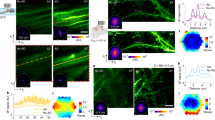
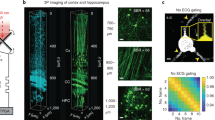
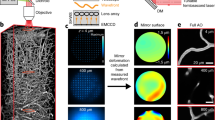
Abstract
Two-photon microscopy, combined with the appropriate optical labelling, enables the measurement and tracking of submicrometer structures within brain cells, as well as the spatiotemporal mapping of spikes in individual neurons and of neurotransmitter release in individual synapses. Yet, the spatial resolution of two-photon microscopy rapidly degrades as imaging is attempted at depths of more than a few scattering lengths into tissue, i.e., below the superficial layers that constitute the top 300–400 µm of the neocortex. To obviate this limitation, we shape the focal volume, generated by the excitation beam, by modulating the incident wavefront via guidestar-assisted adaptive optics. Here, we describe the construction, calibration and operation of a two-photon microscope that incorporates adaptive optics to restore diffraction-limited resolution at depths close to 900 µm in the mouse cortex. Our setup detects a guidestar formed by the excitation of a red-shifted dye in blood serum, used to directly measure the wavefront. We incorporate predominantly commercially available optical, optomechanical, mechanical and electronic components, and supply computer-aided design models of other customized components. The resulting adaptive optics two-photon microscope is modular and allows for expanded imaging and optical excitation capabilities. We demonstrate our methodology in the mouse neocortex by imaging the morphology of somatostatin-expressing neurons that lie 700 µm beneath the pia, calcium dynamics of layer 5b projection neurons and thalamocortical glutamate transmission to L4 neurons. The protocol requires ~30 d to complete and is suitable for users with graduate-level expertise in optics.
Key points
-
The resolution of two-photon microscopy degrades when imaging deep into tissue, limiting its effectiveness beyond the superficial layer of neocortex. To overcome this limitation, the authors describe the construction and operation of a two-photon microscope that incorporates adaptive optics to restore diffraction-limited resolution for imaging structure and function in deep layers.
-
The red-shifted dye cyanine 5.5-conjugated 2,000 kDa dextran is readily delivered to the blood serum and serves as a guidestar for adaptive optics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


















Similar content being viewed by others
Data availability
The data used for generating the time series in Figs. 17 and 18 can be accessed via https://dandiarchive.org/dandiset/000454/0.230302.2331.
Code availability
The MATLAB scripts for calibrating and operating the AO-TPLSM are available in the Supplementary Software.
References
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Kleinfeld, D., Mitra, P. P., Helmchen, F. & Denk, W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl Acad. Sci. USA 95, 15741–15746 (1998).
Svoboda, K., Denk, W., Kleinfeld, D. & Tank, D. W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385, 161–165 (1997).
Dong, C. et al. Fluorescence imaging of neural activity, neurochemical dynamics, and drug-specific receptor conformation with genetically encoded sensors. Annu. Rev. Neurosci. 45, 273–294 (2022).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Marvin, J. S. et al. Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat. Methods 15, 936–939 (2018).
Aggarwal, A. et al. Glutamate indicators with improved activation kinetics and localization for imaging synaptic transmission. Nat. Methods 20, 925–935 (2023).
Villette, V. et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell 179, 1590–1608 e23 (2019).
Kleinfeld, D. et al. Can one concurrently record electrical spikes from every neuron in a mammalian brain? Neuron 103, 1005–1015 (2019).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Ohki, K., Chung, S., Ch’ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Xu, S. et al. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science 370, eabb2494 (2020).
Hampson, K. M. et al. Adaptive optics for high-resolution imaging. Nat. Rev. Methods Prim. 1, 68 (2021).
Wang, K. et al. Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue. Nat. Commun. 6, 7276 (2015).
Wang, K. et al. Rapid adaptive optical recovery of optimal resolution over large volumes. Nat. Methods 11, 625–628 (2014).
Liu, R., Li, Z., Marvin, J. S. & Kleinfeld, D. Direct wavefront sensing enables functional imaging of infragranular axons and spines. Nat. Methods 16, 615–618 (2019).
Debarre, D. et al. Image-based adaptive optics for two-photon microscopy. Opt. Lett. 34, 2495–2497 (2009).
Tang, J., Germain, R. N. & Cui, M. Superpenetration optical microscopy by iterative multiphoton adaptive compensation technique. Proc. Natl Acad. Sci. USA 109, 8434–8439 (2012).
Ji, N., Milkie, D. E. & Betzig, E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat. Methods 7, 141–147 (2010).
Streich, L. et al. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nat. Methods 18, 1253–1258 (2021).
Rodriguez, C. et al. An adaptive optics module for deep tissue multiphoton imaging in vivo. Nat. Methods 18, 1259–1264 (2021).
Aviles-Espinosa, R. et al. Measurement and correction of in vivo sample aberrations employing a nonlinear guide-star in two-photon excited fluorescence microscopy. Biomed. Opt. Express 2, 3135–3149 (2011).
Yardeni, T., Eckhaus, M., Morris, H. D., Huizing, M. & Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab Anim. (NY) 40, 155–160 (2011).
Drew, P. J. et al. Chronic optical access through a polished and reinforced thinned skull. Nat. Methods 7, 981–984 (2010).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Ji, N., Sato, T. R. & Betzig, E. Characterization and adaptive optical correction of aberrations during in vivo imaging in the mouse cortex. Proc. Natl Acad. Sci. USA 109, 22–27 (2012).
Ji, N. Adaptive optical fluorescence microscopy. Nat. Methods 14, 374–380 (2017).
Wang, C. et al. Multiplexed aberration measurement for deep tissue imaging in vivo. Nat. Methods 11, 1037–1040 (2014).
Qin, Z. et al. Adaptive optics two-photon microscopy enables near-diffraction-limited and functional retinal imaging in vivo. Light Sci. Appl. 9, 79 (2020).
Meng, G. et al. High-throughput synapse-resolving two-photon fluorescence microendoscopy for deep-brain volumetric imaging in vivo. eLife 8, e40805 (2019).
Resendez, S. L. et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat. Protoc. 11, 566–597 (2016).
Qin, Z. et al. Adaptive optics two-photon endomicroscopy enables deep-brain imaging at synaptic resolution over large volumes. Sci. Adv. 6, eabc6521 (2020).
Noll, R. J. Zernike polynomials and atmospheric-turbulence. J. Opt. Soc. Am. 66, 207–211 (1976).
Berlage, C. et al. Deep tissue scattering compensation with three-photon F-SHARP. Optica 8, 1613–1619 (2021).
Papadopoulos, I. N., Jouhanneau, J.-S., Poulet, J. F. A. & Judkewitz, B. Scattering compensation by focus scanning holographic aberration probing (F-SHARP). Nat. Photonics 11, 116–123 (2016).
Podgorski, K. & Ranganathan, G. Brain heating induced by near-infrared lasers during multiphoton microscopy. J. Neurophysiol. 116, 1012–1023 (2016).
Prevedel, R. et al. Fast volumetric calcium imaging across multiple cortical layers using sculpted light. Nat. Methods 13, 1021–1028 (2016).
Shih, A. Y. et al. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J. Cereb. Blood Flow. Metab. 32, 1277–1309 (2012).
Yang, G., Pan, F., Parkhurst, C. N., Grutzendler, J. & Gan, W. B. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat. Protoc. 5, 201–208 (2010).
Acknowledgements
We thank S. Adams for assistance with Cy5.5–dextran synthesis and B. Friedman for assistance with animal preparations. This work was funded by the National Science Foundation, grant PHY no. 1532264; the National Institutes of Health, grants U24 EB028942, R35 NS097265 and U19 NS107466; and Early and Advanced Postdoc Mobility fellowships (P2SKP3_164948 and P300PA_177804) from the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
D.K. and R.L. guided this project and R.L. and P.Y. designed the microscope, which is based on a prior design by R.L. P.Y. carried out fabrication and testing of the hardware, T.B., R.L. and M.T. developed the method to synthesize the Cy5.5–dextran, R.L. and P.Y. performed all in vivo measurements, and D.K. and P.Y. wrote the manuscript. D.K. attended to the plethora of university rules and forms that govern research compliance, export control and environmental health and safety, including the ethical use of animals as well as the use of chemicals, hazardous substances, controlled substance, lasers and viruses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Valentina Emiliani, Karen Hampson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Liu, R. et al. Nat. Methods 16, 615–618 (2019): https://doi.org/10.1038/s41592-019-0434-7
Supplementary information
Supplementary Data 1
Assembly drawings.
Supplementary Data 2
Part drawings.
Supplementary Data 3
ZEMAX file.
Supplementary Table 1
Comprehensive parts list including current prices and vendor links.
Supplementary Code 1
MATLAB scripts.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, P., Liu, R., Broggini, T. et al. Construction and use of an adaptive optics two-photon microscope with direct wavefront sensing. Nat Protoc 18, 3732–3766 (2023). https://doi.org/10.1038/s41596-023-00893-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00893-w
This article is cited by
-
More than double the fun with two-photon excitation microscopy
Communications Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.