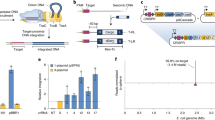
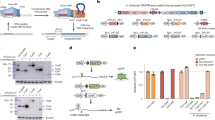
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPR)-associated transposases have the potential to transform the technology landscape for kilobase-scale genome engineering, by virtue of their ability to integrate large genetic payloads with high accuracy, easy programmability and no requirement for homologous recombination machinery. These transposons encode efficient, CRISPR RNA-guided transposases that execute genomic insertions in Escherichia coli at efficiencies approaching ~100%. Moreover, they generate multiplexed edits when programmed with multiple guides, and function robustly in diverse Gram-negative bacterial species. Here we present a detailed protocol for engineering bacterial genomes using CRISPR-associated transposase (CAST) systems, including guidelines on the available vectors, customization of guide RNAs and DNA payloads, selection of common delivery methods, and genotypic analysis of integration events. We further describe a computational CRISPR RNA design algorithm to avoid potential off-targets, and a CRISPR array cloning pipeline for performing multiplexed DNA insertions. The method presented here allows the isolation of clonal strains containing a novel genomic integration event of interest within 1–2 weeks using available plasmid constructs and standard molecular biology techniques.
Key points
-
The protocol describes a novel and versatile CRISPR-associated transposase (CAST) system for the targeted and precise insertion of large DNA payloads into bacterial genomes.
-
Compared with pre-existing methods, this approach allows single and multiplexed insertion events at a desired location, with increased efficiency, reduced population heterogeneity, and improved specificity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Next-generation sequencing (NGS) data used for Figs. 6 and 7 are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (BioProject accession code PRJNA668381). Published genomes used for Tn-seq analyses in Fig. 6 were obtained from the NCBI (accessions codes CP001509.3).
Code availability
The CAST guide RNA design tool and associated documentation are available online via GitHub (https://github.com/sternberglab/CAST-guide-RNA-tool). Custom Python scripts used for the described Tn-seq NGS data analyses used in Fig. 6 are available online via GitHub (https://github.com/sternberglab/Vo_etal_2020).
References
Call, S. N. & Andrews, L. B. CRISPR-based approaches for gene regulation in non-model bacteria. Front. Genome Ed. 4, 892304 (2022).
Lu, L. et al. CRISPR-based metabolic engineering in non-model microorganisms. Curr. Opin. Biotechnol. 75, 102698 (2022).
Ploessl, D., Zhao, Y. & Shao, Z. Engineering of non-model eukaryotes for bioenergy and biochemical production. Curr. Opin. Biotechnol. 79, 102869 (2023).
Gudmunds, E., Wheat, C. W., Khila, A. & Husby, A. Functional genomic tools for emerging model species. Trends Ecol. Evol. 37, 1104–1115 (2022).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Jinek, M. et al. RNA-programmed genome editing in human cells. eLife 2, e00471 (2013).
Cho, S. W., Kim, S., Kim, J. M. & Kim, J.-S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Sander, J. D. & Joung, J. K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
Wang, H., La Russa, M. & Qi, L. S. CRISPR/Cas9 in genome editing and beyond. Annu. Rev. Biochem. 85, 227–264 (2016).
Vento, J. M., Crook, N. & Beisel, C. L. Barriers to genome editing with CRISPR in bacteria. J. Ind. Microbiol. Biotechnol. 46, 1327–1341 (2019).
Fels, U., Gevaert, K. & Van Damme, P. Bacterial genetic engineering by means of recombineering for reverse genetics. Front. Microbiol. 11, 548410 (2020).
Corts, A., Thomason, L. C., Costantino, N. & Court, D. L. Recombineering in non-model bacteria. Curr. Protoc. 2, e605 (2022).
Pyne, M. E., Moo-Young, M., Chung, D. A. & Chou, C. P. Coupling the CRISPR/Cas9 system with Lambda red recombineering enables simplified chromosomal gene replacement in Escherichia coli. Appl. Environ. Microbiol. 81, 5103–5114 (2015).
Reisch, C. R. & Prather, K. L. J. The no-SCAR (Scarless Cas9 Assisted Recombineering) system for genome editing in Escherichia coli. Sci. Rep. 5, 15096 (2015).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR–Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Ikeda, K. et al. Efficient scarless genome editing in human pluripotent stem cells. Nat. Methods 15, 1045–1047 (2018).
Elison, G. L. & Acar, M. Scarless genome editing: progress towards understanding genotype–phenotype relationships. Curr. Genet. 64, 1229–1238 (2018).
Pines, G., Freed, E. F., Winkler, J. D. & Gill, R. T. Bacterial recombineering: genome engineering via phage-based homologous recombination. ACS Synth. Biol. 4, 1176–1185 (2015).
Filsinger, G. T. et al. Characterizing the portability of phage-encoded homologous recombination proteins. Nat. Chem. Biol. 17, 394–402 (2021).
Krishnamurthy, M., Moore, R. T., Rajamani, S. & Panchal, R. G. Bacterial genome engineering and synthetic biology: combating pathogens. BMC Microbiol. 16, 258 (2016).
Charbonneau, M. R., Isabella, V. M., Li, N. & Kurtz, C. B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 11, 1738 (2020).
Fata Moradali, M. & Rehm, B. H. A. in Biopolymers for Biomedical and Biotechnological Applications https://doi.org/10.1002/9783527818310.ch3 (Wiley-Vch, 2021).
McLoughlin, A. J. Plasmid stability and ecological competence in recombinant cultures. Biotechnol. Adv. 12, 279–324 (1994).
Craig, N. L. Tn7: a target site-specific transposon. Mol. Microbiol. 5, 2569–2573 (1991).
Shi, Q. et al. Conformational toggling controls target site choice for the heteromeric transposase element Tn7. Nucleic Acids Res. 43, 10734–10745 (2015).
Herrmann, S. et al. Site-specific recombination strategies for engineering actinomycete genomes. Appl. Environ. Microbiol. 78, 1804–1812 (2012).
van Duyne, G. D. in Mobile DNA III https://doi.org/10.1128/9781555819217.ch5 (ASM Press, 2015).
Cain, A. K. et al. A decade of advances in transposon-insertion sequencing. Nat. Rev. Genet. 21, 526–540 (2020).
van Opijnen, T., Bodi, K. L. & Camilli, A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods 6, 767–772 (2009).
Vo, P. L. H. et al. CRISPR RNA-guided integrases for high-efficiency, multiplexed bacterial genome engineering. Nat. Biotechnol. https://doi.org/10.1038/s41587-020-00745-y (2020).
Klompe, S. E., Vo, P. L. H., Halpin-Healy, T. S. & Sternberg, S. H. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature 571, 219–225 (2019).
Peters, J. E., Makarova, K. S., Shmakov, S. & Koonin, E. V. Recruitment of CRISPR–Cas systems by Tn7-like transposons. Proc. Natl Acad. Sci. USA 114, E7358–E7366 (2017).
Zhang, Y. et al. Programming cells by multicopy chromosomal integration using CRISPR-associated transposases. Cris. J. 4, 350–359 (2021).
Chen, W. et al. Targeted genetic screening in bacteria with a Cas12k-guided transposase. Cell Rep. 36, 109635 (2021).
Rubin, B. E. et al. Species- and site-specific genome editing in complex bacterial communities. Nat. Microbiol. 7, 34–47 (2022).
Yang, S. et al. Orthogonal CRISPR-associated transposases for parallel and multiplexed chromosomal integration. Nucleic Acids Res. 49, 10192–10202 (2021).
Saito, M. et al. Dual modes of CRISPR-associated transposon homing. Cell 184, 2441–2453.e18 (2021).
Halpin-Healy, T. S., Klompe, S. E., Sternberg, S. H. & Fernández, I. S. Structural basis of DNA targeting by a transposon-encoded CRISPR–Cas system. Nature 577, 271–274 (2020).
Klompe, S. E. et al. Evolutionary and mechanistic diversity of Type I-F CRISPR-associated transposons. Mol. Cell 82, 616–628.e5 (2022).
Strecker, J. et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science 365, 48–53 (2019).
Rybarski, J. R., Hu, K., Hill, A. M., Wilke, C. O. & Finkelstein, I. J. Metagenomic discovery of CRISPR-associated transposons. Proc. Natl Acad. Sci. USA 118, e2112279118 (2021).
Hsieh, S.-C. & Peters, J. E. Discovery and characterization of novel Type I-D CRISPR-guided transposons identified among diverse Tn7-like elements in cyanobacteria. Nucleic Acids Res. 51, 765–782 (2023).
Tou, C. J., Orr, B. & Kleinstiver, B. P. Precise cut-and-paste DNA insertion using engineered Type V-K CRISPR-associated transposases. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01574-x (2023).
Vo, P. L. H., Acree, C., Smith, M. L. & Sternberg, S. H. Unbiased profiling of CRISPR RNA-guided transposition products by long-read sequencing. Mob. DNA 12, 13 (2021).
George, J. T. et al. Mechanism of target site selection by type V-K CRISPR-associated transposases. Science 382, eadj8543 (2023).
Schmitz, M., Querques, I., Oberli, S., Chanez, C. & Jinek, M. Structural basis for the assembly of the Type V CRISPR-associated transposon complex. Cell 185, 4999–5010.e17 (2022).
Park, J.-U. et al. Structural basis for target site selection in RNA-guided DNA transposition systems. Science 373, 768–774 (2021).
Peters, J. E. in Mobile DNA III https://doi.org/10.1128/9781555819217.ch30 (ASM Press, 2015).
Hoffmann, F. T. et al. Selective TnsC recruitment enhances the fidelity of RNA-guided transposition. Nature 609, 384–393 (2022).
Skelding, Z., Queen-Baker, J. & Craig, N. L. Alternative interactions between the Tn7 transposase and the Tn7 target DNA binding protein regulate target immunity and transposition. EMBO J. 22, 5904–5917 (2003).
Arciszewska, L. K., Drake, D. & Craig, N. L. Transposon Tn7: cis-acting sequences in transposition and transposition immunity. J. Mol. Biol. 207, 35–52 (1989).
Choi, K. Y., Spencer, J. M. & Craig, N. L. The Tn7 transposition regulator TnsC interacts with the transposase subunit TnsB and target selector TnsD. Proc. Natl Acad. Sci. USA 111, E2858–E2865 (2014).
Yang, J. et al. CRISPR-associated transposase system can insert multiple copies of donor DNA into the same target locus. Cris. J. 4, 789–798 (2021).
Rice, P. A., Craig, N. L. & Dyda, F. Comment on ‘RNA-guided DNA insertion with CRISPR-associated transposases’. Science 368, eabb2022 (2020).
Strecker, J., Ladha, A., Makarova, K. S., Koonin, E. V. & Zhang, F. Response to comment on ‘RNA-guided DNA insertion with CRISPR-associated transposases’. Science 368, eabb2920 (2020).
Tansirichaiya, S., Rahman, M. A. & Roberts, A. P. The transposon registry. Mob. DNA 10, 40 (2019).
Zhang, Y. et al. Multicopy chromosomal integration using CRISPR-associated transposases. ACS Synth. Biol. 9, 1998–2008 (2020).
Petassi, M. T., Hsieh, S.-C. & Peters, J. E. Guide RNA categorization enables target site choice in Tn7–CRISPR–cas transposons. Cell 183, 1757–1771.e18 (2020).
Aziz, R. K., Breitbart, M. & Edwards, R. A. Transposases are the most abundant, most ubiquitous genes in nature. Nucleic Acids Res. 38, 4207–4217 (2010).
Bean, E. L., Herman, C., Anderson, M. E. & Grossman, A. D. Biology and engineering of integrative and conjugative elements: construction and analyses of hybrid ICEs reveal element functions that affect species-specific efficiencies. PLoS Genet. 18, e1009998 (2022).
McKenzie, G. J. & Craig, N. L. Fast, easy and efficient: site-specific insertion of transgenes into Enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol. 6, 39 (2006).
Hartl, D. L. Discovery of the transposable element mariner. Genetics 157, 471–476 (2001).
Lampe, D. J., Akerley, B. J., Rubin, E. J., Mekalanos, J. J. & Robertson, H. M. Hyperactive transposase mutants of the Himar1 mariner transposon. Proc. Natl Acad. Sci. USA 96, 11428–11433 (1999).
Muñoz-López, M. & García-Pérez, J. L. DNA transposons: nature and applications in genomics. Curr. Genomics 11, 115–128 (2010).
Goryshin, I. Y., Miller, J. A., Kil, Y. V., Lanzov, V. A. & Reznikoff, W. S. Tn5/IS50 target recognition. Proc. Natl Acad. Sci. USA 95, 10716–10721 (1998).
Veeranagouda, Y., Husain, F. & Wexler, H. M. Transposon mutagenesis of Bacteroides fragilis using a Mariner transposon vector. Anaerobe 22, 126–129 (2013).
Perry, B. J. & Yost, C. K. Construction of a Mariner-based transposon vector for use in insertion sequence mutagenesis in selected members of the Rhizobiaceae. BMC Microbiol. 14, 298 (2014).
Akerley, B. J. et al. Systematic identification of essential genes by in vitro Mariner mutagenesis. Proc. Natl Acad. Sci. USA 95, 8927–8932 (1998).
Wang, H. H. et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898 (2009).
Wang, H. H. et al. Genome-scale promoter engineering by coselection MAGE. Nat. Methods 9, 591–593 (2012).
Leibig, M. et al. Marker removal in staphylococci via Cre recombinase and different lox sites. Appl. Environ. Microbiol. 74, 1316–1323 (2008).
Fedoryshyn, M., Petzke, L., Welle, E., Bechthold, A. & Luzhetskyy, A. Marker removal from actinomycetes genome using Flp recombinase. Gene 419, 43–47 (2008).
Jensen, S. I. & Nielsen, A. T. Multiplex genome editing in Escherichia coli. Methods Mol. Biol. https://doi.org/10.1007/978-1-4939-7295-1_8 (2018).
Walker, M. W. G., Klompe, S. E., Zhang, D. J. & Sternberg, S. H. Transposon mutagenesis libraries reveal novel molecular requirements during CRISPR RNA-guided DNA integration. Nucleic Acids Res. 51, 4519–4535 (2023).
Stellwagen, A. E. & Craig, N. L. Avoiding self: two Tn7-encoded proteins mediate target immunity in Tn7 transposition. EMBO J. 16, 6823–6834 (1997).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Lampe, G. D. et al. Targeted DNA integration in human cells without double-strand breaks using CRISPR-associated transposases. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01748-1 (2023).
Roberts, A., Nethery, M. A. & Barrangou, R. Functional characterization of diverse type I-F CRISPR-associated transposons. Nucleic Acids Res. 50, 11670–11681 (2022).
Antoine, R. & Locht, C. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from Gram-positive organisms. Mol. Microbiol. 6, 1785–1799 (1992).
Lauritsen, I., Kim, S. H., Porse, A. & Nørholm, M. H. H. Standardized cloning and curing of plasmids. Methods Mol. Biol. https://doi.org/10.1007/978-1-4939-7795-6_28 (2018).
Ronda, C., Chen, S. P., Cabral, V., Yaung, S. J. & Wang, H. H. Metagenomic engineering of the mammalian gut microbiome in situ. Nat. Methods 16, 167–170 (2019).
Llosa, M. & de la Cruz, F. Bacterial conjugation: a potential tool for genomic engineering. Res. Microbiol. 156, 1–6 (2005).
Strand, T. A., Lale, R., Degnes, K. F., Lando, M. & Valla, S. A new and improved host-independent plasmid system for RK2-based conjugal transfer. PLoS One 9, e90372 (2014).
Acknowledgements
We thank S. Pesari for laboratory support, J. Mohabir and S. Acree for assistance with NGS read alignment, and the JP Sulzberger Columbia Genome Center for NGS support. H.H.W. acknowledges funding support from the National Science Foundation (MCB-2025515), National Institutes of Health (1R01EB031935, 2R01AI132403, 1R01DK118044 and 1R21AI146817), Burroughs Wellcome Fund (1016691) and Department of Defense Army Research Office (W911NF-22-2-0210). D.R.G. is supported by the Burroughs Wellcome Fund Postdoctoral Diversity Enrichment Program. S.H.S. acknowledges funding support from the National Institutes of Health (DP2HG011650, R21AI168976, R01EB031935, and R01EB027793), the Pew Biomedical Scholars Program, the Alfred Sloan Foundation Research Fellowship, the Irma T. Hirschl Career Scientist Award, and a generous startup package from the Columbia University Irving Medical Center Dean’s Office and the Vagelos Precision Medicine Fund.
Author information
Authors and Affiliations
Contributions
D.R.G., P.L.H.V. and S.H.S. wrote the manuscript. D.R.G., P.L.H.V. and S.E.K. designed the figures, and C.R. and H.H.W. discussed figure ideas, provided information on conjugations, and provided feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
Columbia University has filed patent applications related to this work. S.H.S. is a cofounder and scientific advisor to Dahlia Biosciences, a scientific advisor to CrisprBits and Prime Medicine, and an equity holder in Dahlia Biosciences and CrisprBits. H.H.W. is a scientific advisor of SNIPR Biome, Kingdom Supercultures, Fitbiomics, Arranta Bio, VecX Biomedicines and Genus PLC, and a scientific cofounder of Aclid, none of whom are involved in the study. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Sheng Yang and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Vo, P. L. H. et al. Nat. Biotechnol. 39, 480–489 (2021): https://doi.org/10.1038/s41587-020-00745-y
Hoffmann, F. T. et al. Nature 609, 384–393 (2022): https://doi.org/10.1038/s41586-022-05059-4
Walker, M. W. G. et al. Nucleic Acids Res. 51, 4519–4535 (2023): https://doi.org/10.1093/nar/gkad270
Supplementary information
Supplementary Tables 1–3
Supplementary Tables 1–3
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gelsinger, D.R., Vo, P.L.H., Klompe, S.E. et al. Bacterial genome engineering using CRISPR-associated transposases. Nat Protoc 19, 752–790 (2024). https://doi.org/10.1038/s41596-023-00927-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00927-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.