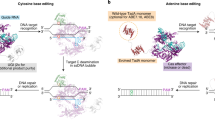
Abstract
Fusing apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like cytidine deaminase with catalytically impaired Cas proteins (e.g., nCas9 or dCas9) provides a novel gene-editing technology, base editing, that grants targeted base substitutions with high efficiency. However, genome-wide and transcriptome-wide off-target mutations are observed in base editing, which raises safety concerns regarding therapeutic applications. Previously, we developed a new base editing system, the transformer base editor (tBE), to induce efficient editing with no observable genome-wide or transcriptome-wide off-target mutations both in mammalian cells and in mice. Here we describe a detailed protocol for the design and application of the tBE. Steps for designing single-guide RNA (sgRNA) and helper sgRNA pairs, making constructs, determining the genome-wide and transcriptome-wide off-target mutations, producing the tBE-containing adeno-associated viruses, delivering adeno-associated viruses into mice and examining the in vivo editing effects are included in this protocol. High-precision base editing by the tBE can be completed within 2–3 weeks (in mammalian cells) or within 6–8 weeks (in mice), with sgRNA–helper sgRNA pairs. The whole process can be collaboratively accomplished by researchers using standard techniques from molecular biology, bioinformatics and mouse husbandry.
Key points
-
This protocol describes the transformer base editor system to induce efficient editing with no observable genome-wide or transcriptome-wide off-target mutations, both in mammalian cells and in mice.
-
The transformer base editor system overcomes the problems of guide RNA-independent and guide RNA-dependent off-target editing as it remains inactive at off-target sites but can be transformed to be active for base editing after binding at the on-target site.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data used to generate the example results shown in Fig. 5 were originally published in ref. 27. All sequencing datasets have been deposited in the Gene Expression Omnibus under the accession code GSE164837 and GSE164477, at the NCBI BioProject under the accession code PRJNA692761 and in the National Omics Data Encyclopedia under the accession codes OEP001688, OEP001689 and OEP001690. All other data supporting the finding of this study are available from the corresponding authors on reasonable requests. Source data are provided with this paper.
Code availability
The custom Perl and Shell scripts for CFBI are available at GitHub (https://github.com/YangLab/CFBI). The computational pipeline of BEIDOU to identify high-confidence base substitution or indel events from WGS data is available at GitHub (https://github.com/YangLab/BEIDOU). The workflow of RADAR to detect and visualize all 12 possible types of RNA-editing event from RNA-seq data is available at GitHub (https://github.com/YangLab/RADAR).
References
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Nishida, K. et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, aaf8729 (2016).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Yang, B., Yang, L. & Chen, J. Development and application of base editors. CRISPR J. 2, 91–104 (2019).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Yang, L. & Chen, J. A tale of two moieties: rapidly evolving CRISPR/Cas-based genome editing. Trends Biochem. Sci. 45, 874–888 (2020).
Porto, E. M., Komor, A. C., Slaymaker, I. M. & Yeo, G. W. Base editing: advances and therapeutic opportunities. Nat. Rev. Drug Discov. 19, 839–859 (2020).
Nambiar, T. S., Baudrier, L., Billon, P. & Ciccia, A. CRISPR-based genome editing through the lens of DNA repair. Mol. Cell 82, 348–388 (2022).
Zuo, E. et al. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 364, 289–292 (2019).
Jin, S. et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 364, 292–295 (2019).
Zhou, C. et al. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature 571, 275–278 (2019).
Grunewald, J. et al. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 569, 433–437 (2019).
Yang, B., Li, X., Lei, L. & Chen, J. APOBEC: from mutator to editor. J. Genet. Genomics 44, 423–437 (2017).
Lei, L. et al. APOBEC3 induces mutations during repair of CRISPR–Cas9-generated DNA breaks. Nat. Struct. Mol. Biol. 25, 45–52 (2018).
Olson, M. E., Harris, R. S. & Harki, D. A. APOBEC enzymes as targets for virus and cancer therapy. Cell Chem. Biol. 25, 36–49 (2018).
Chen, J., Yang, B. & Yang, L. To BE or not to BE, that is the question. Nat. Biotechnol. 37, 520–522 (2019).
Rees, H. A. et al. Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat. Commun. 8, 15790 (2017).
Wang, X. et al. Efficient base editing in methylated regions with a human APOBEC3A–Cas9 fusion. Nat. Biotechnol. 36, 946–949 (2018).
Billon, P. et al. CRISPR-mediated base editing enables efficient disruption of Eukaryotic genes through induction of STOP codons. Mol. Cell 67, 1068–1079 e1064 (2017).
Kuscu, C. et al. CRISPR-STOP: gene silencing through base-editing-induced nonsense mutations. Nat. Methods 14, 710–712 (2017).
Kim, K. et al. Highly efficient RNA-guided base editing in mouse embryos. Nat. Biotechnol. 35, 435–437 (2017).
Diorio, C. et al. Cytosine base editing enables quadruple-edited allogeneic CART cells for T-ALL. Blood 140, 619–629 (2022).
Wang, L. et al. Reactivation of gamma-globin expression through Cas9 or base editor to treat beta-hemoglobinopathies. Cell Res. 30, 276–278 (2020).
Zeng, J. et al. Therapeutic base editing of human hematopoietic stem cells. Nat. Med. 26, 535–541 (2020).
Liang, P. et al. Correction of beta-thalassemia mutant by base editor in human embryos. Protein Cell 8, 811–822 (2017).
Koblan, L. W. et al. In vivo base editing rescues Hutchinson–Gilford progeria syndrome in mice. Nature 589, 608–614 (2021).
Wang, L. et al. Eliminating base-editor-induced genome-wide and transcriptome-wide off-target mutations. Nat. Cell Biol. 23, 552–563 (2021).
Sternberg, S. H., Redding, S., Jinek, M., Greene, E. C. & Doudna, J. A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 507, 62–67 (2014).
Richardson, C. D., Ray, G. J., DeWitt, M. A., Curie, G. L. & Corn, J. E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR–Cas9 using asymmetric donor DNA. Nat. Biotechnol. 34, 339–344 (2016).
Wang, D., Tai, P. W. L. & Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 18, 358–378 (2019).
Ma, Y. et al. Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells. Nat. Methods 13, 1029–1035 (2016).
Komor, A. C. et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv. 3, eaao4774 (2017).
Wang, L. et al. Enhanced base editing by co-expression of free uracil DNA glycosylase inhibitor. Cell Res. 27, 1289–1292 (2017).
Koblan, L. W. et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 36, 843–846 (2018).
Li, X. et al. Base editing with a Cpf1-cytidine deaminase fusion. Nat. Biotechnol. 36, 324–327 (2018).
Wang, X. et al. Cas12a base editors induce efficient and specific editing with low DNA damage response. Cell Rep. 31, 107723 (2020).
Zhang, X. et al. Increasing the efficiency and targeting range of cytidine base editors through fusion of a single-stranded DNA-binding protein domain. Nat. Cell Biol. 22, 740–750 (2020).
Levy, J. M. et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 4, 97–110 (2020).
Chadwick, A. C., Wang, X. & Musunuru, K. In vivo base editing of PCSK9 (proprotein convertase subtilisin/kexin type 9) as a therapeutic alternative to genome editing. Arterioscler. Thromb. Vasc. Biol. 37, 1741–1747 (2017).
Wu, Y. et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 25, 776–783 (2019).
Kunkel, T. A. & Erie, D. A. Eukaryotic mismatch repair in relation to DNA replication. Annu. Rev. Genet. 49, 291–313 (2015).
Liu, Z. et al. Highly efficient RNA-guided base editing in rabbit. Nat. Commun. 9, 2717 (2018).
Xie, J. et al. Efficient base editing for multiple genes and loci in pigs using base editors. Nat. Commun. 10, 2852 (2019).
Li, G. et al. Base pair editing in goat: nonsense codon introgression into FGF5 results in longer hair. FEBS J. 286, 4675–4692 (2019).
Zhang, Y. et al. Programmable base editing of zebrafish genome using a modified CRISPR–Cas9 system. Nat. Commun. 8, 118 (2017).
Zuo, E. et al. A rationally engineered cytosine base editor retains high on-target activity while reducing both DNA and RNA off-target effects. Nat. Methods 17, 600–604 (2020).
Jin, S. et al. Rationally designed APOBEC3B cytosine base editors with improved specificity. Mol. Cell 79, 728–740 e726 (2020).
Lee, S. et al. Single C-to-T substitution using engineered APOBEC3G-nCas9 base editors with minimum genome- and transcriptome-wide off-target effects. Sci. Adv. 6, eaba1773 (2020).
Kleinstiver, B. P. et al. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Chen, J. S. et al. Enhanced proofreading governs CRISPR–Cas9 targeting accuracy. Nature 550, 407–410 (2017).
Nishimasu, H. et al. Engineered CRISPR–Cas9 nuclease with expanded targeting space. Science 361, 1259–1262 (2018).
Walton, R. T., Christie, K. A., Whittaker, M. N. & Kleinstiver, B. P. Unconstrained genome targeting with near-PAMless engineered CRISPR–Cas9 variants. Science 368, 290–296 (2020).
Kim, Y. B. et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 35, 371–376 (2017).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Jinek, M. et al. RNA-programmed genome editing in human cells. eLife 2, e00471 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Bae, S., Park, J. & Kim, J. S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014).
Gao, R. et al. Genomic and transcriptomic analyses of prime editing guide RNA-independent off-target effects by prime editors. CRISPR J. 5, 276–293 (2022).
Li, J. et al. Efficient base editing in G/C-rich regions to model androgen insensitivity syndrome. Cell Res. 29, 174–176 (2019).
Villiger, L. et al. In vivo cytidine base editing of hepatocytes without detectable off-target mutations in RNA and DNA. Nat. Biomed. Eng. 5, 179–189 (2021).
Concordet, J. P. & Haeussler, M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 46, W242–W245 (2018).
Listgarten, J. et al. Prediction of off-target activities for the end-to-end design of CRISPR guide RNAs. Nat. Biomed. Eng. 2, 38–47 (2018).
Cancellieri, S. et al. Human genetic diversity alters off-target outcomes of therapeutic gene editing. Nat. Genet. 55, 34–43 (2023).
Sena-Esteves, M. & Gao, G. Purification of recombinant adeno-associated viruses (rAAVs) by iodixanol gradient centrifugation. Cold Spring Harb. Protoc. 2020, 095612 (2020).
Hwang, G. H. et al. Web-based design and analysis tools for CRISPR base editing. BMC Bioinform. 19, 542 (2018).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Ramaswami, G. et al. Identifying RNA editing sites using RNA sequencing data alone. Nat. Methods 10, 128–132 (2013).
Kent, W J. BLAT—the BLAST-like alignment tool. Genome Res. 12, 656–664 (2002).
Acknowledgements
This work was supported by 2018YFA0801401 (J.C.) and 2019YFA0802804 (L.Y.) from the National Key R&D Program of China, 81872305 (J.C.) and 31925011 (L.Y.) from the National Natural Science Foundation of China, NK2022010207 (J.C.) from Ministry of Agriculture and Rural Affairs, 21JC1404600 (J.C.), 20PJ1410200 (J.L.) and 23XD1422500 (J.C.) from Shanghai Municipal Science and Technology Commission. We thank Shanghai Frontiers Science Center for Biomacromolecules and Precision Medicine, ShanghaiTech University, Shanghai Clinical Research and Trial Center, Animal Core Facility, ShanghaiTech University and Molecular and Cell Biology Core Facility, School of Life Science and Technology, ShanghaiTech University for providing support.
Author information
Authors and Affiliations
Contributions
J.C., L.Y. and J.L. conceived, designed and supervised the project. W.H., B.-Q.G., J.Z., Z.H., J.L., L.Y. and J.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Yongsub Kim, Liangxue Lai, Zhanjun Li, Shaohua Yao, Erwei Zuo and the other, anonymous, reviwer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Wang, L. et al. Nat. Cell Biol. 23, 552-563 (2021): https://doi.org/10.1038/s41556-021-00671-4
Extended data
Extended Data Fig. 1 Procedure to construct the designed plasmids each of which expresses a pair of hsgRNA-MS2 and sgRNA-boxB.
a, The schematic diagram demonstrating the processes of constructing one plasmid expressing a pair of designed hsgRNA-MS2 and sgRNA-boxB. b, The schematic diagram demonstrating the construction of plasmids designed for the screening of all combinations of sgRNAs and hsgRNAs against one on-target site.
Supplementary information
Supplementary Table 1
Supplementary Table 1.
Source data
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, W., Gao, BQ., Zhu, J. et al. Design and application of the transformer base editor in mammalian cells and mice. Nat Protoc 18, 3194–3228 (2023). https://doi.org/10.1038/s41596-023-00877-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00877-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.