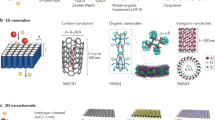
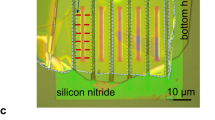
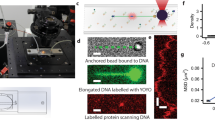
Abstract
Fluidic channels at atomic scales regulate cellular trafficking and molecular filtration across membranes, and thus play crucial roles in the functioning of living systems. However, constructing synthetic channels experimentally at these scales has been a significant challenge due to the limitations in nanofabrication techniques and the surface roughness of the commonly used materials. Angstrom (Å)-scale slit-like channels overcome such challenges as these are made with precise control over their dimensions and can be used to study the fluidic properties of gases, ions and water at unprecedented scales. Here we provide a detailed fabrication method of the two-dimensional Å-scale channel devices that can be assembled to contain a desired number of channels, a single channel or up to hundreds of channels, made with atomic-scale precision using layered crystals. The procedure includes the fabrication of the substrate, flake, spacer layer, flake transfers, van der Waals assembly and postprocessing. We further explain how to perform molecular transport measurements with the Å-channels to directly probe the intriguing and anomalous phenomena that help shed light on the physics governing ultra-confined transport. The procedure requires a total of 1–2 weeks for the fabrication of the two-dimensional channel device and is suitable for users with prior experience in clean room working environments and nanofabrication.
Key points
-
Angstrom-scale fluidic devices enable measurement of ballistic gas flows, ultrafast water permeation, steric ion exclusion and voltage gating of currents. The authors detail the fabrication of the substrate, flake, spacer layer and flake transfers, as well as van der Waals assembly and postprocessing.
-
Compared with nanofluidic devices such as nanotubes, lithographically patterned/etched channels and two-dimensional laminates, angstrom-scale channels have atomically flat, pristine and mechanically robust walls with programmable lengths, widths and heights.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




















Similar content being viewed by others
References
Eijkel, J. C. T. & van den Berg, A. Nanofluidics: what is it and what can we expect from it? Microfluid. Nanofluidics. 1, 249–267 (2005).
Bocquet, L. & Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 39, 1073–1095 (2010).
Convery, N. & Gadegaard, N. 30 years of microfluidics. Micro Nano. Eng. 2, 76–91 (2019).
van den Berg, A., Craighead, H. G. & Yang, P. From microfluidic applications to nanofluidic phenomena. Chem. Soc. Rev. 39, 899–900 (2010).
Agre, P. The aquaporin water channels. Proc. Am. Thorac. Soc. 3, 5–13 (2006).
Israelachvili, J. N. Intermolecular and Surface Forces (Academic Press, 2011).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Deng, H. et al. Multiple functional groups of varying ratios in metal–organic frameworks. Science 327, 846 (2010).
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469 (2002).
Koenig, S. P., Wang, L., Pellegrino, J. & Bunch, J. S. Selective molecular sieving through porous graphene. Nat. Nanotechnol. 7, 728–732 (2012).
Celebi, K. et al. Ultimate permeation across atomically thin porous graphene. Science 344, 289 (2014).
Jiang, D.-E., Cooper, V. R. & Dai, S. Porous graphene as the ultimate membrane for gas separation. Nano Lett. 9, 4019–4024 (2009).
Berezhkovskii, A. & Hummer, G. Single-file transport of water molecules through a carbon nanotube. Phys. Rev. Lett. 89, 064503 (2002).
Lee, C. Y., Choi, W., Han, J.-H. & Strano, M. S. Coherence resonance in a single-walled carbon nanotube ion channel. Science 329, 1320 (2010).
Secchi, E. et al. Massive radius-dependent flow slippage in carbon nanotubes. Nature 537, 210–213 (2016).
Won, C. Y. & Aluru, N. R. Water permeation through a subnanometer boron nitride nanotube. J. Am. Chem. Soc. 129, 2748–2749 (2007).
Siria, A. et al. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 494, 455–458 (2013).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752 (2014).
Nair, R. R., Wu, H. A., Jayaram, P. N., Grigorieva, I. V. & Geim, A. K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 335, 442 (2012).
Liu, G., Jin, W. & Xu, N. Graphene-based membranes. Chem. Soc. Rev. 44, 5016–5030 (2015).
Gogotsi, Y. & Anasori, B. The rise of MXenes. ACS Nano 13, 8491–8494 (2019).
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017).
Anasori, B. et al. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 9, 9507–9516 (2015).
Majumder, M., Chopra, N., Andrews, R. & Hinds, B. J. Enhanced flow in carbon nanotubes. Nature 438, 44 (2005).
Feng, J. et al. Observation of ionic coulomb blockade in nanopores. Nat. Mater. 15, 850–855 (2016).
Ayuk, E., Ugwu, M. & Aronimo, S. B. A review on synthetic methods of nanostructured materials. Chem. Res. J. 2, 97–123 (2017).
Biswas, A. et al. Advances in top-down and bottom-up surface nanofabrication: techniques, applications and future prospects. Adv. Colloid Interface Sci. 170, 2–27 (2012).
Chen, Q. & Liu, Z. Fabrication and applications of solid-state nanopores. Sensors 19, 1886 (2019).
Storm, A., Chen, J., Ling, X., Zandbergen, H. & Dekker, C. Fabrication of solid-state nanopores with single-nanometre precision. Nat. Mater. 2, 537–540 (2003).
Kim, M. J., McNally, B., Murata, K. & Meller, A. Characteristics of solid-state nanometre pores fabricated using a transmission electron microscope. Nanotechnology 18, 205302 (2007).
Lin, Y., Ying, Y.-L., Shi, X., Liu, S.-C. & Long, Y.-T. Direct sensing of cancer biomarkers in clinical samples with a designed nanopore. Chem. Commun. 53, 11564–11567 (2017).
Krapf, D. et al. Fabrication and characterization of nanopore-based electrodes with radii down to 2 nm. Nano Lett. 6, 105–109 (2006).
Lo, C. J., Aref, T. & Bezryadin, A. Fabrication of symmetric sub-5 nm nanopores using focused ion and electron beams. Nanotechnology 17, 3264 (2006).
Gierak, J. et al. Sub-5 nm FIB direct patterning of nanodevices. Microelectron. Eng. 84, 779–783 (2007).
O’Hern, S. C. et al. Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes. Nano Lett. 14, 1234–1241 (2014).
Russo, C. J. & Golovchenko, J. A. Atom-by-atom nucleation and growth of graphene nanopores. Proc. Natl Acad. Sci. USA 109, 5953–5957 (2012).
Walker, M. I. et al. Extrinsic cation selectivity of 2D membranes. ACS Nano 11, 1340–1346 (2017).
Zhou, Z. et al. DNA translocation through hydrophilic nanopore in hexagonal boron nitride. Sci. Rep. 3, 3287 (2013).
Danda, G. et al. Monolayer WS2 nanopores for DNA translocation with light-adjustable sizes. ACS Nano 11, 1937–1945 (2017).
Mojtabavi, M., VahidMohammadi, A., Liang, W., Beidaghi, M. & Wanunu, M. Single-molecule sensing using nanopores in two-dimensional transition metal carbide (MXene) membranes. ACS Nano 13, 3042–3053 (2019).
Murray, D. J. et al. Large area synthesis of a nanoporous two-dimensional polymer at the air/water interface. J. Am. Chem. Soc. 137, 3450–3453 (2015).
Guan, C. Z., Wang, D. & Wan, L. J. Construction and repair of highly ordered 2D covalent networks by chemical equilibrium regulation. Chem. Commun. 48, 2943–2945 (2012).
Dey, K. et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 139, 13083–13091 (2017).
Spitzer, S. et al. Solvent-free on-surface synthesis of boroxine COF monolayers. Chem. Commun. 53, 5147–5150 (2017).
Kambe, T. et al. pi-Conjugated nickel bis(dithiolene) complex nanosheet. J. Am. Chem. Soc. 135, 2462–2465 (2013).
Motoyama, S., Makiura, R., Sakata, O. & Kitagawa, H. Highly crystalline nanofilm by layering of porphyrin metal–organic framework sheets. J. Am. Chem. Soc. 133, 5640–5643 (2011).
Kidambi, P. R. et al. Facile fabrication of large-area atomically thin membranes by direct synthesis of graphene with nanoscale porosity. Adv. Mater. 30, 1804977 (2018).
Liu, J. et al. Hydrophobic, flexible, and lightweight mxene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 29, 1702367 (2017).
Moreno, C. et al. Bottom-up synthesis of multifunctional nanoporous graphene. Science 360, 199–203 (2018).
Shao, J. J., Raidongia, K., Koltonow, A. R. & Huang, J. Self-assembled two-dimensional nanofluidic proton channels with high thermal stability. Nat. Commun. 6, 7602 (2015).
Geim, A. K. & Grigorieva, I. V. Van der Waals heterostructures. Nature 499, 419–425 (2013).
Frisenda, R. et al. Recent progress in the assembly of nanodevices and van der Waals heterostructures by deterministic placement of 2D materials. Chem. Soc. Rev. 47, 53–68 (2018).
Kang, K. et al. Layer-by-layer assembly of two-dimensional materials into wafer-scale heterostructures. Nature 550, 229–233 (2017).
Huang, Y. et al. Emerging magnetic interactions in van der Waals heterostructures. Nano Lett. 20, 7852–7859 (2020).
Pham, D. K. Electronic properties of a two-dimensional van der Waals MoGe2N4/MoSi2N4 heterobilayer: effect of the insertion of a graphene layer and interlayer coupling. RSC Adv. 11, 28659–28666 (2021).
Zhang, Q. et al. Interface nano-optics with van der Waals polaritons. Nature 597, 187–195 (2021).
Radha, B. et al. Molecular transport through capillaries made with atomic-scale precision. Nature 538, 222–225 (2016).
Esfandiar, A. et al. Size effect in ion transport through angstrom-scale slits. Science 358, 511–513 (2017).
Keerthi, A. et al. Ballistic molecular transport through two-dimensional channels. Nature 558, 420–424 (2018).
Gopinadhan, K. et al. Complete steric exclusion of ions and proton transport through confined monolayer water. Science 363, 145–148 (2019).
Mouterde, T. et al. Molecular streaming and its voltage control in ångström-scale channels. Nature 567, 87–90 (2019).
Fumagalli, L. et al. Anomalously low dielectric constant of confined water. Science 360, 1339–1342 (2018).
Yang, W. et al. Translocation of DNA through ultrathin nanoslits. Adv. Mater. 33, 2007682 (2021).
Knudsen, M. Die gesetze der molekularströmung und der inneren reibungsströmung der gase durch röhren. Ann. der Phys. 333, 75–130 (1909).
Smoluchowski, M. V. Zur kinetischen theorie der transpiration und diffusion verdünnter gase. Ann. der Phys. 338, 1559–1570 (1910).
Livesey, R. G. & Lafferty, J. M. Foundations of Vacuum Science and Technology (Wiley, 1998).
Lei, W., Rigozzi, M. K. & McKenzie, D. R. The physics of confined flow and its application to water leaks, water permeation and water nanoflows: a review. Rep. Prog. Phys. 79, 025901 (2016).
Han, Y.-L., Phillip Muntz, E., Alexeenko, A. & Young, M. Experimental and computational studies of temperature gradient–driven molecular transport in gas flows through nano/microscale channels. Nanoscale Microscale Thermophys. Eng. 11, 151–175 (2007).
Scorrano, G. et al. Gas flow at the ultra-nanoscale: universal predictive model and validation in nanochannels of ångstrom-level resolution. ACS Appl. Mater. Interfaces 10, 32233–32238 (2018).
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034 (2006).
Majumder, M., Chopra, N. & Hinds, B. J. Mass transport through carbon nanotube membranes in three different regimes: Ionic diffusion and gas and liquid flow. ACS Nano 5, 3867–3877 (2011).
Steckelmacher, W. A review of the molecular flow conductance for systems of tubes and components and the measurement of pumping speed. Vacuum 16, 561–584 (1966).
Bhatia, S., Bonilla, M. & Nicholson, D. Molecular transport in nanopores: a theoretical perspective. Phys. Chem. Chem. Phys. 13, 15350–15383 (2011).
Cohen-Tanugi, D. & Grossman, J. C. Water desalination across nanoporous graphene. Nano Lett. 12, 3602–3608 (2012).
Heiranian, M., Farimani, A. B. & Aluru, N. R. Water desalination with a single-layer MoS2 nanopore. Nat. Commun. 6, 8616 (2015).
Konatham, D., Yu, J., Ho, T. A. & Striolo, A. Simulation insights for graphene-based water desalination membranes. Langmuir 29, 11884–11897 (2013).
Gai, J.-G., Gong, X.-L., Wang, W.-W., Zhang, X. & Kang, W.-L. An ultrafast water transport forward osmosis membrane: porous graphene. J. Mater. Chem. A. 2, 4023–4028 (2014).
Hummer, G., Rasaiah, J. C. & Noworyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001).
Qin, X., Yuan, Q., Zhao, Y., Xie, S. & Liu, Z. Measurement of the rate of water translocation through carbon nanotubes. Nano Lett. 11, 2173–2177 (2011).
Falk, K., Sedlmeier, F., Joly, L., Netz, R. R. & Bocquet, L. Molecular origin of fast water transport in carbon nanotube membranes: superlubricity versus curvature dependent friction. Nano Lett. 10, 4067–4073 (2010).
Mücksch, C., Rösch, C., Müller−Renno, C., Ziegler, C. & Urbassek, H. M. Consequences of hydrocarbon contamination for wettability and protein adsorption on graphite surfaces. J. Phys. Chem. C. 119, 12496–12501 (2015).
Yang, Q. et al. Capillary condensation under atomic-scale confinement. Nature 588, 250–253 (2020).
Haigh, S. J. et al. Cross-sectional imaging of individual layers and buried interfaces of graphene-based heterostructures and superlattices. Nat. Mater. 11, 764–767 (2012).
Bao, W. et al. Controlled ripple texturing of suspended graphene and ultrathin graphite membranes. Nat. Nanotechnol. 4, 562–566 (2009).
Garaj, S. et al. Graphene as a subnanometre trans-electrode membrane. Nature 467, 190–193 (2010).
Kim, K., Luo, H., Zhu, T., Pierron, O. N. & Graham, S. Influence of polymer substrate damage on the time dependent cracking of SiNx barrier films. Sci. Rep. 8, 4560 (2018).
Garcia, S. P., Bao, H. & Hines, M. A. Etchant anisotropy controls the step bunching instability in KOH etching of silicon. Phys. Rev. Lett. 93, 166102 (2004).
Graf, M. et al. Fabrication and practical applications of molybdenum disulfide nanopores. Nat. Protoc. 14, 1130–1168 (2019).
Sato, K. et al. Characterization of orientation-dependent etching properties of single-crystal silicon: effects of KOH concentration. Sens. Actuator A Phys. 64, 87–93 (1998).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Blake, P. et al. Making graphene visible. Appl. Phys. Lett. 91, 063124 (2007).
Gorbachev, R. V. et al. Hunting for monolayer boron nitride: optical and Raman signatures. Small 7, 465–468 (2011).
Nair, R. R. et al. Graphene as a transparent conductive support for studying biological molecules by transmission electron microscopy. Appl. Phys. Lett. 97, 3 (2010).
Tu, J.-S. in Alignment Controlled Graphene on hBN Substrate for Graphene Based Capacitor and Tunneling Transistor (University of Manchester, 2015).
Sajja, R. et al. Hydrocarbon contamination in angström-scale channels. Nanoscale 13, 9553–9560 (2021).
Wright, M. R. in An Introduction to Aqueous Electrolyte Solutions (John Wiley, 2007).
Haynes, W. M. in CRC Handbook of Chemistry and Physics (CRC Press, 2016).
Perram, J. W. & Stiles, P. J. On the nature of liquid junction and membrane potentials. Phys. Chem. Chem. Phys. 8, 4200–4213 (2006).
Keerthi, A. et al. Water friction in nanofluidic channels made from two-dimensional crystals. Nat. Commun. 12, 3092 (2021).
Robin, P. et al. Long-term memory and synapse-like dynamics in two-dimensional nanofluidic channels. Science 379, 161–167 (2023).
Acknowledgements
A.K. acknowledges Royal Society research grants (RGS\R2\202036 and IES\R3\203066), EPSRC new horizons grant (EP/V048112/1). B.R. and A.K. acknowledge the EPSRC strategic equipment grant (EP/W006502/1). B.R. acknowledges funding from the Royal Society University Research Fellowship (URF\R1\180127, RF\ERE\210016), Philip Leverhulme Prize PLP-2021-262, EPSRC New Horizons grant EP/X019225/1 and funding from the European Union’s H2020 Framework Programme/European Research Council Starting Grant (852674–AngstroCAP). R.Q. acknowledges a CSC scholarship. All authors are thankful to M. Sellers and D. Mccullagh for their technical support for custom design and manufacturing of electrochemical cells and gas transport holders.
Author information
Authors and Affiliations
Contributions
A.K., A.B., Y.Y., R.S., R.Q., S.A.D. and M.R. carried out the fabrication of Å-channel devices. A.K., B.R., A.B., R.S. and M.V.S.M. carried out the device characterization. S.G. performed the ion conductance measurements and their analysis. A.B., M.V.S.M., Y.Y., B.R. and A.K. wrote the manuscript with inputs from M.R., R.S. and S.G. All the authors contributed to discussions. A.K. and B.R. provided supervision for the protocol.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Radha, B. et al. Nature 538, 222–225 (2016): https://doi.org/10.1038/nature19363
Keerthi, A. et al. Nature 558, 420–424 (2018): https://doi.org/10.1038/s41586-018-0203-2
Gopinadhan, K. et al. Science 363, 145–148 (2019): https://doi.org/10.1126/science.aau6771
Mouterde, T. et al. Nature 567, 87–90 (2019): https://doi.org/10.1038/s41586-019-0961-5
Robin, P. et al. Science 379, 161–167 (2023): https://doi.org/10.1126/science.adc9931
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhardwaj, A., Surmani Martins, M.V., You, Y. et al. Fabrication of angstrom-scale two-dimensional channels for mass transport. Nat Protoc 19, 240–280 (2024). https://doi.org/10.1038/s41596-023-00911-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00911-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.