Abstract
The Wingless-type MMTV integration site family member 2b (Wnt2b) has been found to be a principal mediator of liver development and regeneration. However, the significance of Wnt2b in the pathogenesis of fibrosis-related liver diseases remains undefined. Here, we report that Wnt2b was highly expressed in the fibrotic liver tissues, exhibiting protective effects against activation of hepatic stellate cells (HSCs) and fibrosis progression. We identified a negative regulation of Wnt2b on the toll-like receptor 4 (TLR4) activation-mediated pro-fibrogenic effects. Wnt2b was shown not only to directly suppress LPS-induced HSCs activation, but also to inhibit TLR4-enhanced the sensitivity of HSCs to transforming growth factor beta (TGF-β). Mechanistic study showed that Wnt2b suppresses TLR4 signaling through inhibiting the expression of TLR4 as well as the activation of NF-κB and MAPKs. These findings provided new insights into the pathophysiology of liver fibrosis by characterizing Wnt2b as a novel endogenous suppressor of TLR4 signaling, maintaining tissue homeostasis during the early stage of hepatic fibrosis-associated liver diseases.
Similar content being viewed by others
Introduction
Liver fibrosis is clinically associated with the development of cirrhosis and hepatocellular carcinoma (HCC), and has become one of the most significant public health concerns worldwide1, 2. Liver fibrosis is a wound-healing response to repeated liver injury and chronic liver inflammation of different etiologies. Fibrogenesis is usually considered as a dynamic process, with the potential to regress by cessation of injury, or to progress into cirrhosis if the key pathways involved in this process were not interrupted successfully at the right time3. The activation of hepatic stellate cells (HSCs) is the pivotal event in liver fibrosis. Following the onset of liver injury, the quiescent vitamin A-rich HSCs are activated and differentiate to pro-fibrogenic myofibroblasts (MFBs), which are considered to be the main source of extracellular matrix (ECM) and the major effectors during fibrogenesis4,5,6. Although innovations have been made recent years, the intricate mechanisms underlying HSCs activation in hepatic fibrogenesis are not fully clarified and no anti-fibrotic therapy has yet been approved by FDA7.
As an important pattern recognition receptor (PRR), TLR4 has shown to be a primary mediator for HSCs activation and fibrosis progression in the context of chronic liver injury8, 9. In experimental hepatic fibrosis models and patients with cirrhosis, the bacterial translocation and lipopolysaccharide (LPS) levels were increased10. HSCs highly express TLR4 and are hyperresponsive to LPS11. TLR4 promotes HSCs activation either by down-regulating the TGF-β pseudoreceptor Bambi, augmenting TGF-β–mediated HSCs activation11, or by negative modulating the functions of miR-146a-5p and miR-2912, 13. Moreover, TLR4 augments the accumulation of bone marrow-derived monocytes and Kupffer cells at the damaged sites via enhancing the expression of adhesion molecules and chemokines14. Importantly, hyporesponsive TLR4 mutations, such as D299G and T399I single nucleotide polymorphisms (SNPs), have shown to reduce the risk of liver fibrosis in patients with hepatitis C virus infection, promising the pro-fibrogenic role of TLR4 in a clinically relevant setting15, 16. Therefore, modulation of the TLR4 signaling might represent a feasible strategy for the treatment or prevention of chronic liver disease.
Wnt2b, also referred to as Wnt13, is a highly conserved secretary glycoprotein of the Wingless-type MMTV integration site (Wnt) family. The Wnt family members regulate multiple biological processes from embryonic development to adult life of all animals17, and also show to be implicated in various pathologies such as human malignancies18 and metabolic diseases19, 20. The functions of Wnt2b in liver physiology have been well demonstrated. Impaired function of Wnt2b in embryos results in a complete absence or severe decrease in hepatic tissue21. Additionally, genetic evidence supported a central role of Wnt2b in coordinating early liver development from the multipotent foregut endoderm17. The conversion of cholangiocytes to hepatocytes in zebrafish with Wnt2b mutants was impaired following substantial hepatocyte depletion22. Shackel et al. observed that Wnt2b mRNA increased in human primary biliary cirrhosis (PBC) by using cDNA array analysis23. However, the role of Wnt2b in TLR4-associated hepatic inflammation and fibrosis-related liver diseases is still unclear.
In this study, we present the evidence that the level of Wnt2b expression was elevated in the fibrotic liver tissues. Wnt2b displayed an inhibitory effect on HSCs activation and fibrosis progression. Our data demonstrate that Wnt2b may serve as a novel endogenous suppressor of TLR4 signaling-mediated liver fibrosis.
Results
Wnt2b expression is elevated in hepatic fibrosis
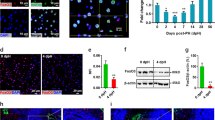
To investigate the potential association of Wnt2b with the development of human fibrotic liver disease, the expressions of Wnt2b were evaluated in Tissue microarrays (TMAs) containing 10 fibrotic subjects (with a mean age of 48.8 ± 2.9 years) and 9 normal controls (with a mean age of 43.3 ± 1.4 years). As shown in Fig. 1a, the fibrotic liver tissues exhibited higher levels of Wnt2b compared to normal controls as demonstrated by immunohistochemistry analysis. Consistently, in liver fibrosis mouse model established by repeated administration of thioacetamide (TAA) (Fig. 1b), hepatic Wnt2b expression was also markedly increased compared to healthy controls, accompanied with highly expressed α-SMA (Fig. 1c). Furthermore, both elevated mRNA (Fig. 1d upper) and protein levels (Fig. 1d lower) of Wnt2b in the fibrotic liver tissues were confirmed by RT-PCR and western blotting, respectively. The amount of Wnt2b in the fibrotic liver homogenate was increased from 143.885 ± 34.63 ng/g to 1972.23 ± 365.10 ng/g (Fig. 1e). Similar results were observed in CCl4-treated mice (Supplementary Fig. S1). These findings suggested that Wnt2b might be involved in the pathogenesis of liver fibrosis.
Wnt2b is elevated in hepatic fibrosis. (a) Representative Sirius Red staining, IHC staining of Wnt2b in liver tissue microarrays containing healthy controls (n = 9, with a mean age of 43.3 ± 1.4 years) and patients with fibrosis (n = 10, with a mean age of 48.8 ± 2.9 years). Hepatic fibrosis mouse model was induced by TAA, and then the following analyses were performed. (b) H&E, Sirius Red staining. (c) Immunofluorescence staining of α-SMA and IHC staining of Wnt2b. (d) RT-PCR (upper) and Western blotting (lower) of Wnt2b. Cropped blots are displayed; Full-length blots are presented in Supplementary Fig. S5. (e) ELISA analysis of Wnt2b in liver homogenate. Statistical analyses provided the mean ± SE (n = 8/group); *P < 0.05, **P < 0.01, ***P < 0.001.
Wnt2b is mainly produced by hepatocytes during liver fibrogenesis
To identify the main cellular source of Wnt2b in fibrotic livers, hepatocytes and non-parenchymal cells (NPCs) were isolated from TAA-challenged mice and controls, respectively. As shown in Fig. 2a, the expression of Wnt2b was markedly up-regulated in liver parenchyma obtained from fibrotic mice compared to controls (left), while Wnt2b was slight elevated in NPCs (right). Moreover, when primary hepatocytes isolated from healthy control mice were challenged with hepatotoxic chemicals in vitro, both the mRNA and protein levels of Wnt2b were increased in a dose-dependent manner (Fig. 2b), suggesting hepatocytes were the primary cellular source of Wnt2b within fibrotic livers. We further analyzed the changes of Wnt2b in HSCs. As shown in Fig. 2c, the level of Wnt2b in HSCs obtained from fibrotic mice was higher than controls. As quiescent HSCs isolated from healthy control mice were cultured for 14 days in vitro, these self-activated HSCs (Supplementary Fig. S2) exhibited higher expression of Wnt2b than the quiescent phenotype (1 d) (Fig. 2d), indicating the levels of Wnt2b in activated HSCs were also increased during liver fibrogenesis.
Wnt2b is mainly produced by hepatocytes during liver fibrogenesis. (a) mRNA and protein levels of Wnt2b in hepatocytes (left) and NPCs (right) from control and TAA-treated mice, respectively. (b) mRNA (upper) and protein (lower) levels of Wnt2b in primary mouse hepatocytes treated with CCl4 in vitro. (c) Immunofluorescence staining of Wnt2b in HSCs from control and TAA-treated mice. (d) RT-PCR analysis of Wnt2b in cultured HSCs from naive mice at the indicated points of time. Mouse embryonic tissues served as positive controls. Cropped blots are displayed; Full-length blots are presented in Supplementary Fig. S5. Data are mean ± SEM of three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001.
Wnt2b attenuates the progression of liver fibrosis
Next, we evaluated whether the level of hepatic Wnt2b expression influenced the degree of liver fibrosis. As shown in Fig. 3a,b, the level of Wnt2b in livers from TAA-challenged mice was knockdown or upregulated by hydrodynamic injection (HD) of shRNA targeting mouse Wnt2b (sh-Wnt2b) and Wnt2b-overexpression plasmid (pRK5-mWnt2b), respectively. Interestingly, sh-Wnt2b markedly promoted total hepatic collagen deposition and CD45 positive cell infiltration, indicating that the decrease in Wnt2b expression accelerated fibrosis progression24, 25 (Fig. 3c and Supplementary Fig. S3b). Consistently, an enhanced activation of HSCs was observed in Wnt2b-silencing livers (Fig. 3d), characterized by an increased expression of α-SMA. In contrast to Wnt2b silencing, Wnt2b overexpression resulted in decreased hepatic collagen deposition (Fig. 3e) and CD45 positive cell infiltration (Supplementary Fig. S3c), as well as a marked down-regulation of α-SMA (Fig. 3f). These results collectively suggested that Wnt2b attenuates the progression of liver fibrosis.
Wnt2b protects against liver fibrosis. (a) The schedule for Wnt2b knockdown with shRNA targeting mouse Wnt2b (sh-Wnt2b) or Wnt2b-overexpression with plasmid pRK5-mWnt2b (Over-Wnt2b) under TAA treatment. (b) The effects of sh-Wnt2b (left) and pRK5-mWnt2b (right) in TAA-challenged mice livers. (c) H&E and Sirius Red staining, Western blotting of Collagen-I, (d) Western blotting (upper) and immunofluorescence staining of α-SMA (lower) for liver tissues from mice treated with sh-Wnt2b or control vector. (e) H&E and Sirius Red staining, Western blotting of Collagen-I, (f ) Western blotting (upper) and immunofluorescence staining of α-SMA (lower) for liver tissues from mice treated with pRK5-mWnt2b construct or control vector. Cropped blots are displayed; Full-length blots are presented in Supplementary Fig. S5. Statistical analysis are mean ± SE (n = 6/group); *P < 0.05, **P < 0.01, ***P < 0.001.
Wnt2b exerts a direct inhibitory effect on HSCs activation
Since most of the receptors for Wnt signaling, such as Fzd4 and Fzd726, 27, were confirmed to be presented in HSCs, we analyzed whether Wnt signaling is involved in HSCs activation. As shown in Fig. 4a, Fzd4 and Fzd7 receptors were increased in activated HSCs compared to the quiescent phenotype, indicating a direct regulation of Wnt2b signaling on HSCs. To confirm the influence of Wnt2b on HSCs in vitro, an immortalized human HSC line LX2 was then used. As shown in Fig. 4b, the expressions of α-SMA and Collagen-Ι were markedly inhibited in LX2 cells cultured with the conditioned medium (CM) from Wnt2b-overexpressing HEK293 cells. Additionally, as LX2 cells transfected with Wnt2b-overexpressing vector, both α-SMA and Collagen-Ι were down-regulated (Fig. 4c). These findings indicated a direct inhibitory effect of Wnt2b on HSCs activation.
Wnt2b exerts a direct inhibitory effect on HSCs activation. (a) RT-PCR analysis of Fzds and LRP5/6 in cultured HSCs from naive mice at the indicated points of time. Mouse embryonic tissues served as positive controls. (b,c) mRNA and protein levels of α-SMA and Collagen-I in LX2 cells cultured in conditioned medium (CM) collected from HEK293 cells transfected with active Wnt2B-V5 (CM-Wnt2b) or control plasmid (CM-Control) (b), as well as in LX2 cells transfected with active Wnt2B-V5 or control plasmid (c). Cropped blots are displayed; Full-length blots are presented in Supplementary Fig. S6. Data are mean ± SEM of three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001.
Wnt2b suppresses TLR4 activation-mediated pro-fibrogenic effects
The activation of TLR4 signaling has been demonstrated to be crucial in HSCs activation and fibrosis progression. Consistent with the publications10, 28, the small intestinal bacterial overgrowth (SIBO) and bacterial translocation were observed in WT mice fibrotic models (Fig. 5a). Moreover, liver fibrosis was more difficult to be induced in TLR4−/− mice than in WT mice, characterized by lower deposition of collagen (Fig. 5b). Notably, as shown in Fig. 5c, the inhibition of TLR4 pathway with TAK242 abrogated the exacerbated fibrosis effect mediated by silencing Wnt2b. In vitro studies, LPS exposure increased α-SMA and Collagen-Ι expressions in LX2 cells in a dose-dependent manner (Fig. 5d), and such pro-fibrogenic effects were markedly attenuated by the presence of Wnt2b (Fig. 5e). In addition, TLR4 signaling was shown to sensitize HSCs to TGF-β stimulation11. As shown in Fig. 5f, at the suboptimal dose29, TGF-β alone could not induce marked activation of HSCs, while the pre-treatment of LPS enhanced the response of LX2 cells to TGF-β, resulting in strong induction of α-SMA and Collagen-Ι. Importantly, Wnt2b afforded the resistance of LX2 cells to LPS-mediated TGF-β sensitization (Fig. 5g). These results demonstrated a negative regulation of Wnt2b on TLR4-mediated pro-fibrogenic effects, indicating that Wnt2b attenuated HSCs activation and fibrosis progression by suppressing TLR4 pathway.
Wnt2b suppresses TLR4 activation-mediated pro-fibrogenic effects. (a) Representative images for bacterial growth of jejunum (left) and liver tissues (right) after cultivation on Blood Agar Plates. (b) Representative H&E (upper) and Sirius Red staining (lower) of liver tissues from WT mice and TLR4 −/− mice after 12 intraperitoneal injections of TAA. (c) H&E and Sirius Red staining,Western blotting of α-SMA in liver tissues from mice treated with TAA alone, or combined with sh-Wnt2b construct/TLR4 inhibitor TAK242 (4 mg/kg, i.p.), or all of the three factors given above in combination for 4 weeks. (d) Protein levels of α-SMA and Collagen-I in LX2 cells stimulated with LPS (10, 100 ng/ml) for 24 h. (e) Effects of Wnt2b on the α-SMA and Collagen-I expressions in LX2 cells stimulated with LPS (100 ng/ml). (f) Protein levels of α-SMA and Collagen-I in LX2 cells stimulated with LPS (100 ng/ml) or vehicle for 24 h, and TGF-β (500 pg/ml) or vehicle for an additional 48 h. (g) LX2 cells were transfected with active Wnt2B-V5 or control plasmids for 24 h, followed by treatment with LPS ± TGF-β as described in Fig. 5f. The expression of α-SMA and Collagen-I were then detected by Western blot analysis. Cropped blots are displayed; Full-length blots are presented in Supplementary Fig. S7. Statistical analysis provided the mean ± SE (n = 6/group), *P < 0.05, **P < 0.01, ***P < 0.001.
Wnt2b disturbs TLR4 signaling transduction
Subsequently, we wanted to understand the mechanism underlying the inhibitory effect of Wnt2b on TLR4 pathway. We observed the expressions of TLR4 and NF-κB signal transduction were reduced in LX2 cells treated with Wnt2b-CM (Fig. 6a), as well as in LX2 cells transfected with Wnt2b-overexpressing vector (Fig. 6b). Furthermore, the phosphorylation of TLR4 signaling transduction-associated molecules mitogen-activated protein (MAP) kinases30, 31, including c-Jun N-terminal protein kinase (JNK), extracellular-regulated kinase (ERK) and P38, was also suppressed in the presence of Wnt2b (Fig. 6c,d). In the parallel in vivo experiments, the expressions of TLR4 and TLR4-related signaling intermediates in tissues from fibrotic Wnt2b-overexpressing livers were downregulated compared to WT controls, whereas TLR4 signaling transduction was augmented in Wnt2b-silencing livers (Fig. 6e,f). Meanwhile, Wnt2b overexpression inhibited expression and nuclear translocation of NF-κB in HSCs compared to control, whereas Wnt2b silencing promoted the activation of NF-κB in HSCs (Fig. 6g). These findings collectively suggested that Wnt2b disturbed TLR4 signaling transduction though modulating the expression of TLR4 as well as the activation of TLR4-related signaling intermediates.
Wnt2b disturbs the TLR4 signaling transduction. (a,b) Protein levels of TLR4, p-NF-κB p65 (Ser536) and NF-κB p65 (upper), and mRNA levels of RELA and TNF (lower) in LX2 cells cultured in Wnt2b-CM (a), as well as in LX2 cells transfected with active Wnt2B-V5 or control plasmids (b). (c,d) Western blotting of the phosphorylation of MAPKs in LX2 cells cultured in Wnt2b-CM (c), and in LX2 cells transfected with active Wnt2B-V5 or control plasmids (d). (e,f ) Protein levels of TLR4, p-NF-κB p65 (Ser536) and NF-κB p65 (e), and mRNA levels of RELA and TNF (f ) in liver tissues from mice challenged with TAA combined with HD injection of pRK5-mWnt2b/sh-Wnt2b construct or control vector as depicted in Fig. 3. (g) Immunofluorescence staining of NF-κB p65 in HSCs isolated from mice challenged with TAA combined with HD injection of pRK5-mWnt2b (upper) / sh-Wnt2b (lower) construct or control vector as depicted in Fig. 3. Cropped blots are displayed; Full-length blots are presented in Supplementary Fig. S8. Statistical analysis provided the mean ± SE (n = 6/group), *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The activation of TLR4 has a prominent role in driving HSC activation and fibrogenesis10, 11. As the first extraintestinal organ to encounter portal venous blood from the gut, liver is prone to exposure to microbial components. Especially, the abnormal quantities of bacterial products could translocate from the gut lumen into the liver because of the increased intestinal permeability under liver injury14, 28. Elevations of LPS and TLR4 activation have shown to be not only a feature of end-stage liver disease32, 33, but also actively promote HSCs activation and influence disease progression even at the initial stage34. Therefore, the investigation for the potential inhibitors of TLR4 represents an attractive strategy for the treatment or prevention of chronic liver disease. The roles of Wnt2b in liver physiology have been thoroughly described, while its significance in hepatic inflammation and fibrosis remains undefined. In the current study, we found Wnt2b was markedly up-regulated in fibrotic livers and acted as an endogenous inhibitor of liver fibrogenesis. Stress-induced Wnt2b exerts a suppressive effect on TLR4 pathway, by which Wnt2b protected against HSCs activation and fibrosis progression. Wnt2b was not only to directly suppress LPS-induced HSCs activation, but also shown to inhibit TLR4-enhanced the sensitivity of HSCs to TGF-β, an important mediator of hepatic fibrosis35. Similar to Seki et al. reported11, we found TLR4 activation could sensitize HSCs to TGF-β stimulation. Pre-treatment of LPS enhanced the response of LX2 cells to a suboptimal dose of TGF-β29, while Wnt2b was able to induce the resistance of LX2 cells to LPS-mediated TGF-β sensitization. The mechanism for the inhibitory effect of Wnt2b on TLR4-mediated pro-fibrogenic effects might relate to the following findings that Wnt2b suppressed the expression of TLR4 and the activation of TLR4 downstream signal transduction, including NF-κB and MAPKs. TLR4 induced the enhancement of HSCs activation to TGF-β in an NF-κB-dependent manner11. Accordingly, inhibition of NF-κB by Wnt2b could largely cancel the sensitivity of HSCs to TGF-β. In addition, by analyzing the 5′-regulatory region of TLR4 gene, we identified the putative binding sites for the transcription factor activator protein-1 (AP-1) and NF-κB at the upstream of the transcription start site in TLR4 promoter. Since MAPKs could be activated by LPS stimulation and in turn promote the transcription of AP-1 and NF-κB36, 37, a TLR4-MAPKs-AP-1/NF-κB positive feedback loop might exist and be involved in HSCs activation. Here, Wnt2b was shown to inhibit the activation of MAPKs in HSCs, raising the possibility that Wnt2b broke TLR4-boosted transcriptional positive feedback. However, the details of the mechanism underlying the inhibitory effect of Wnt2b on TLR4 pathway need to be determined through more exact experiments. And, the possible correlation between Wnt2b and TLR4 in the progression of fibrosis-related liver diseases will be continued in our future work. In addition to HSCs, hepatocytes also expressed most of the receptors for Wnt signaling (data not shown). Although serum ALT levels were not differentially changed by Wnt2b-overexpressing/silencing (Supplementary Fig. S4), suggesting that the exacerbated fibrosis effect mediated by Wnt2b silencing might not be directly associated with hepatocytes injury38, further investigation on the roles of hepatocytes on liver fibrosis is still important in the future39. Nonetheless, the current study expanded our understanding of the complex relationship between the Wnt pathway and the development of fibrosis. Significantly, Wnt2b was identified as a novel endogenous TLR4 suppressor, which displayed suppressive effects on HSCs activation and fibrosis progression (Fig. 7). These findings provided a potential therapeutic candidate which could be suitable for restoring tissue homeostasis during the early stage of liver disease, and indicated a possible role for Wnt2b in sterile inflammation and inflammation-related oncogenesis.
Materials and Methods
General methodology
General methodology such as H&E staining, Immunohistochemistry analysis, Sirius red staining, and Isolation of primary HSCs were described in the supplementary materials. The information of the patients and control subjects was available in the Supplementary Table 1. The source of Abs used was listed in Supplementary Table 2. The RT-PCR and qPCR primers used were shown in Supplementary Tables 3 and 4, respectively.
Ethics Statement
All animal study proposal and protocol were approved by Ethical Committee of Shandong University (License No: LL-201602065). Animal care provided was conformed to the principles in “Guide for the Care and Use of Laboratory Animals” (NIH, 1996). All animal experimental protocols were performed in accordance with “Regulations for the Administration of Affairs Concerning Experimental Animals” (Approved by State Science and Technology Commission, People’s Republic of China, 10/31/1988). For the studies on human tissue samples, the Tissue microarrays (TMAs) were constructed by Shanghai Biochip Co., Ltd. and AlenaBio Co., Ltd., approved by Ethical Committee of Zhejiang province Taizhou Hospital and Henan province Tongxu People’s Hospital. The methods were carried out in accordance with the approved guidelines. All written informed consents were obtained from all subjects in this study.
Animals and Models of Fibrosis
Pathogen-free male C57BL/6 mice (4–6 weeks) were obtained from HuaFuKang Biological Technology Co., Ltd. (Beijing, China). C57BL/6-derived TLR4-knockout mice (TLR4 −/−, male, 4–6 weeks)40 were kindly provided by S.B. Sun (Sun Yat-Sen University, Guangdong, China). Chronic hepatocellular stress-dependent liver fibrosis was induced by 12 intraperitoneal injections of thioacetamide (TAA) (T104039, Aladdin, Shanghai, China) at 200 mg/kg or 8 such injections of carbon tetrachloride (CCl4) (Fuyu, Tianjin, China) at 0.8 ml/kg41.
Cell Line and Reagents
LX-2, an immortalized human HSC line with a stable phenotype and biochemical characteristics of activated HSCs42, was verified by checking the ICLAC and NCBI databases and used for studies in vitro. LPS was purchased from Sigma-Aldrich (L2630, St. Louis, MO, U.S.). Recombinant human TGF-β1 was purchased from PeproTech (100–21C, Rocky Hill, U.S.). TLR4 inhibitor (TAK-242) was purchased from Calbiochem (614316, Darmstadt, Germany). NF-κB inhibitor (BAY11–7082) was purchased from Beyotime Biotechnology (S1523, Shanghai, China).
Mouse Plasmids and Hydrodynamic Injection
pRK5-mWnt2b encoding murine Wnt2b was provided by Chris Garcia and Jeremy Nathans (Addgene plasmid # 42275)43. The shRNA plasmid targeting mouse Wnt2b was purchased from OriGene (Gene ID 22414, Beijing, China). Plasmids were diluted in 2 ml saline (0.9% NaCl) for each mouse, and then hydrodynamically injected through the caudal vein within 8 to 10 s. The efficacy of plasmids delivering into the livers was examined by Western blot.
Human Plasmids and Cell Transfection
Active Wnt2B-V5 encoding homonine Wnt2b was a gift from Xi He (Addgene plasmid # 43808)44. LX-2 cells were plated at 5 × 105 cells/well in 6-well plates, and the transfection was performed with Lipofectamine 2000 (11668–019, InvitrogenTM, CA, U.S.) in accordance with the manufacturer’s instructions. Cells were analyzed 24 h after transfection unless stated otherwise.
Statistical analysis
Data were presented as the mean ± standard error of the mean (SEM). All the in vitro experiments were performed at least three times independently. In vivo studies, mice were randomly assigned to each group and the minimum number of mice used for each assay was three. Statistical analysis between two groups was performed using Student’s t-test and analysis between multiple groups was analyzed by one-way ANOVA with Bonferroni correction. Differences were considered statistically significant at P value < 0.05. (*P value < 0.05; **P value < 0.01; ***P value < 0.001).
Data Availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nature reviews. Immunology 14, 181–194, doi:10.1038/nri3623 (2014).
Friedman, S. L. Evolving challenges in hepatic fibrosis. Nature reviews. Gastroenterology & hepatology 7, 425–436, doi:10.1038/nrgastro.2010.97 (2010).
Schuppan, D. & Kim, Y. O. Evolving therapies for liver fibrosis. The Journal of clinical investigation 123, 1887–1901, doi:10.1172/JCI66028 (2013).
Liang, S. et al. Novel fate-tracing strategies show that hepatic stellate cells mediate fibrosis in vivo. Gastroenterology 146, 1823–1825, doi:10.1053/j.gastro.2014.04.010 (2014).
Tacke, F. & Trautwein, C. Mechanisms of liver fibrosis resolution. Journal of hepatology 63, 1038–1039, doi:10.1016/j.jhep.2015.03.039 (2015).
Ebrahimkhani, M. R. et al. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nature medicine 17, 1668–1673, doi:10.1038/nm.2490 (2011).
Poelstra, K. Liver fibrosis in 2015: Crucial steps towards an effective treatment. Nature reviews. Gastroenterology & hepatology 13, 67–68, doi:10.1038/nrgastro.2015.224 (2016).
Darnaud, M., Faivre, J. & Moniaux, N. Targeting gut flora to prevent progression of hepatocellular carcinoma. Journal of hepatology 58, 385–387, doi:10.1016/j.jhep.2012.08.019 (2013).
Paik, Y. H. et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 37, 1043–1055, doi:10.1053/jhep.2003.50182 (2003).
Pradere, J. P., Troeger, J. S., Dapito, D. H., Mencin, A. A. & Schwabe, R. F. Toll-like receptor 4 and hepatic fibrogenesis. Seminars in liver disease 30, 232–244, doi:10.1055/s-0030-1255353 (2010).
Seki, E. et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nature medicine 13, 1324–1332, doi:10.1038/nm1663 (2007).
Chen, Y. et al. MicroRNA-146a-5p negatively regulates pro-inflammatory cytokine secretion and cell activation in lipopolysaccharide stimulated human hepatic stellate cells through inhibition of toll-like receptor 4 signaling pathways. International journal of molecular sciences 17, 1076, doi:10.3390/ijms17071076 (2016).
Roderburg, C. et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 53, 209–218, doi:10.1002/hep.23922 (2011).
Mencin, A., Kluwe, J. & Schwabe, R. F. Toll-like receptors as targets in chronic liver diseases. Gut 58, 704–720, doi:10.1136/gut.2008.156307 (2009).
Huang, H. et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology 46, 297–306, doi:10.1002/hep.21695 (2007).
Dhillon, N. et al. A single nucleotide polymorphism of Toll-like receptor 4 identifies the risk of developing graft failure after liver transplantation. Journal of hepatology 53, 67–72, doi:10.1016/j.jhep.2009.12.044 (2010).
Poulain, M. & Ober, E. A. Interplay between Wnt2 and Wnt2bb controls multiple steps of early foregut-derived organ development. Development 138, 3557–3568, doi:10.1242/dev.055921 (2011).
Liu, D. et al. Adenoviral vector expressing short hairpin RNA targeting Wnt2B has an effective antitumour activity against Wnt2B2-overexpressing tumours. European Journal of Cancer 48, 1208–1218, doi:10.1016/j.ejca.2011.05.003 (2012).
Zhou, J. B., Yang, J. K., Zhang, B. H. & Lu, J. Interaction of Wnt pathway related variants with type 2 diabetes in a Chinese Han population. PeerJ 3, 1304, doi:10.7717/peerj.1304 (2015).
Liu, B. L. et al. The Protective effects of curcumin on obesity-related glomerulopathy are associated with inhibition of Wnt/β-Catenin signaling activation in podocytes. Evidence-based complementary and alternative medicine 2015, 827472, doi:10.1155/2015/827472 (2015).
Ober, E. A., Verkade, H., Field, H. A. & Stainier, D. Y. Mesodermal Wnt2b signalling positively regulates liver specification. Nature 442, 688–691, doi:10.1038/nature04888 (2006).
Choi, T. Y., Ninov, N., Stainier, D. Y. & Shin, D. Extensive conversion of hepatic biliary epithelial cells to hepatocytes after near total loss of hepatocytes in zebrafish. Gastroenterology 146, 776–788, doi:10.1053/j.gastro.2013.10.019 (2014).
Shackel, N. A., McGuinness, P. H., Abbott, C. A., Gorrell, M. D. & McCaughan, G. W. Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression. Gut 49, 565–576, doi:10.1136/gut.49.4.565 (2001).
Robinson, M. W., Harmon, C. & O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cellular & molecular immunology 13, 267–276, doi:10.1038/cmi.2016.3 (2016).
Chen, Y. W. et al. Preservation of basal AcSDKP attenuates carbon tetrachloride-induced fibrosis in the rat liver. Journal of hepatology 53, 528–536, doi:10.1016/j.jhep.2010.03.027 (2010).
Ohta, K. et al. Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4. Proceedings of the National Academy of Sciences of the United States of America 108, 14962–14967, doi:10.1073/pnas.1100513108 (2011).
Flanagan, D. J. et al. Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5(+) stem cells. Stem cell reports 4, 759–767, doi:10.1016/j.stemcr.2015.03.003 (2015).
Abhilash, P. A., Harikrishnan, R. & Indira, M. Ascorbic acid suppresses endotoxemia and NF-κB signaling cascade in alcoholic liver fibrosisin guinea pigs: a mechanistic approach. Toxicology and applied pharmacology 274, 215–224, doi:10.1016/j.taap.2013.11.005 (2014).
Fabre, T., Kared, H., Friedman, S. L. & Shoukry, N. H. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. The Journal of immunology 193, 3925–3933, doi:10.4049/jimmunol.1400861 (2014).
Liu, J. & Cao, X. Cellular and molecular regulation of innate inflammatory responses. Cellular & molecular immunology 13, 711–721, doi:10.1038/cmi.2016.58 (2016).
Whitmarsh, A. J. & Davis, R. J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. Journal of molecular medicine 74, 589–607 (1996).
Vespasiani-Gentilucci, U. et al. Hepatic toll-like receptor 4 expression is associated with portal inflammation and fibrosis in patients with NAFLD. Liver international 35, 569–581, doi:10.1111/liv.12531 (2015).
Wiest, R., Lawson, M. & Geuking, M. Pathological bacterial translocation in liver cirrhosis. Journal of hepatology 60, 197–209, doi:10.1016/j.jhep.2013.07.044 (2014).
Zhu, Q. et al. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. Journal of hepatology 56, 893–899, doi:10.1016/j.jhep.2011.11.013 (2012).
Fabregat, I. et al. TGF-β signalling and liver disease. The FEBS Journal 283, 2219–2232, doi:10.1111/febs.13665 (2016).
Tomalka, J. A., de Jesus, T. J. & Ramakrishnan, P. Sam68 is a regulator of Toll-like receptor signaling. Cellular & molecular immunology 14, 107–117, doi:10.1038/cmi.2016.32 (2017).
Guha, M. & Mackman, N. LPS induction of gene expression in human monocytes. Celluar signalling 13, 85–94, doi:10.1016/S0898-6568(00)00149-2 (2001).
Lee, W. Y., Salmi, M., Kelly, M. M., Jalkanen, S. & Kubes, P. Therapeutic advantage of anti-VAP-1 over anti-α4 integrin antibody in concanavalin a-induced hepatitis. Hepatology 58, 1413–1423, doi:10.1002/hep.26469 (2013).
Zhou, Z., Xu, M. J. & Gao, B. Hepatocytes: a key cell type for innate immunity. Cellular & molecular immunology 13, 301–315, doi:10.1038/cmi.2015.97 (2016).
Su, S. B. et al. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. The Journal of immunology 175, 6303–6310, doi:10.4049/jimmunol.175.10.6303 (2005).
McHedlidze, T. et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 39, 357–371, doi:10.1016/j.immuni.2013.07.018 (2013).
Xu, L. et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 54, 142–151, doi:10.1136/gut.2004.042127 (2005).
Yu, H., Ye, X., Guo, N. & Nathans, J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development 139, 4383–4394, doi:10.1242/dev.083352 (2012).
MacDonald, B. T. et al. Disulfide bond requirements for active Wnt ligands. The Journal of biological chemistry 289, 18122–18136, doi:10.1074/jbc.M114.575027 (2014).
Acknowledgements
All authors are very grateful to the principal investigators for the constructs we obtained from addgene. We are also very thankful to Dr. S.B. Sun for donating the TLR4 −/− mice. This work was supported by grants from National Basic Research Program of China (No. 2013CB531503), Natural Science Foundation of China (No.81172789, No.30972692).
Author information
Authors and Affiliations
Contributions
Jian Zhang, Zhigang Tian, Qiuju Han, and Yi Yuan conceived and designed the experiments. Yi Yuan and Siyu Li performed the experiments and analyzed the data. Yi Yuan wrote the manuscript. Jian Zhang revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, Y., Han, Q., Li, S. et al. Wnt2b attenuates HSCs activation and liver fibrosis through negative regulating TLR4 signaling. Sci Rep 7, 3952 (2017). https://doi.org/10.1038/s41598-017-04374-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04374-5
This article is cited by
-
Hsa_circ_0008360 promotes high glucose-induced damage in HK-2 cells via miR-346/WNT2B axis
Journal of Endocrinological Investigation (2024)
-
ADAM12 is an independent predictor of poor prognosis in liver cancer
Scientific Reports (2022)
-
The Anti-fibrotic Effects of Heat-Killed Akkermansia muciniphila MucT on Liver Fibrosis Markers and Activation of Hepatic Stellate Cells
Probiotics and Antimicrobial Proteins (2021)
-
Long non-coding RNA CDKN2B-AS1 regulates high glucose-induced human mesangial cell injury via regulating the miR-15b-5p/WNT2B axis
Diabetology & Metabolic Syndrome (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.