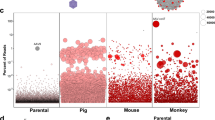
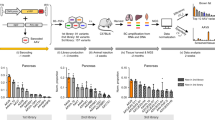
Abstract
Over the past 5 years, our laboratory has systematically developed a structure-guided library approach to evolve new adeno-associated virus (AAV) capsids with altered tissue tropism, higher transduction efficiency and the ability to evade pre-existing humoral immunity. Here, we provide a detailed protocol describing two distinct evolution strategies using structurally divergent AAV serotypes as templates, exemplified by improving CNS gene transfer efficiency in vivo. We outline four major components of our strategy: (i) structure-guided design of AAV capsid libraries, (ii) AAV library production, (iii) library cycling in single versus multiple animal models, followed by (iv) evaluation of lead AAV vector candidates in vivo. The protocol spans ~95 d, excluding gene expression analysis in vivo, and can vary depending on user experience, resources and experimental design. A distinguishing attribute of the current protocol is the focus on providing biomedical researchers with 3D structural information to guide evolution of precise ‘hotspots’ on AAV capsids. Furthermore, the protocol outlines two distinct methods for AAV library evolution consisting of adenovirus-enabled infectious cycling in a single species and noninfectious cycling in a cross-species manner. Notably, our workflow can be seamlessly merged with other RNA transcript-based library strategies and tailored for tissue-specific capsid selection. Overall, the procedures outlined herein can be adapted to expand the AAV vector toolkit for genetic manipulation of animal models and development of human gene therapies.
Key points
-
This protocol uses structure-guided design of AAV capsid libraries, infectious and noninfectious library cycling and evaluation of lead candidates in vivo to evolve new AAV vectors for enhanced CNS gene delivery.
-
This approach evolves novel AAV variants iteratively across multiple species. The vectors produced can display altered tissue tropism, higher transduction efficiency and the ability to evade pre-existing humoral immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The example data generated for the protocol has not been previously published; however, the protocol has previously been used to produce similar results shown in refs. 12,13,14. All data associated with the figures of this study are included in this paper. Any additional data to support these findings can be made available upon reasonable request to the corresponding author. Source data are provided with this paper.
Code availability
The Perl scripts used for NGS analysis of AAV variants have been described previously12. The scripts have been deposited into the Zenodo repository and can be accessed via https://doi.org/10.5281/zenodo.7075694.
References
Mendell, J. R. et al. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 29, 464–488 (2021).
Kuzmin, D. A. et al. The clinical landscape for AAV gene therapies. Nat. Rev. Drug Discov. 20, 173–174 (2021).
Mingozzi, F. & High, K. A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet 12, 341–355 (2011).
Hudry, E. & Vandenberghe, L. H. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron 101, 839–862 (2019).
Deverman, B. E. et al. Gene therapy for neurological disorders: progress and prospects. Nat. Rev. Drug Discov. 17, 641–659 (2018).
Rafii, M. S. et al. Adeno-associated viral vector (serotype 2)-nerve growth factor for patients with Alzheimer disease: a randomized clinical trial. JAMA Neurol. 75, 834–841 (2018).
Li, C. & Samulski, R. J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet 21, 255–272 (2020).
Barnes, C., Scheideler, O. & Schaffer, D. Engineering the AAV capsid to evade immune responses. Curr. Opin. Biotechnol. 60, 99–103 (2019).
Kotterman, M. A. & Schaffer, D. V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 15, 445–451 (2014).
Wang, D., Tai, P. W. L. & Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 18, 358–378 (2019).
Zolotukhin, S. & Vandenberghe, L. H. AAV capsid design: a Goldilocks challenge. Trends Mol. Med 28, 183–193 (2022).
Gonzalez, T. J. et al. Cross-species evolution of a highly potent AAV variant for therapeutic gene transfer and genome editing. Nat. Commun. 13, 5947 (2022).
Havlik, L. P. et al. Coevolution of adeno-associated virus capsid antigenicity and tropism through a structure-guided approach. J. Virol. 94, e00976-20 (2020).
Tse, L. V. et al. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc. Natl Acad. Sci. USA 114, E4812–E4821 (2017).
Havlik, L. P. et al. Receptor switching in newly evolved adeno-associated viruses. J. Virol. 95, e0058721 (2021).
Albright, B. H. et al. Mapping the structural determinants required for AAVrh.10 transport across the blood-brain barrier. Mol. Ther. 26, 510–523 (2018).
Murlidharan, G. et al. Unique glycan signatures regulate adeno-associated virus tropism in the developing brain. J. Virol. 89, 3976–3987 (2015).
Shen, S. et al. Multiple roles for sialylated glycans in determining the cardiopulmonary tropism of adeno-associated virus 4. J. Virol. 87, 13206–13213 (2013).
Davidsson, M. et al. A systematic capsid evolution approach performed in vivo for the design of AAV vectors with tailored properties and tropism. Proc. Natl Acad. Sci. USA 116, 27053–27062 (2019).
Deverman, B. E. et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016).
Hordeaux, J. et al. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol. Ther. 26, 664–668 (2018).
Shen, S. et al. Glycan binding avidity determines the systemic fate of adeno-associated virus type 9. J. Virol. 86, 10408–10417 (2012).
Shen, S. et al. Engraftment of a galactose receptor footprint onto adeno-associated viral capsids improves transduction efficiency. J. Biol. Chem. 288, 28814–28823 (2013).
Asokan, A. et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 28, 79–82 (2010).
Madigan, V. J. & Asokan, A. Engineering AAV receptor footprints for gene therapy. Curr. Opin. Virol. 18, 89–96 (2016).
Tarantal, A. F. et al. Systemic and persistent muscle gene expression in rhesus monkeys with a liver de-targeted adeno-associated virus vector. Hum. Gene Ther. 28, 385–391 (2017).
Li, C. et al. Development of patient-specific AAV vectors after neutralizing antibody selection for enhanced muscle gene transfer. Mol. Ther. 24, 53–65 (2016).
Ogden, P. J. et al. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science 366, 1139–1143 (2019).
Bryant, D. H. et al. Deep diversification of an AAV capsid protein by machine learning. Nat. Biotechnol. 39, 691–696 (2021).
Tervo, D. G. et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382 (2016).
Jose, A. et al. High-resolution structural characterization of a new adeno-associated virus serotype 5 antibody epitope toward engineering antibody-resistant recombinant gene delivery vectors. J. Virol. 93, e01394-18 (2019).
Meyer, N. L. & Chapman, M. S. Adeno-associated virus (AAV) cell entry: structural insights. Trends Microbiol 30, 432–451 (2022).
Emmanuel, S. N. et al. Parvovirus capsid-antibody complex structures reveal conservation of antigenic epitopes across the family. Viral Immunol. 34, 3–17 (2021).
Tseng, Y. S. et al. Adeno-associated virus serotype 1 (AAV1)- and AAV5-antibody complex structures reveal evolutionary commonalities in parvovirus antigenic reactivity. J. Virol. 89, 1794–1808 (2015).
Mietzsch, M. et al. Structural study of Aavrh.10 receptor and antibody interactions. J. Virol. 95, e0124921 (2021).
Emmanuel, S. N. et al. Structurally mapping antigenic epitopes of adeno-associated virus 9: development of antibody escape variants. J. Virol. 96, e0125121 (2022).
Hemmi, S. & Spindler, K. R. Murine adenoviruses: tools for studying adenovirus pathogenesis in a natural host. FEBS Lett. 593, 3649–3659 (2019).
Gralinski, L. E. et al. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J. Virol. 83, 9398–9410 (2009).
Guida, J. D. et al. Mouse adenovirus type 1 causes a fatal hemorrhagic encephalomyelitis in adult C57BL/6 but not BALB/c mice. J. Virol. 69, 7674–7681 (1995).
Weinberg, J. B. et al. Acute respiratory infection with mouse adenovirus type 1. Virology 340, 245–254 (2005).
Kajon, A. E., Brown, C. C. & Spindler, K. R. Distribution of mouse adenovirus type 1 in intraperitoneally and intranasally infected adult outbred mice. J. Virol. 72, 1219–1223 (1998).
Klempa, B. et al. A novel cardiotropic murine adenovirus representing a distinct species of mastadenoviruses. J. Virol. 83, 5749–5759 (2009).
Ashley, S. L. et al. Matrix metalloproteinase activity in infections by an encephalitic virus, mouse adenovirus type 1. J. Virol. 91, e01412–e01416 (2017).
Stier, M. T. & Spindler, K. R. Polymorphisms in Ly6 genes in Msq1 encoding susceptibility to mouse adenovirus type 1. Mamm. Genome 23, 250–258 (2012).
Maheshri, N. et al. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 24, 198–204 (2006).
Grimm, D. et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 82, 5887–5911 (2008).
Gray, S. J. et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB). Mol. Ther. 18, 570–578 (2010).
Weinmann, J. et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat. Commun. 11, 5432 (2020).
Tabebordbar, M. et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184, 4919–4938.e22 (2021).
El Andari, J. et al. Semirational bioengineering of AAV vectors with increased potency and specificity for systemic gene therapy of muscle disorders. Sci. Adv. 8, eabn4704 (2022).
Nonnenmacher, M. et al. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol. Ther. Methods Clin. Dev. 20, 366–378 (2021).
Rapti, K. et al. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol. Ther. 20, 73–83 (2012).
Smola, M. J. et al. Selective 2′-hydroxyl acylation analyzed by primer extension and mutational profiling (SHAPE-MaP) for direct, versatile and accurate RNA structure analysis. Nat. Protoc. 10, 1643–1669 (2015).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br. J. Pharm. 177, 3617–3624 (2020).
Montiel-Garcia, D. et al. VIPERdb v3.0: a structure-based data analytics platform for viral capsids. Nucleic Acids Res. 49, D809–D816 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. et al. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Grieger, J. C., Choi, V. W. & Samulski, R. J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 1, 1412–1428 (2006).
Schmit, P. F. et al. Cross-packaging and capsid mosaic formation in multiplexed AAV libraries. Mol. Ther. Methods Clin. Dev. 17, 107–121 (2020).
Xiao, X., Li, J. & Samulski, R. J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72, 2224–2232 (1998).
Mietzsch, M., Penzes, J. J. & Agbandje-McKenna, M. Twenty-five years of structural parvovirology. Viruses 11, 362 (2019).
Tseng, Y. S. & Agbandje-McKenna, M. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front. Immunol. 5, 9 (2014).
Challis, R. C. et al. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. 14, 379–414 (2019).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Goertsen, D. et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 25, 106–115 (2022).
Nonnenmacher, M. et al. High capsid-genome correlation facilitates creation of AAV libraries for directed evolution. Mol. Ther. 23, 675–682 (2015).
Govindasamy, L. et al. Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J. Virol. 80, 11556–11570 (2006).
Mietzsch, M. et al. Characterization of AAV-specific affinity ligands: consequences for vector purification and development strategies. Mol. Ther. Methods Clin. Dev. 19, 362–373 (2020).
Huang, L. Y. et al. Characterization of the adeno-associated virus 1 and 6 sialic acid binding site. J. Virol. 90, 5219–5230 (2016).
Meyer, N. L. et al. Structure of the gene therapy vector, adeno-associated virus with its cell receptor, AAVR. Elife 8, e44707 (2019).
Earley, L. F. et al. Adeno-associated virus (AAV) assembly-activating protein is not an essential requirement for capsid assembly of AAV serotypes 4, 5, and 11. J. Virol. 91, e01980-16 (2017).
Bleker, S., Sonntag, F. & Kleinschmidt, J. A. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J. Virol. 79, 2528–2540 (2005).
Bleker, S., Pawlita, M. & Kleinschmidt, J. A. Impact of capsid conformation and Rep-capsid interactions on adeno-associated virus type 2 genome packaging. J. Virol. 80, 810–820 (2006).
Grieger, J. C. et al. Surface-exposed adeno-associated virus Vp1-NLS capsid fusion protein rescues infectivity of noninfectious wild-type Vp2/Vp3 and Vp3-only capsids but not that of fivefold pore mutant virions. J. Virol. 81, 7833–7843 (2007).
Kronenberg, S. et al. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J. Virol. 79, 5296–5303 (2005).
Kotchey, N. M. et al. A potential role of distinctively delayed blood clearance of recombinant adeno-associated virus serotype 9 in robust cardiac transduction. Mol. Ther. 19, 1079–1089 (2011).
Judd, J. et al. Random insertion of mCherry into VP3 domain of adeno-associated virus yields fluorescent capsids with no loss of infectivity. Mol. Ther. Nucleic Acids 1, e54 (2012).
Jackson, C. B. et al. AAV vectors engineered to target insulin receptor greatly enhance intramuscular gene delivery. Mol. Ther. Methods Clin. Dev. 19, 496–506 (2020).
DiMattia, M. A. et al. Structural insight into the unique properties of adeno-associated virus serotype 9. J. Virol. 86, 6947–6958 (2012).
Mietzsch, M. et al. Characterization of the serpentine adeno-associated virus (SAAV) capsid structure: receptor interactions and antigenicity. J. Virol. 96, e0033522 (2022).
Li, W. et al. Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium. Mol. Ther. 17, 2067–2077 (2009).
Gurda, B. L. et al. Capsid antibodies to different adeno-associated virus serotypes bind common regions. J. Virol. 87, 9111–9124 (2013).
Pulicherla, N. et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 19, 1070–1078 (2011).
Afione, S. et al. Identification and mutagenesis of the adeno-associated virus 5 sialic acid binding region. J. Virol. 89, 1660–1672 (2015).
Lerch, T. F., Xie, Q. & Chapman, M. S. The structure of adeno-associated virus serotype 3B (AAV-3B): insights into receptor binding and immune evasion. Virology 403, 26–36 (2010).
Naumer, M. et al. Development and validation of novel AAV2 random libraries displaying peptides of diverse lengths and at diverse capsid positions. Hum. Gene Ther. 23, 492–507 (2012).
Michelfelder, S. et al. Peptide ligands incorporated into the threefold spike capsid domain to re-direct gene transduction of AAV8 and AAV9 in vivo. PLoS ONE 6, e23101 (2011).
DiPrimio, N. et al. Surface loop dynamics in adeno-associated virus capsid assembly. J. Virol. 82, 5178–5189 (2008).
Horowitz, E. D., Finn, M. G. & Asokan, A. Tyrosine cross-linking reveals interfacial dynamics in adeno-associated viral capsids during infection. ACS Chem. Biol. 7, 1059–1066 (2012).
Acknowledgements
We acknowledge L. Edwards and K. Gleason for their assistance in completing in-life portions for our pig studies, N. Olby for her assistance in performing the IT infusions in pigs, J. McNamara and E. Matthews for their assistance with dissection of pig brains and Duke DLAR for their assistance with mouse care. We acknowledge the Duke light microscopy core facility and Translating Duke Health initiative for their support. Figures were created in part by using BioRender.
Author information
Authors and Affiliations
Contributions
T.J.G., A.M.-D. and A.A. conceptualized and wrote the manuscript. T.J.G. and J.A.H. conducted the structural modeling. T.J.G. and S.M.-T. designed and cloned the AAV libraries. T.J.G., A.M.-D., S.M.-T. and R.M.C.R. optimized viral production and purification protocols and conducted in vivo cycling. T.J.G., A.M.-D. and A.A. designed experiments and created the figures. T.J.G., A.M.-D., L.O.B., M.M.F. and D.K.O. conducted experiments and analyzed data. A.A. and J.A.P. acquired funding.
Corresponding author
Ethics declarations
Competing interests
T.J.G. and A.A. have filed patent applications on the subject matter of this paper. A.A. is a co-founder at StrideBio and TorqueBio and serves on the advisory boards of Atsena Therapeutics, Isolere Bio, Mammoth Bio, Ring Therapeutics and Kriya Therapeutics. S.M.-T. is a current employee of Regeneron Pharmaceuticals. R.M.C.R. is a current employee of Kriya Therapeutics.
Peer review
Peer review information
Nature Protocols thanks David Schaffer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Tse, L. V. et al. Proc. Natl. Acad. Sci. USA 114, E4812–E4821 (2017): https://doi.org/10.1073/pnas.1704766114
Havlik, L. P. et al. J. Virol. 94, e00976-20 (2020): https://doi.org/10.1128/JVI.00976-20
Gonzalez, T. J. et al. Nat. Commun. 13, 5947 (2022): https://doi.org/10.1038/s41467-022-33745-4
Extended data
Extended Data Fig. 1 Representative Sanger sequencing traces for library quality control.
a, Representative sequencing traces of wild-type AAV9 VR VIII. Samples that match this would be excluded from library insert generation. b, Representative sequencing traces for AAV9 VR VIII containing high diversity at the library site. Users should confirm that the wild-type sequence is not called as the majority population and confirm overlapping histogram peak base calls at each position as shown. Samples that match this would be included during library insert generation. Figure created with BioRender.com.
Extended Data Fig. 2 Generation of iodixanol gradients for AAV purification.
Gradients can be created either by overlaying (a) or underlaying (b) of different densities in 17-ml ultracentrifuge tubes. These densities include 17%, 25%, 40% and 60% iodixanol. Virus is added on top of the completed gradient before spinning in an ultracentrifuge. Either method can be used successfully, and the choice of method is based on user preference. Figure created with BioRender.com.
Extended Data Fig. 3 ICV injection station.
a, Ice anesthetization setup. Two large gloves are filled with ice to create a sandwich chamber. When ready to begin, pups are placed between the two gloves of ice and incubated for 3–5 min. During this time, the pup will become unresponsive, and a loss of its pink body color will be observed. b, Stereotaxic injection apparatus with injection pump and Hamilton syringe. Once pups are anesthetized, mount pups on the stage and zero out your X and Y coordinates by moving your needle above the lambda point. The injection site is located 0.8 mm (X), followed by 1.5 mm (Y) up toward the coronal suture and −1.5 mm (Z).
Supplementary information
Source data
Source Data Fig. 5
Statistical source data for panels b, c and d
Source Data Fig. 6
Statistical source data for panel h
Source Data Fig. 7
Statistical source data for panels b and c
Source Data Fig. 9
Statistical source data for panel b
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonzalez, T.J., Mitchell-Dick, A., Blondel, L.O. et al. Structure-guided AAV capsid evolution strategies for enhanced CNS gene delivery. Nat Protoc 18, 3413–3459 (2023). https://doi.org/10.1038/s41596-023-00875-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00875-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.