented through the analysis of 8 to 11 years of continuous (every hour) in situ temperature records.
Average annual temperature cycle (mean ± SD of daily T) at 20 and 40 m depths for Medes, Calanques and Scandola are shown in Fig. 2a–c respectively. The three sites share common features, typical for the NW-Mediterranean, starting by a strong seasonal component and significant vertical gradients from May-June to October-November (seasonal thermal stratification dynamics). Minima were recorded in February-March and maxima in August-September for 20 m depth and in October for 40 m depth. Overall, annual mean T ranged between 16.2–17.4 °C at 20 m and between 15.1–15.5 °C at 40 m depth. Considering the July to October period (hereafter referred to as warm period) notorious differences in thermal regimes (mean ± SD) between depths (inside localities) and between localities (same depth) (Fig. 2a–c) can be observed. Figure 2d provides additional information on the distribution of hourly temperature records for comparison across localities and depth levels considering the warm period.
First, it is worth noting the differences inside localities between the 20 and 40 m depth (warm period median temperature range 18.8–21.2 °C vs. 15.5–17.1 °C for 20 and 40 m depth respectively) (Fig. 2d). The most important vertical gradients were found at Scandola (∼4 °C), followed by Medes (∼3 °C) and then by Calanques (∼2 °C) (Fig. 2d).
At 20 m depth, Scandola is the warmest locality both considering the median (21.2 °C) and the maximum temperature (26.9 °C) (Fig. 2c,d). This locality also presented the most stable thermal regime with a narrow interquartile range of 2.3 °C (Fig. 2d). Coldest and most variable regimes were found in Calanques (Fig. 2b,d) presenting a median temperature of 18.8 °C, and interquartile range of 5 °C and clearly higher frequency of low temperature values (1st quartile value of 16 °C) (Fig. 2d) which are a signature of the N winds driven by coastal upwelling of deep and cold waters at this locality. Intermediate conditions were found in Medes (Fig. 2a,d) where median and maximum temperatures were respectively 20.2 °C and 25.6 °C (Fig. 2a,d).
Even though at 40 m depth differences in mean temperature are attenuated, there are still clear patterns in thermal regimes. Medes is the warmest locality in depth, taking into account the median (17.1 °C) and maximum temperature values (24.9 °C) as well (Fig. 2d). Medes also experiences the most variable conditions during the warm period (Fig. 2a,d). For Calanques and Scandola, median values are respectively 15.5 °C and 16.7 °C; and maximum was 23.6 °C for both localities (Fig. 2d).
Finally, extreme hot conditions of the warm period were characterized. Figure 2e presents boxplots of the warm tail of hourly temperature records distribution (records over the 90th percentile, considering the warm period of all available years). This representation of extreme temperatures reinforces the patterns observed on thermal regimes at seasonal scale in Fig. 2d. Differences from 1.4 to 3.4° Care observed between depths inside localities and of up to 1 °C between localities (considering mean of extreme temperatures) (Fig. 2e). At 20 m depth Scandola clearly experienced the most extreme temperatures, with no overlapping of Scandola interquartile range (23.1–24.3 °C) with respect to those of Medes and Calanques (Fig. 2e). In depth, Medes clearly present higher values of most extreme temperature values in comparison with Calanques and Scandola (Fig. 2e).
From a heat stress perspective, information analyzed here account for important differences in the thermal regimes and extreme hot temperatures distribution both among depths (inside localities) and between localities (same depths).
Indeed, global Kruskal-Wallis statistical test evidenced significant differences (p-value < 0.001) between temperature regimes (considering data of the warm period of all available years). Pair-wise comparisons (using Dunn-Sidak’s approach) accounted for significant differences among all pairs of localities and depths (all p-values < 0.01).
Assessing the features of Paramuricea clavata thermotolerance
We identified the critical temperature of P. clavata as 25 °C because after one week of exposure, 20 colonies from seven of the eight populations (8%) were affected by necrosis. After 30 days of exposure at 25 °C, the mean % of affected colonies increased gradually from 3% to 97% (Fig. 3).
Results of the experiments to evaluate the thermotolerance thresholds of P. clavata. Daily mean (±SE) percentage of affected colonies (tissue necrosis >0%) for each temperature treatment: 25 °C, 26 °C, 27 °C and 28 °C. The 25 °C results correspond with the results of the ladder experiment from the day when T reached 25 °C. For clarification purposes, the mean (±SE) values of all tested populations (pooling sites and depths) are presented for each experiment.
Exposure to temperatures of 26, 27 and 28 °C resulted in a much more abrupt increase in necrosis, and a high level of mean affectation (90–100%) was observed after the 8th day of the 26 °C experiment. For the 27 and 28 °C experiments, 90–100% mean affectation was reached on the 4th and 3rd days, respectively (Fig. 3). These results suggest that exposure to temperatures of 26 °C, or higher, for a short time is lethal to the red gorgonian P. clavata.
Analyzing the potential factors that condition the response of populations to thermal stress
Importance of environmental factors
Figure 4 illustrates the differences among localities (Fig. 4a) and depths (Fig. 4b) in the response to thermal stress (25 °C) of P. clavata populations dwelling in contrasting thermal regimes, and these results do not fully support our hypotheses. Considering the response to temperature of populations from different localities, the results were opposite to those expected. Scandola which was clearly the warmest locality at 20 m depth was the most affected while Calanques, which was the coldest locality both at 20 and 40 m depth (lowest median values), was the least affected (Figs 2 and 4a). Between depths, very little differences were observed being shallow populations less affected than the deep populations (Fig. 4b). The two-way multivariate PERMANOVA indicated significant results for the Locality (p-value < 0.001) and Depth factors (p-value < 0.05 but very close to the significance threshold: p-value = 0.049) while the interaction term (Locality x Depth) was not significant (p-value > 0.05) (Supplementary Table S1). Regarding localities, the pairwise post-hoc results of the two-way PERMANOVA indicated significant differences between the Medes and Scandola (p-value < 0.01) and the Calanques and Scandola (p-value < 0.001) populations, but no significant differences were found between Medes and Calanques (p-value > 0.05) (Fig. 4a). When considering the results of the one-way PERMANOVA, the Population factor was significant (p-value < 0.001) (Supplementary Table S2), and the pairwise post-hoc results revealed that many pairs of populations display significant differences in the response to thermal stress, most of them involving Scandola (Supplementary Table S3). However, several pairs of populations from different localities and depths did not exhibit significant differences (p-value > 0.05, Supplementary Table S3, Fig. S1). For example, no significant differences were found between the pairs Medes shallow - Calanques 1 shallow and Medes deep – Scandola deep (Supplementary Table S3, Fig. S1). Moreover, no significant differences were found between depths at any of the three localities (Supplementary Table S3, Fig. S1).
Results of the common garden experiment. Mean percentage (±SE) of tissue necrosis of colonies from the study localities (a) and depths (b) during the 25 °C experiment. The levels of significance of the differences between pairs of localities (a) and depths (b) from the two-way pairwise PERMANOVA are indicated as follows: ***p-value < 0.001, **p-value < 0.01, *p-value < 0.05. The lack of indications between pairs denotes the absence of significant differences.
Importance of biological processes
Population genetic structure. Genetic characteristics of the populations including the level of null alleles, heterozygosity and f computations are presented on the Supplementary Results S1. The global θ was 0.075 (95% CI: 0.044–0.134), and the pairwise values and 95% CI for θ ranged from 0.028 (95% CI: 0.010–0.041) for Calanques 3 shallow vs. Calanques 1 shallow to 0.137 (95% CI: 0.052–0.230) for Calanques 1 shallow vs. Scandola. The population-specific F ST values varied from 0.052 (95% CI: 0.033–0.073) for Calanques 2 shallow to 0.090 (95% CI: 0.058–0.127) for Calanques 1 shallow (Table 1).
Relationship between neutral genetic structure and phenotypic response. The global P ST under the null assumption (c/h 2 = 1) was 0.25 (95% CI: 0.007–0.436), and the pairwise P ST values ranged from 0 for Calanques 3 shallow vs. Calanques 1 shallow, Calanques 3 shallow vs. Calanques 1 deep and Calanques 1shallow vs. Calanques 1 deep (95% CI were 0–0.135, 0–0.095 and 0–0.08, respectively) to 0.235 (95% CI: 0–0.521) for Calanques 2 shallow vs. Scandola (Table 1). Pairwise P ST were positively and significantly correlated to pairwise F ST (Mantel r coefficient = 0.55, p value < 0.05, Fig. 5), but the global P ST was not significantly different from the global F ST based on the respective 95% CIs. In all of the pairwise P ST and F ST comparisons, the 95% CIs overlapped, suggesting a lack of significant differences between the two measures (Table 1). Therefore, the patterns of neutral genetic and phenotypic differentiations were identical and likely driven by the same process.
A positive and significant correlation was obtained between the population-specific F ST and the mean percentage of necrosis at the last day of the experiment (Spearman Rho = 0.94; p-value < 0.01). Accordingly, genetically isolated populations seemed to be more impacted by thermal stress (Fig. 6).
Affectation vs. local F ST . Relation between affectation (mean percentage of necrosis on the last day of the experiment) and local F ST . The points and their labels are shown. The Spearman Rho statistic and its corresponding p-value are also presented. M: Medes; C1sh, C2sh and C3sh: Calanques1, 2 and 3 shallow; C1d: Calanques 1 deep; S: Scandola.
Discussion
In this study, we conducted a comprehensive characterization of the response to thermal stress of Paramuricea clavata populations thriving in distinct temperature regimes, i.e. from localities separated by hundreds of kilometers and from different depths. Through a common garden thermotolerance experiment, we demonstrated the limited influence of local temperature regimes and suggested that genetic drift may hinder the impact of divergent selection and modulate the response of populations to thermal stress.
Our study indicated that 25 °C is a critical temperature for P. clavata because all of the analyzed populations exhibited the first signs of necrosis after one week of exposure. When populations were exposed to higher temperatures (≥26 °C), colonies were affected by necrosis from the first day, and most of them suffered extensive damage in less than 5 days. Other experimental studies also indicated that similar short-term exposure to 25 °C was critical for triggering necrosis in P. clavata 38, 39. At lower temperatures (23–24 °C), 49 days of exposure were required for the first signs of necrosis to be observed35. Overall, these results are consistent with in-situ temperature surveys conducted during the mortalities events in 1999 and 2003 in different areas of the NWM7, 37. They are also supported by previous experimental studies focused on other anthozoans affected during these events. Indeed, in experimental conditions, 25 °C was identified as critical temperature for aposymbiotic species such as the red coral Corallium rubrum and the yellow gorgonian Eunicella cavolini 31, 32.
Since temperatures higher than 25 °C are rarely observed in the studied localities36, 37, we suggest that the shallow (marginal) populations of P. clavata are living near their upper thermal limit. This hypothesis is strengthened by the fact that Mediterranean gorgonians, in general, and P. clavata, in particular, shift their distribution to greater depths in warmer areas40, 41. Specifically, Linares et al.42 noted this upper depth limit distribution on the latitudinal scale with populations inhabiting from 10–15 m at 42° 22′N and from 30–35 m at 37° 38′N associated to the changes in the temperature conditions. Coupling this information on geographic and bathymetric species distributions to projected ocean warming and to population response variability would enable the identification of areas that are particularly vulnerable to climate change43,44,45 and the design of efficient specific conservation measures45.
The populations of P. clavata dwelling in warm conditions did not exhibit a higher resistance to thermal stress than those found in colder conditions. This result contrasts with previous studies conducted in various marine species suggesting that populations from warm habitats were more tolerant to thermal stress than populations from cold habitats (e.g. refs 15, 17, 46 and 47). In these cases, local adaptation and acclimatization processes related to local temperature conditions were used to explain the differential responses to thermal stress. Our study takes the opposing view of these results since the differential responses reported here seems only slightly related to local thermal conditions. While the observed positive correlation between phenotypic divergence (P ST ) and the neutral genetic divergence among populations (F ST ) may be due alternatively to selective or neutral processes48, the low and mostly similar pairwise P ST s and F ST s values combined to the positive correlation between the population-specific F ST and the level of necrosis at the end of the experiment support the influence of genetic drift. Accordingly, we hypothesize that the differential responses to thermal stress in P. clavata is mainly driven by genetic drift, a stochastic and neutral evolutionary processes.
In line with this hypothesis, recent studies suggest that genetic drift may have a dominant impact on phenotypic differentiation in Mediterranean octocorals. The level of genetic drift was suggested as a limiting factor in the adaptation of red coral populations to their local environments29 while, a genetic survey of P. clavata populations recently impacted by a mortality event demonstrated a positive link between the extent of genetic drift and the level of tissue necrosis49. Moreover, this hypothesis is consistent with model predictions50, 51 and the results of a meta-analysis52. Indeed, the influence of genetic drift on local adaptation is expected to be stronger in species with restricted connectivity and low effective population size50,51,52 as reported in P. clavata 53, 54. Considering these life history traits are shared by other Mediterranean anthozoans55, 56, the lack of local adaptation to thermal conditions may be widespread in these species questioning their abilities to deal with on-going climate change.
One should highlight the limitations linked to the P ST computations57,58,59. Genomic studies60 are thus needed to further decipher the respective roles of selective and neutral processes on the evolution of P. clavata and similar anthozoans in the environmental shift.
Understanding the response of populations to climate change is a central topic in conservation biology as a way to better assess species viability in the currently changing ocean. Our study highlights the potential impact of genetic drift in the response to thermal stress, which complements the picture of previously identified modulating factors (e.g., physiological status35, thermodependent pathogens61, sex53). Taking this information into consideration should improve our capacity to more accurately characterize the vulnerability of marine coastal ecosystems. In this sense, we addressed the importance of the assessment of genetic drift on populations of P. clavata at different spatial scales, which is especially urgent in areas where warming is expected to reach critical temperatures, such as the North Western Mediterranean62.
This approach will allow the more effective design of conservation measures encompassing a comprehensive range of spatial scales to preserve the adaptive potential of marine populations: from the design of MPA networks to specific restoration actions at the population level in view to promote the connectivity and controlling density erosion of populations. Including this information in management plans will contribute to the conservation rich Mediterranean marine biodiversity, in the face of expected warming62.
Methods
Model species
The red gorgonian Paramuricea clavata (Risso, 1826) (Cnidaria, Anthozoa, Octocorallia) was the model species chosen for this study. This species is considered a key species of Mediterranean coralligenous assemblages and is also one of the species that has been most affected by recent mass mortality events7, 33, 34.
Study localities and temperature regimes
We considered three localities separated by hundreds of kilometers within the NWM region: the Medes Islands (Catalonia, NE Spain), Calanques (Provence, France) and Scandola (NW Corsica, France) (Fig. 1a). These three localities were chosen based on 1) their contrasted temperature regimes and features of thermal anomalies (Fig. 2, subsection Temperature regimes of the study localities of the Results Section36, 37), 2) their differential degrees of impact during the recent regional mass mortality events7, 36, 37 and 3) their association with distinct genetic clusters54.
At each locality, two populations dwelling at shallow (20 m) and deep (40 m) depths, and thus separated by tens of meters, were sampled (Fig. 1b–d). In Calanques, two additional shallow (20 m) populations separated between 4 to 6 km were sampled (Fig. 1c). Throughout the text, the studied populations are referred as Medes shallow, Medes deep, Calanques 1 shallow, Calanques 1 deep, Calanques 2 shallow, Calanques 3 shallow, Scandola shallow and Scandola deep.
Temperature regimes of the studied localities were characterized in previous studies36, 37. Here we provide a description of the main attributes of thermal regimes of the different localities and depths where the populations were sampled through the analysis of hourly temperature time series provided by TMEDNet (http://www.t-mednet.org). The longest possible period for which data was available at the 3 sites for a given depth were considered, i.e. 11 years (2004–2014) for 20 m depth and 8 years (2004–2011) for 40 m depth. From these long term series, average annual cycle and statistics on the distribution of hourly records were calculated. Annual cycle is based on daily average temperatures. For each julian day, mean ± SD were calculated. Boxplots were used to summarize information on central tendency, dispersion and extreme values of hourly temperature records: first, to characterize conditions during warm months, from July to end of October (hereafter warm period); then, to characterize distribution of extreme hot temperatures during the warm period (from 90th percentile).
Experimental setting and biological material
We carried out a common garden experiment (i.e. individuals from different origins are transplanted to a new habitat where they are submitted to a common biotic -e.g. high predation- or abiotic -e.g. high temperature- environmental factor18) in aquaria with the selected Paramuricea clavata populations. We performed four independent common garden thermotolerance experiments in aquaria, considering for each, four different temperatures respectively: 25 °C, 26 °C, 27 °C and 28 °C (see Experimental Design subsection). All the experiments were conducted between July and November of 2009 with apical tips (between 5 and 7 cm in length) of healthy, mature red gorgonian colonies (20–40 cm in height63). Apical tips (hereafter colonies) were randomly sampled in the study localities by SCUBA diving after 2009 spawning occurred. All the colonies were sampled in June 2009 except for those of the 28 °C experiment which were sampled in November 2009 (see Supplementary Table S4).
Immediately after sampling, each colony was assembled on experimental plates and placed in coolers equipped with air pumps (battery powered) to be transported to experimental aquarium facilities at the Institute of Marine Sciences-CSIC (Barcelona, Spain). Experimental plates were built up by three PVC rectangular pieces (5 × 30 cm in size) and two rubber layers. Each PVC piece had 10 holes (1 cm in diameter) to place the colonies. The colonies were fixed in the experimental plates without using any putty, instead the colonies were hold mechanically thanks to the perpendicular cuts previously done in the rubber layers (one cut in each layer) (see Fig. S2).
The maximum transportation time was of 36 h for the colonies collected in Scandola (Corsica, France) while the minimum time was of several hours for the colonies collected in Medes (Catalonia, Spain). The temperature in the coolers was monitored regularly and seawater ice packs were used when necessary to maintain the water temperature between 15–18 °C. Once in the aquarium, colonies were maintained at 16–17 °C until the experiments started (Supplementary Methods S1). At the moment of starting with the experiments 100% of the colonies were healthy, presenting expanded polyps during feeding events and no tissue necrosis. All of the experiments used the same setting with two aquarium sets: Control and Treatment. Both Control and Treatment sets were composed by three replicates (three tanks of 105 l each), where colonies were placed, plus one large buffer tank (≈ 100 l) (Fig. 7). The buffer tanks were supplied with filtered Mediterranean seawater (pumped at 15 m depth) and from there, water was pumped continuously into the experimental tanks. Buffer tanks were used to control the temperature of experimental tanks (Fig. 7).
Experimental setting. Experimental setting of all the performed experiments. Control and Treatment sets where colonies were placed were composed by three replicates (C1, C2 and C3, and T1, T2 and T3 respectively) plus one large buffer tank which was supplied with filtered Mediterranean seawater and from there, water was pumped continuously into the experimental tanks. Buffer tanks were used to control the temperature of experimental tanks.
In the Control, seawater was maintained at 16 to 17 °C. We decided to set this temperature as control temperature because it corresponds to low temperatures during the summer period in all localities and it was also the Control temperature used in previous experimental studies with corals of the same study region31, 38, 64. In Treatment sets, seawater was heated (see the Experimental design subsection) in the buffer tank with submersible resistance heaters and was regulated by temperature controllers (Aqua Medic T controller). All the tanks were equipped with submersible pumps to facilitate water circulation within the tanks. Temperature was registered in the Control and Treatment aquariums with Tidbit Stowaway autonomous temperature sensors every half an hour.
Depending on the experiment, 15 to 30 colonies were used per population and per Control/Treatment set (Table S4). The experimental sets functioned as an open system.
To check the physiological status of the colonies during the experiments, the proxy of the surface area (cm2/branch linear cm35) was used. Overall the results showed that colonies placed in the Control followed similar variations in surface area than those in the field (Supplementary Methods S1).
Colonies feeding
In the Control and Treatment sets, colonies were fed two days per week, one day with nauplii of Artemia salina (1076.7 ± 156.7 org/l) and the other with phytoplankton diatom Tetraselmis chuii (chl-a = 3.3 ± 0.4 ug/l) (these concentrations correspond to those of each experimental tank). After the addition of food, the tanks were maintained in closed system mode for 5 h. During this period, water circulation within tanks was supplied by submersible pumps.
The strategy of supplying pulses of food instead of a constant supply was based on the results of in situ studies of food abundance and prey capture at short time scales in benthic suspension feeders65. The results of sampling food abundance near the organisms and their gut contents demonstrated that organisms commonly acquire available resources in short time pulses. There was estimated that these pulses of food abundance occurred between 2 and 3 times per week on average65, so that was simulated in the performed experiments. The period of 5 h was chosen on the basis of the duration of the pulses of food abundance in the field65 and because previous experimental studies with P. clavata indicated that 5 h is an appropriate period, after which food abundance reaches similar levels prior to starting the feeding66. Furthermore, we measured food concentrations before, during and after the feeding events, and we arrived to the same results (results not shown). To obtain an estimate of food concentration during the experiments, we measured the particulate organic carbon (POC) concentration which is the most integrative descriptor of food conditions66. The POC concentration throughout the experimental treatment was 323 ± 50 ug C/l. POC values that characterize coastal waters in the NW Mediterranean are on the order 300 ug C/l67, 68, hence, provided food concentrations were similar to those observed in the summer period in the field.
Overall, the frequency and amount of food together with the maintenance of the system in a close mode after feeding, guaranteed that colonies were supplied with a surplus of food as observed in previous experimental studies66.
Response variable
For all of the experiments, the response variable was the level of tissue necrosis, which was visually monitored daily and assessed as the percentage of the colony exhibiting dead tissue (from clear grey to black) or denuded axis (see Fig. S3). From these observations, the percentage of affected colonies (i.e., the proportion of colonies displaying tissue necrosis >0%) and mean necrosis values per day were computed to assess the response to thermal stress.
Experimental design
The common garden experiments were designed to assess the thermotolerance thresholds of Paramuricea clavata and to evaluate the importance of environmental and neutral genetic factors in the response of P. clavata populations to thermal stress. Four experiments considering the following temperatures were carried out: 25, 26, 27 and 28 °C (see next subsection).
Assessing the thermotolerance thresholds of Paramuricea clavata
We carried out four experiments to evaluate the thermotolerance thresholds of P. clavata. The first experiment consisted of a stepwise temperature augmentation from 18 °C to 22 °C by 2 °C per week and then by 1 °C per week until 25 °C, which was identified as the critical temperature for P. clavata (see Results). Once this temperature was reached, the conditions were kept constant for 30 days (until at least 95% of the colonies were affected by tissue necrosis).
To explore the response to thermal stress above this temperature, we performed three additional experiments with constant temperature of 26, 27 and 28 °C. These temperatures were attained after one day of gradual augmentation from 18 °C to the treatment temperature, and the experiments were conducted until at least 95% of the colonies were affected by necrosis. The 26, 27 and 28 °C experiments had a total duration of 13, 6 and 4 days respectively (Table S4). The 25, 26 and 27 °C experiments were carried out using the eight populations while the 28 °C experiment was only performed with two populations (Medes shallow and deep) due to logistical constraints (Table S4).
For each population and experiment, we reported the daily percentage of affected colonies. Then, to characterize the thermotolerance features of P. clavata, for each experiment, we calculated the mean (±SE) daily percentage of affected colonies across populations.
Exploring the factors and processes modulating the response of populations to thermal stress
To explore the influence of environmental and genetic factors on the response of P. clavata populations, we considered the common garden experiment at 25 °C because this temperature was identified as critical for P. clavata (see Results).
Environmental factors. We postulated that populations from depths/localities with colder thermal regimes will be more affected by thermal stress than populations subject warmer thermal regimes. In particular, and bearing in mind the temperature regimes of the selected localities and depths36, 37 (see Results section), we expected shallow populations to be less affected by thermal stress than those in deeper water. Regarding the localities, the Scandola and Calanques populations should be the least and most affected, respectively, while the Medes population should present an intermediate response. To analyze these hypotheses, we focused on the response to temperature of six populations coming from three localities and two depths with contrasting thermal regimes: Medes shallow and deep, Calanques 1 shallow and deep and Scandola shallow and deep36, 37. The two populations from Calanques 2 and Calanques 3 shallow were not included to maintain a balanced design in the analysis (see below).
To assess statistical differences in tissue necrosis in the Treatment between localities and depths, we performed a two-way multivariate PERMANOVA69, 70 with Locality (three levels) and Depth (two levels) as fixed factors. A multivariate approach, taking into account tissue necrosis from three days covering the whole experiment (the 10th, 20th and 30th days) as the multivariate dependent variable was considered69. Post-hoc pair-wise PERMANOVA tests were performed to identify significant differences between pairs of localities. Complementarily, we ran a second PERMANOVA taking into account the six populations but accounting for only one factor (Population) that was considered fixed. The aim of this one-way analysis was to obtain pair-wise (through post-hoc pair-wise tests) results for all of the populations without restricting comparisons within depths or localities. The same multivariate approach of the previous experiment was considered. The colonies from the Control sets were not considered in these analyses because they remained healthy (no tissue necrosis) until the end of the experiment. For all of the tests, 9999 permutations were achieved. PERMANOVA analyses were performed in PRIMER v671 in conjunction with the Windows PERMANOVA + module72. For a more detailed description of the statistical analyses, we refer to Supplementary Methods S2.
Biological processes. Phenotypic divergence between populations, such as the differential response to thermal stress, results from the interaction between neutral and adaptive processes73, 74, and one way to characterize this interaction is to estimate and compare the neutral genetic divergence to the phenotypic divergence for the considered trait58. Through this characterization, we aimed to obtain the first insight into the role of population genetic features on the observed differential responses to thermal stress.
Neutral genetic divergence: Before initiating the experiments, a small tissue sample was taken from each apical tip and stored in 96% ethanol and at −20 °C for subsequent genetic analysis. Two hundred and forty colonies belonging to the eight populations were genotyped using six microsatellite loci (Parcla-09, Parcla-10, Parcla-12, Parcla-14, Parcla-17 and Par-d) following54 DNA extraction and genotyping protocols are detailed in Supplementary Methods S3.
As a preliminary step, we identified the different genetic pools (i.e., genetically distinct populations) within our dataset by conducting exact tests of the overall genotypic differentiation among populations and between all pairs of populations in GENEPOP75 using the default parameters. These analyses showed that the two populations from Medes (shallow and deep) and the two populations from Scandola (shallow and deep) were not significantly genetically different (p-values > 0.001). Accordingly, we grouped Medes shallow and Medes deep in the Medes genetic pool and Scandola shallow and Scandola deep in the Scandola genetic pool, so the following analyses were conducted considering six different genetic pools (Calanques 1 shallow, Calanques 1 deep, Calanques 2 shallow, Calanques 3 shallow, Medes and Scandola).
We estimated the neutral genetic divergence and respective 95% confidence intervals (95% CIs) by computing the global and pairwise differentiation between the six genetic pools using θ, the Weir & Cockerham estimator of F ST 76 in FREENA77. We also calculated the population-specific F ST as implemented in GESTE78 for the six genetic pools. This method measures how genetically distinct a population is compared to all of the populations combined and provides insight into the relative impact of genetic drift and gene flow on the differentiation of each population78.
Phenotypic divergence: The global and pairwise phenotypic divergences were estimated between the six genetic pools using P ST 58, 79, 80, which is defined as P ST = cσ 2 GB /(cσ 2 GB + 2h 2 σ 2 GW ), where σ 2 GB and σ 2 GW represent the among-sample (in this context one sample refers to one genetic pool) and the within-sample variance components for the considered phenotypic trait, respectively; h 2 represents the assumed additive genetic proportion of the differences between individuals within samples (“narrow-sense heritability”); and c represents the proportion of the total variance presumed to occur because of additive genetic effects across samples81. We considered the null assumption that the genetic architecture of the trait (i.e., the level of tissue necrosis) remained equal across samples with c/h 2 = 180, 81. Among- and within-sample variances were computed based on PERMANOVAs, which were conducted considering “the level of tissue necrosis of the 10th, 20th and 30th experiment day” of the common garden experiment as a multivariate dependent variable and “genetic pool” as the only random factor (with six levels: Medes, Calanques 1 shallow, Calanques 1 deep, Calanques 2 shallow, Calanques 3 shallows and Scandola).
Variances were estimated by extracting the within-sample and between-sample mean squares (MS B and MS W , respectively) from the PERMANOVA output. The within-sample variance (σ 2 GW ) was directly estimated based on the within-sample MS w . The between-sample variance (σ 2 GB ) was estimated as σ 2 GB = (MS B − MS W )/n 0 with n 0 being a weighted average of the sample size for each comparison:
where a is the number of samples to be compared, and n i is the number of individuals in the i th sample82, 83. Negative variance values were set to zero84.
We obtained each P ST by calculating 1000 P ST from 1000 PERMANOVAs considering 1000 bootstrap samples of the multivariate dependent variable. This allowed us to estimate the final P ST values as the median values over the 1000 permutations along with the 95% CI, which corresponds to the 25th and 975th values of the sorted P ST respectively. Finally, we considered 100 permutations for each PERMANOVA.
Comparison between genetic and phenotypic divergences: We conducted a Mantel test (n = 1000) to analyze the relationship between pairwise genetic (F ST ) and phenotypic (P ST ) estimates of divergence. A significant relationship between the two estimates of divergence suggests that the pattern of phenotypic divergence is mainly explained by genetic drift58, 85 or, alternatively, that isolation by adaptation occurs with divergent selection driving the evolution of the phenotypic trait and the differentiation at neutral loci86, 87. To disentangle the two hypotheses, we then compared the P ST (under c/h 2 = 1) and F ST estimates for each population pair. If divergent selection is involved in the observed patterns, we expect the P ST estimates to be significantly higher than F ST 58. The comparison of the estimators was based on their 95% CIs.
Using a non-parametric Spearman correlation, we further examined the impact of genetic differentiation on the response to thermal stress by testing for the relationship between the population-specific F ST and the level of necrosis of each population observed on the last day of the experiment. A positive correlation is expected when isolated populations, which are subjected to a stronger genetic drift effect, are more impacted by thermal stress.
The PERMANOVA (adonis function), P ST computations (personal scripts available in Supplementary Methods S4), Mantel test (mantel function) and Spearman correlation (cor.test function) were implemented in R version 3.2.1 (http://cran.r-project.org).
References
Hoegh-Guldberg, O. & Bruno, J. F. The Impact of Climate Change on the World’s Marine Ecosystems. Science 328, 1523–1528 (2010).
Carpenter, K. E. et al. One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 321, 560–563 (2008).
Hughes, T. P. et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 301, 929–933 (2003).
Harvell, C. D. et al. Emerging Marine Diseases-Climate Links and Anthropogenic Factors. Science 285, 1505–1510 (1999).
Berkelmans, R. & Oliver, J. K. Large Scale Bleaching of Corals on the Great Barrier Reef. Coral Reefs 18, 55–60 (1999).
Cerrano, C. et al. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecol. Lett. 3, 284–293 (2000).
Garrabou, J. et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Change Biol. 15, 1090–1103 (2009).
Hoegh-Guldberg. Climate Change, coral bleaching (1999).
Berkelmans, R., De’ath, G., Kininmonth, S. & Skirving, W. J. A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: spatial correlation, patterns, and predictions. Coral Reefs 23, 74–83 (2004).
Garrabou, J., Perez, T., Sartoretto, S. & Harmelin, J. G. Mass mortality event in red coral Corallium rubrum populations in the Provence region (France, NW Mediterranean). Mar. Ecol. Prog. Ser. 217, 263–272 (2001).
Kersting, D. K., Bensoussan, N. & Linares, C. Long-Term Responses of the Endemic Reef-Builder Cladocora caespitosa to Mediterranean Warming. PLoS ONE 8, e70820 (2013).
Pratchett, M. S., McCowan, D., Maynard, J. A. & Heron, S. F. Changes in Bleaching Susceptibility among Corals Subject to Ocean Warming and Recurrent Bleaching in Moorea, French Polynesia. PLoS ONE 8, e70443 (2013).
Császár, N. B. M., Ralph, P. J., Frankham, R., Berkelmans, R. & van Oppen, M. J. H. Estimating the Potential for Adaptation of Corals to Climate Warming. PLoS ONE 5, e9751 (2010).
Howells, E. J. et al. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Change 2, 116–120 (2012).
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N. & Bay, R. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014).
Ateweberhan, M. & McClanahan, T. R. Relationship between historical sea-surface temperature variability and climate change-induced coral mortality in the western Indian Ocean. Mar. Pollut. Bull. 60, 964–970 (2010).
Howells, E. J. et al. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Change 2, 116–120 (2012).
Kawecki, T. J. & Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241 (2004).
Reusch, T. B. H. Climate change in the oceans: evolutionary versus phenotypically plastic responses of marine animals and plants. Evol. Appl. 7, 104–122 (2014).
Williams, G. Adaptation and Natural Selection (Princeston University Press, 1996).
Wilson, R. S. & Franklin, C. E. Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 17, 66–70 (2002).
Reusch, T. B. H. & Wood, T. E. Molecular ecology of global change. Mol. Ecol. 16, 3973–3992 (2007).
Charlesworth, B., Charlesworth, D. & Barton, N. H. The Effects of Genetic and Geographic Structure on Neutral Variation. Annu. Rev. Ecol. Evol. Syst. 34, 99–125 (2003).
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M. & Marshall, D. J. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500 (2013).
Sanford, E. & Kelly, M. W. Local Adaptation in Marine Invertebrates. Annu. Rev. Mar. Sci. 3, 509–535 (2011).
Somero, G. N. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 (2010).
Stillman, J. H. Acclimation capacity underlies susceptibility to climate change. Science 301, 65–65 (2003).
Franssen, S. U. et al. Transcriptomic resilience to global warming in the seagrass Zostera marina, a marine foundation species. Proc. Natl. Acad. Sci. 108, 19276–19281 (2011).
Ledoux, J.-B. et al. Potential for adaptive evolution at species range margins: contrasting interactions between red coral populations and their environment in a changing ocean. Ecol. Evol. 5, 1178–1192 (2015).
Haguenauer, A., Zuberer, F., Ledoux, J.-B. & Aurelle, D. Adaptive abilities of the Mediterranean red coral Corallium rubrum in a heterogeneous and changing environment: from population to functional genetics. J. Exp. Mar. Biol. Ecol. 449, 349–357 (2013).
Torrents, O., Tambutté, E., Caminiti, N. & Garrabou, J. Upper thermal thresholds of shallow vs. deep populations of the precious Mediterranean red coral Corallium rubrum (L.): Assessing the potential effects of warming in the NW Mediterranean. J. Exp. Mar. Biol. Ecol. 357, 7–19 (2008).
Pivotto, I. D. et al. Highly contrasted responses of Mediterranean octocorals to climate change along a depth gradient. R. Soc. Open Sci. 2, 140493–140493 (2015).
True, M. A. Étude quantitative de quatre peuplements sciaphiles sur substrat rocheux dans la région marseillaise. Bull. L’Institut Oceanogr. Monaco 1410, 1–48 (1970).
Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Annu. Rev. 44, 123–195 (2006).
Coma, R. et al. Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc. Natl. Acad. Sci. 106, 6176–6181 (2009).
Bensoussan, N., Romano, J.-C., Harmelin, J.-G. & Garrabou, J. High resolution characterization of northwest Mediterranean coastal waters thermal regimes: To better understand responses of benthic communities to climate change. Estuar. Coast. Shelf Sci. 87, 431–441 (2010).
Crisci, C., Bensoussan, N., Romano, J.-C. & Garrabou, J. Temperature Anomalies and Mortality Events in Marine Communities: Insights on Factors behind Differential Mortality Impacts in the NW Mediterranean. PLoS ONE 6, e23814 (2011).
Bally, M. & Garrabou, J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: a new case of emerging disease linked to climate change. Glob. Change Biol. 13, 2078–2088 (2007).
Previati, M., Scinto, A., Cerrano, C. & Osinga, R. Oxygen consumption in Mediterranean octocorals under different temperatures. J. Exp. Mar. Biol. Ecol. 390, 39–48 (2010).
Kipson, S. et al. Population structure and conservation status of the red gorgonian Paramuricea clavata (Risso, 1826) in the Eastern Adriatic Sea. Mar. Ecol. 36, 982–993 (2015).
Sini, M., Kipson, S., Linares, C., Garrabou, J. & Koutsoubas, D. Distribution of Eunicella cavolini (Koch, 1887) across the Mediterranean. in (2014).
Linares, C., Coma, R., Garrabou, J., Díaz, D. & Zabala, M. Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. J. Appl. Ecol. 45, 688–699 (2008).
van Hooidonk, R., Maynard, J. A. & Planes, S. Temporary refugia for coral reefs in a warming world. Nat. Clim. Change 3, 508–511 (2013).
Pairaud, I. L., Bensoussan, N., Garreau, P., Faure, V. & Garrabou, J. Impacts of climate change on coastal benthic ecosystems: assessing the current risk of mortality outbreaks associated with thermal stress in NW Mediterranean coastal areas. Ocean Dyn. 64, 103–115 (2014).
Peters, H., O’Leary, B. C., Hawkins, J. P. & Roberts, C. M. Identifying species at extinction risk using global models of anthropogenic impact. Glob. Change Biol. 21, 618–628 (2015).
Jensen, L. F. et al. Local adaptation in brown trout early life-history traits: implications for climate change adaptability. Proc. R. Soc. B Biol. Sci. 275, 2859–2868 (2008).
Gleason, L. U. & Burton, R. S. Genomic Evidence for Ecological Divergence Against a Background of Population Homogeneity in the Marine Snail Chlorostoma funebralis. Mol. Ecol. 25, 3557–3573 (2016).
Leinonen, T., Cano, J. M., MäKinen, H. & Merilä, J. Contrasting patterns of body shape and neutral genetic divergence in marine and lake populations of threespine sticklebacks. J. Evol. Biol. 19, 1803–1812 (2006).
Arizmendi-Mejía, R. et al. Combining Genetic and Demographic Data for the Conservation of a Mediterranean Marine Habitat-Forming Species. PLOS ONE 10, e0119585 (2015).
Alleaume-Benharira, M., Pen, I. R. & Ronce, O. Geographical patterns of adaptation within a species’ range: interactions between drift and gene flow. J. Evol. Biol. 19, 203–215 (2006).
Bridle, J. R., Polechová, J., Kawata, M. & Butlin, R. K. Why is adaptation prevented at ecological margins? New insights from individual-based simulations. Ecol. Lett. 13, 485–494 (2010).
Leimu, R. & Fischer, M. A Meta-Analysis of Local Adaptation in Plants. PLoS ONE 3, e4010 (2008).
Arizmendi-Mejía, R. et al. Demographic responses to warming: reproductive maturity and sex influence vulnerability in an octocoral. Coral Reefs 34, 1207–1216 (2015).
Mokhtar-Jamaï, K. et al. From global to local genetic structuring in the red gorgonian Paramuricea clavata: the interplay between oceanographic conditions and limited larval dispersal. Mol. Ecol. 20, 3291–3305 (2011).
Ledoux, J.-B. et al. Genetic survey of shallow populations of the Mediterranean red coral [Corallium rubrum (Linnaeus, 1758)]: new insights into evolutionary processes shaping nuclear diversity and implications for conservation. Mol. Ecol. 19, 675–690 (2010).
Ledoux, J. B. et al. Fine-scale genetic structure and inferences on population biology in the threatened Mediterranean red coral. Corallium rubrum. Mol. Ecol. 19, 4204–4216 (2010).
Edelaar, P. & BjöRklund, M. If FST does not measure neutral genetic differentiation, then comparing it with QST is misleading. Or is it?: is comparing FST with QST misleading? Mol. Ecol. 20, 1805–1812 (2011).
Leinonen, P. H., Remington, D. L., Leppälä, J. & Savolainen, O. Genetic basis of local adaptation and flowering time variation. Arabidopsis lyrata. 22, 709–723 (2013).
Pujol, B., Wilson, A. J., Ross, R. I. C. & Pannell, J. R. Are Q ST - F ST comparisons for natural populations meaningful? Mol. Ecol. 17, 4782–4785 (2008).
De Wit, P. et al. The simple fool’s guide to population genomics via RNA-Seq: an introduction to high-throughput sequencing data analysis. Mol. Ecol. Resour. 12, 1058–1067 (2012).
Vezzulli, L. et al. Vibrio infections triggering mass mortality events in a warming Mediterranean Sea: Vibrio infections triggering sea-fan mass mortality. Environ. Microbiol. 12, 2007–2019 (2010).
Somot, S., Sevault, F., Déqué, M. & Crépon, M. 21st century climate change scenario for the Mediterranean using a coupled atmosphere–ocean regional climate model. Glob. Planet. Change 63, 112–126 (2008).
Coma, R., Zabala, M. & Gilli, J.-M. Sexual reproductive effort in Mediterranean gorgonian Paramuricea clavata. Mar. Ecol. Prog. Ser. 117, 185–192 (1995).
Haguenauer, A., Zuberer, F., Ledoux, J.-B. & Aurelle, D. Adaptive abilities of the Mediterranean red coral Corallium rubrum in a heterogeneous and changing environment: from population to functional genetics. J. Exp. Mar. Biol. Ecol. 449, 349–357 (2013).
Rossi, S. Environmental factors affecting the trophic ecology of benthic suspension feeders (2002).
Coma, R. et al. Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc. Natl. Acad. Sci. 106, 6176–6181 (2009).
Coma, R. & Ribes, M. Seasonal energetic constraints in Mediterranean benthic suspension feeders: effects at different levels of ecological organization. Oikos 101, 205–215 (2003).
Rossi, S. & Gili, J.-M. Composition and temporal variation of the near-bottom seston in a Mediterranean coastal area. Estuar. Coast. Shelf Sci. 65, 385–395 (2005).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46 (2001).
Anderson, M. J. Permutational multivariate analysis of variance. Dep. Stat. Univ. Auckl. Auckl (2005).
Clarke, K. R. & Gorley, R. N. PRIMER v6: user anual/tutorial. PRIMER-E (2006).
Anderson, M. J., Gorley, R. N. & Clarke, K. R. PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E (2008).
Slatkin, M. Gene flow and selection in a cline. Genetics 75, 733–756 (1973).
Lynch, M. The evolution of genetic networks by non-adaptative processes. Nat. Rev. Genet. 8, 803–813 (2007).
Rousset, F. genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 8, 103–106 (2008).
Weir, B. S. & Cockerham, C. C. Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (1984).
Chapuis, M. P. & Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 24, 621–631 (2007).
Foll, M. & Gaggiotti, O. E. Identifying the environmental factors that determine the genetic structure of populations. Genetics 174, 875–891 (2006).
Leinonen, T., O’Hara, R. B., Cano, J. M. & Merilä, J. Comparative studies of quantitative trait and neutral marker divergence: a meta-analysis. J. Evol. Biol. 21, 1–17 (2008).
Saether, S. A. et al. Inferring local adaptation from Q ST -F ST comparisons: neutral genetic and quantitative trait variation in European populations of great snipe. J. Evol. Biol. 20, 1563–1576 (2007).
Brommer, J. E. Whither Pst? The approximation of Qst by Pst in evolutionary and conservation biology: Whither Pst? J. Evol. Biol. 24, 1160–1168 (2011).
Sokal, R. R. & Rohlf, F. J. Biometry: the principles and practice of statistics in biological research (W.H. Freeman, 2012).
Storz, J. F. Contrasting patterns of divergence in quantitative traits and neutral DNA markers: analysis of clinal variation: genetic and phenotypic divergence. Mol. Ecol. 11, 2537–2551 (2002).
Gitzen, R. A., Millspaugh, J. J., Cooper, A. B. & Licht, D. S. Design and Analysis of Longterm Ecological Monitoring Studies (Cambridge University Press, 2012).
Ozerov, M. Y. et al. Generation of a neutral F ST baseline for testing local adaptation on gill raker number within and between European whitefish ecotypes in the Baltic Sea basin. J. Evol. Biol. 28, 1170–1183 (2015).
Nosil, P., Funk, D. J. & Ortiz-Barrientos, D. Divergent selection and heterogeneous genomic divergence. Mol. Ecol. 18, 375–402 (2009).
Orsini, L. et al. The evolutionary time machine: using dormant propagules to forecast how populations can adapt to changing environments. Trends Ecol. Evol. 28, 274–282 (2013).
Pante, E. & Simon-Bouhet, B. marmap: A Package for Importing, Plotting and Analyzing Bathymetric and Topographic Data in R. PLoS ONE 8(9), e73051 (2013).
Acknowledgements
The authors gratefully acknowledge the funding support given by the Alan Program (CC Doctoral Fellowship), by CSIC-Uruguay (Call Programa de pasantías en el exterior 2015) and by the Portuguese Foundation for Science and Technology (Fundação para a Ciència e a Tecnologia; FCT) which funded JBL Postdoctoral grant (SFRH/BPD/74400/2010). Authors are also grateful to the MEDCHANGE project funded by the Agence Nationale pour la Recherche (ANR) and to the Spanish Ministry of Economy and Competitivity ( http://www.mineco.gob.es) through the Biorock (CTM2009-08045), Smart (CGL2012-32194) and CSI-Coral (CGL2013-43106-R). Temperature data series data is part of the regional temperature observation network T-MEDNet (www.t-mednet.org); we thank J.M. Dominici for providing the data for the Réserve Naturelle de Scandola. The authors also thank the staff of Experimental Aquarium Facilities at the Institute of Marine Sciences E. Martinez and M. Delgado for the technical support provided. EC, JG, CL, PL and NT are part of the Marine Conservation research group MEDRECOVER www.medrecover.org (2009 SGR 1174) while RC and MR are part of the Marine Biogeochemistry and Global Change Research group (2014SGR1029) from the Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
C.C., J.B.L. and J.G. wrote the first draft of the manuscript and K.M.H., M.B., N.B., D.A., E.C., R.C., J.P.F., C.L., M.L., P.L.S., C.M., M.R., N.T., F.Z. contributed with revisions. C.C., J.B.L., K.M.H., M.B., N.B., D.A., E.C., R.C., J.P.F., C.L., M.L., P.L.S., C.M., M.R., N.T., F.Z. and J.G. contributed to the study design. C.C., R.C., J.G., J.B.L., M.B., E.C., C.L., M.R., N.T. and F.Z. participate in the experimental work with P. clavata colonies. C.C. performed the statistical analyses on mortality data. K.M.H. obtained the genetic data. J.B.L. and C.C. performed statistical analyses of the genetic data. N.B. obtained temperature descriptors for the studied localities. P.L.S. and C.M. provided technical support.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crisci, C., Ledoux, JB., Mokhtar- Jamaï, K. et al. Regional and local environmental conditions do not shape the response to warming of a marine habitat-forming species. Sci Rep 7, 5069 (2017). https://doi.org/10.1038/s41598-017-05220-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05220-4
This article is cited by
-
Comparative population genetics of habitat-forming octocorals in two marine protected areas: eco-evolutionary and management implications
Conservation Genetics (2024)
-
Exploring the response of a key Mediterranean gorgonian to heat stress across biological and spatial scales
Scientific Reports (2022)
-
The constraint of ignoring the subtidal water climatology in evaluating the changes of coralligenous reefs due to heating events
Scientific Reports (2020)
-
Biodiversity loss in a Mediterranean ecosystem due to an extreme warming event unveils the role of an engineering gorgonian species
Scientific Reports (2019)
-
Divergent responses to warming of two common co-occurring Mediterranean bryozoans
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.