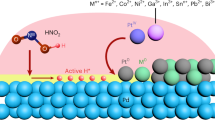
Abstract
Intermetallic nanomaterials consist of two or more metals in a highly ordered atomic arrangement. There are many possible combinations and morphologies, and exploring their properties is an important research area. Their strict stoichiometry requirement and well-defined atom binding environment make intermetallic compounds an ideal research platform to rationally optimize catalytic performance. Making mesoporous intermetallic materials is a further advance; crystalline mesoporosity can expose more active sites, facilitate the mass and electron transfer, and provide the distinguished mesoporous nanoconfinement environment. In this Protocol, we describe how to prepare ordered mesoporous intermetallic nanomaterials with controlled compositions, morphologies/structures and phases by a general concurrent template strategy. In this approach, the concurrent template used is a hybrid of mesoporous platinum or palladium and Korea Advanced Institute of Science and Technology-6 (KIT-6) (meso-Pt/KIT-6 or meso-Pd/KIT-6) that can be transformed by the second precursors under reducing conditions. The second precursor can either be a second metal or a metalloid/non-metal, e.g., boron/phosphorus. KIT-6 is a silica scaffold that is removed using NaOH or HF to form the mesoporous product. Procedures for example catalytic applications include the 3-nitrophenylacetylene semi-hydrogenation reaction, p-nitrophenol reduction reaction and electrochemical hydrogen evolution reaction. The synthetic strategy for preparation of ordered mesoporous intermetallic nanoparticles would take almost 5 d; the physical characterization by electron microscope, X-ray diffraction and inductively coupled plasma–mass spectrometry takes ~2 days and the function characterization depends on the research question, but for catalysis it takes 1–5 h.
Key points
-
Ordered mesoporous intermetallic compounds are generated by first hybridizing platinum or palladium with a silica scaffold (KIT-6), adding a second metal or metalloid/non-metal under reducing conditions, followed by removal of KIT-6 using either NaOH or HF.
-
Other synthetic approaches do not allow the preparation of ordered mesostructures. The advantage of creating ordered mesostructures is that it is easier to optimize the structure–activity profile of the resulting materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout














Similar content being viewed by others
References
Akhmetshina, T. G. et al. Topology of intermetallic structures: from statistics to rational design. Acc. Chem. Res. 51, 21–30 (2018).
Li, J. et al. Intermetallic nanoparticles: synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 52, 2015–2025 (2019).
Rößner, L. et al. Electrochemical energy conversion on intermetallic compounds: a review. ACS Catal. 9, 2018–2062 (2019).
Furukawa, S. et al. Intermetallic compounds: promising inorganic materials for well-structured and electronically modified reaction environments for efficient catalysis. ACS Catal. 7, 735–765 (2016).
Luo, M. et al. Tuning multimetallic ordered intermetallic nanocrystals for efficient energy electrocatalysis. Adv. Energy Mater. 7, 1602073 (2017).
Yan, Y. et al. Intermetallic nanocrystals: syntheses and catalytic applications. Adv. Mater. 29, 1605997 (2017).
Bueno, S. L. A. et al. Building durable multimetallic electrocatalysts from intermetallic seeds. Acc. Chem. Res. 54, 1662–1672 (2021).
Yoo, T. Y. et al. Direct synthesis of intermetallic platinum-alloy nanoparticles highly loaded on carbon supports for efficient electrocatalysis. J. Am. Chem. Soc. 142, 14190–14200 (2020).
Zhou, M. et al. Noble-metal based random alloy and intermetallic nanocrystals: syntheses and applications. Chem. Rev. 121, 736–795 (2021).
Zhang, B. et al. General strategy for synthesis of ordered Pt3M intermetallics with ultrasmall particle size. Angew. Chem. Int. Ed. 59, 7857–7863 (2020).
Tsai, A. P. et al. Intermetallic: a pseudoelement for catalysis. Acc. Chem. Res. 50, 2879–2885 (2017).
Zhao, E. W. et al. Silica-Encapsulated Pt-Sn intermetallic nanoparticles: a robust catalytic platform for parahydrogen-induced polarization of gases and liquids. Angew. Chem. Int. Ed. 56, 3925–3929 (2017).
Cheng, H. et al. Subsize Pt-based intermetallic compound enables long-term cyclic mass activity for fuel-cell oxygen reduction. Proc. Natl Acad. Sci. USA 118, e2104026118 (2021).
Luo, Z. et al. Pd2Sn [010] nanorods as a highly active and stable ethanol oxidation catalyst. J. Mater. Chem. A 4, 16706–16713 (2016).
Wang, Q. et al. Pd2Ga nanorods as highly active bifunctional catalysts for electrosynthesis of acetic acid coupled with hydrogen production. Chem. Eng. J. 446, 136878 (2022).
Guo, J. et al. Template-directed rapid synthesis of Pd-based ultrathin porous intermetallic nanosheets for efficient oxygen reduction. Angew. Chem. Int. Ed. 60, 10942–10949 (2021).
Chen, S. et al. Propane dehydrogenation on single-site [PtZn4] intermetallic catalysts. Chem 7, 387–405 (2021).
Chen, M. et al. Thermal unequilibrium of PdSn intermetallic nanocatalysts: from in situ tailored synthesis to unexpected hydrogenation selectivity. Angew. Chem. Int. Ed. 60, 18309–18317 (2021).
Chen, M. et al. Silica-encapsulated intermetallic nanoparticles for highly active and selective heterogeneous catalysis. Acc. Mater. Res. 2, 1190–1202 (2021).
Li, F. et al. Atomistic imaging of competition between surface diffusion and phase transition during the intermetallic formation of faceted particles. ACS Nano 15, 5284–5293 (2021).
Kresge, C. T. et al. Ordered mesoporous molecular sieves synthesized by a lquid-crystal template mechanism. Nature 359, 710–712 (1992).
Qiu, P. et al. Spherical mesoporous materials from single to multilevel architectures. Acc. Chem. Res. 52, 2928–2938 (2019).
Teng, Z. et al. Mesoporous organosilica hollow nanoparticles: synthesis and applications. Adv. Mater. 31, 1707612 (2019).
Ren, Y. et al. Ordered mesoporous metal oxides: synthesis and applications. Chem. Soc. Rev. 41, 4909–4927 (2012).
Han, L. et al. Anionic surfactant templated mesoporous silicas (AMSs). Chem. Soc. Rev. 42, 3740–3752 (2013).
Lv, H. et al. Single-crystalline mesoporous palladium and palladium-copper nanocubes for highly efficient electrochemical CO2 reduction. CCS Chem. 4, 1376–1385 (2022).
Yan, Y. et al. Mesoporous nanoarchitectures for electrochemical energy conversion and storage. Adv. Mater. 32, e2004654 (2020).
Sun, L. et al. Noble-metal-based hollow mesoporous nanoparticles: synthesis strategies and applications. Adv. Mater. 34, 2201954 (2022).
Chaikittisilp, W. et al. Material evolution with nanotechnology, nanoarchitectonics, and materials informatics: what will be the next paradigm shift in nanoporous materials? Adv. Mater. 34, e2107212 (2022).
Lv, H. et al. Mesoporosity-enabled selectivity of mesoporous palladium-based nanocrystals catalysts in semihydrogenation of alkynes. Angew. Chem. Int. Ed. 61, e202114539 (2022).
Chen, H. et al. Active site engineering in porous electrocatalysts. Adv. Mater. 32, 2002435 (2020).
Lv, H. et al. Asymmetric multimetallic mesoporous nanospheres. Nano Lett. 19, 3379–3385 (2019).
Lv, H. et al. Mesoporous gold nanospheres via Thiolate-Au(I) intermediates. Chem. Sci. 10, 6423–6430 (2019).
Li, C. et al. Pore-tuning to boost the electrocatalytic activity of polymeric micelle-templated mesoporous Pd nanoparticles. Chem. Sci. 10, 4054–4061 (2019).
Lv, H. et al. Ternary palladium–boron–phosphorus alloy mesoporous nanospheres for highly efficient electrocatalysis. ACS Nano 13, 12052–12061 (2019).
Lv, H. et al. Synthesis and crystal-phase engineering of mesoporous palladium–boron alloy nanoparticles. ACS Cent. Sci. 6, 2347–2353 (2020).
Dasgupta, A. et al. Intermetallics in catalysis: an exciting subset of multimetallic catalysts. Catal. Today 330, 2–15 (2019).
Xiao, W. et al. Recent advances of structurally ordered intermetallic nanoparticles for electrocatalysis. ACS Catal. 8, 3237–3256 (2018).
Gamler, J. T. L. et al. Random alloyed versus intermetallic nanoparticles: a comparison of electrocatalytic performance. Adv. Mater. 30, e1801563 (2018).
Yang, K. D. et al. Morphology-directed selective production of ethylene or ethane from CO2 on a Cu mesopore electrode. Angew. Chem. Int. Ed. 56, 796–800 (2017).
Kleitz, F. et al. Cubic Ia3d large mesoporous silica: synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 12, 2136–2137 (2003).
Lv, H. et al. A general concurrent template strategy for ordered mesoporous intermetallic nanoparticles with controllable catalytic performance. Angew. Chem. Int. Ed. 61, e202116179 (2022).
Xie, M. et al. Pt–Co@Pt octahedral nanocrystals: enhancing their activity and durability toward oxygen reduction with an intermetallic core and an ultrathin shell. J. Am. Chem. Soc. 143, 8509–8518 (2021).
Walter, C. et al. Perspective on intermetallics towards efficient electrocatalytic water-splitting. Chem. Sci. 12, 8603–8631 (2021).
Zhou, M. et al. Improvement of oxygen reduction performance in alkaline media by tuning phase structure of Pd-Bi nanocatalysts. J. Am. Chem. Soc. 143, 15891–15897 (2021).
Nakaya, Y. et al. Single-atom Pt in intermetallics as an ultrastable and selective catalyst for propane dehydrogenation. Nat. Commun. 11, 2838 (2020).
Nakaya, Y. et al. Doubly decorated platinum-gallium intermetallics as stable catalysts for propane dehydrogenation. Angew. Chem. Int. Ed. 60, 19715–19719 (2021).
Wordsworth, J. et al. The influence of nanoconfinement on electrocatalysis. Angew. Chem. Int. Ed. 61, e202200755 (2022).
Han, A. et al. Isolating contiguous Pt atoms and forming Pt-Zn intermetallic nanoparticles to regulate selectivity in 4-nitrophenylacetylene hydrogenation. Nat. Commun. 10, 3787 (2019).
Feng, Y. et al. On-demand, ultraselective hydrogenation system enabled by precisely modulated Pd-Cd nanocubes. J. Am. Chem. Soc. 142, 962–972 (2020).
Feng, Y. et al. Electroreduction of carbon dioxide in metallic nanopores through a pincer mechanism. Angew. Chem. Int. Ed. 59, 19297–19303 (2020).
Min, X. et al. Porous metal nanocrystal catalysts: can crystalline porosity enable catalytic selectivity? CCS Chem. 4, 1829–1842 (2022).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Zhang, L. et al. A polymer solution to prevent nanoclustering and improve the selectivity of metal nanoparticles for electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 58, 15834–15840 (2019).
Dong, Y. et al. Triblock copolymer syntheses of mesoporoussilica with periodic 50 to 300 angstrom pore. Science 279, 548–552 (1998).
Kruk, M. et al. Characterization of the porous structure of SBA-15. Chem. Mater. 12, 1961–1968 (2000).
Fang, J. et al. A general soft-enveloping strategy in the templating synthesis of mesoporous metal nanostructures. Nat. Commun. 9, 521 (2018).
Lai, X. et al. Ordered mesoporous copper oxide with crystalline walls. Angew. Chem. Int. Ed. 46, 738–741 (2007).
Lau, S. et al. Amine-boranes as transfer hydrogenation and hydrogenation reagents: a mechanistic perspective. Angew. Chem. Int. Ed. 60, 14272–14294 (2021).
Wu, Y. et al. Selective transfer semihydrogenation of alkynes with H2O(D2O) as the H(D) source over a Pd-P cathode. Angew. Chem. Int. Ed. 59, 21170–21175 (2020).
Shen, M. et al. Room-temperature chemoselective reduction of 3-nitrostyrene to 3-vinylaniline by ammonia borane over Cu nanoparticles. J. Am. Chem. Soc. 140, 16460–16463 (2018).
Gu, D. et al. Controllable synthesis of mesoporous Peapod-like Co3O4@Carbon nanotube arrays for high-performance lithium-ion batteries. Angew. Chem. Int. Ed. 54, 7060–7064 (2015).
Wang, H. et al. Shape-and size-controlled synthesis in hard templates: sophisticated chemical reduction for mesoporous monocrystalline platinum nanoparticles. J. Am. Chem. Soc. 133, 14526–14529 (2011).
Li, Z. et al. Size tunable gold nanorods evenly distributed in the channels of mesoporous silica. ACS Nano 2, 1205–1212 (2008).
Doi, Y. et al. Tailored synthesis of mesoporous platinum replicas using double gyroid mesoporous silica (KIT-6) with different pore diameters via vapor infiltration of a reducing agent. Chem. Commun. 46, 6365–6367 (2010).
Feng, J. et al. Self-templating approaches to hollow nanostructures. Adv. Mater. 31, e1802349 (2019).
Lv, H. et al. Precise synthesis of hollow mesoporous palladium–sulfur alloy nanoparticles for selective catalytic hydrogenation. CCS Chem. 4, 2854–2863 (2021).
Wang, Y. et al. Ordered mesoporous intermetallic trimetals for efficient and pH-universal hydrogen evolution electrocatalysis. Adv. Energy Mater. 12, 2201478 (2022).
He, T. et al. Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature 598, 76–81 (2021).
Wang, Y. et al. Ordered mesoporous intermetallic PtP2 nanoparticles with enhanced electrocatalytic activity and stability for hydrogen evolution. CCS Chem. 5, 1896–1907 (2023).
Acknowledgements
B.L. acknowledges financial supports from the Natural Science Foundation of Sichuan Province (2023NSFC0080) and the Fundamental Research Funds for the Central Universities. Y.Y. thanks the supports from the JST-ERATO Yamauchi Materials Space-Tectonics Project (JPMJER2003) and the Queensland node of the NCRIS-enabled Australian National Fabrication Facility (ANFF). We thank Y. Huang (Center of Engineering Experimental Teaching, School of Chemical Engineering, Sichuan University) for the discussion of SEM images and F. Yang (the Comprehensive Training Platform of the Specialized Laboratory, College of Chemistry, Sichuan University) for the discussion of TEM images.
Author information
Authors and Affiliations
Contributions
B.L. conceived the idea and provided design guidelines. H.L., Y.Y. and B.L. developed the protocol and co-drafted the manuscript. Y.W. and L.S. contributed to the discussion and manuscript modification.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Lv, H. et al. Angew. Chem. Int. Ed. 61, e202116179/1-8 (2022): https://doi.org/10.1002/anie.202116179
Wang, Y. et al. Adv. Energy Mater. 12, 2201478/1-8 (2022): https://doi.org/10.1002/aenm.202201478
Lv, H. et al. Angew. Chem. Int. Ed. 62, e202304420/1-8 (2023): https://doi.org/10.1002/anie.202304420
Key data used in this protocol
Wang, H. et al. J. Am. Chem. Soc. 133, 14526–14529 (2011): https://doi.org/10.1021/ja2058617
Lv, H. et al. Angew. Chem. Int. Ed. 61, e202116179/1-8 (2022): https://doi.org/10.1002/anie.202116179
Lv, H. et al. ACS Cent. Sci. 6, 2347–2353 (2020): https://doi.org/10.1021/acscentsci.0c01262
Wang, Y. et al. Adv. Energy Mater. 12, 2201478/1-8 (2022): https://doi.org/10.1002/aenm.202201478
Wang, Y. et al. CCS Chem. 5, 1896–1907 (2023): https://doi.org/10.31635/ccschem.022.202202451
Extended data
Extended Data Fig. 1 The comparison of soft and hard-templating method for the syntheses of mesoporous nanomaterials.
(a) Soft-templating method and (b) hard-templating method for the syntheses of mesoporous nanomaterials.
Extended Data Fig. 2 TEM images of mesoporous silica used in the Protocol.
TEM images of (a,b) KIT-6 and (c,d) SBA-15. Figure adapted with permission from ref. 36, American Chemical Society.
Extended Data Fig. 3 The schematic procedures of the other morphologies and compositions of mesoporous intermetallics.
The schematic procedures of the concurrent templates toward the syntheses of the mesoporous intermetallic nanoparticles of (a) meso-i-Pt1Sn1(SBA-15), (b) h-meso-i-Pt1Sn1, (c) meso-i-PtZnCo and (d) meso-i-Pd2B.
Extended Data Fig. 4 TEM images of meso-i-Pt1Sn1 nanoparticles with different nanoparticle size.
TEM images of meso-i-Pt1Sn1 nanoparticles with a nanoparticle size of (a) 121 nm, (b) 164 nm, and (c) 208 nm. Figure adapted with permission from ref. 42, Wiley.
Extended Data Fig. 5 STEM EDS mapping images of meso-i-PtX2.
STEM EDS mapping images of meso-i-PtP2, meso-i-PtS2, meso-i-PtSe2 and meso-i-PtTe2. Figure adapted with permission from ref. 70, Chinese Chemical Society.
Extended Data Fig. 6 STEM/TEM and STEM EDX images of meso-i-Pt1Sn1, meso-i-Pt3Sn1, and meso-Pt nanoparticles after catalysis.
STEM/TEM and STEM EDX images of (a,b) meso-i-Pt1Sn1, (c,d) meso-i-Pt3Sn1, and (e–h) meso-Pt nanoparticles after catalysis. All the nanoparticles retained their structure/morphology and composition, indicating a good catalytic stability. Figure adapted with permission from ref. 42, Wiley.
Extended Data Fig. 7 PXRD patterns of meso-Pt, meso-i-Pt3Sn1, and meso-i-Pt1Sn1 nanoparticles after catalytic stability tests.
All samples retained PXRD peaks well, indicating they are chemically stable for catalysis. Figure adapted with permission from ref. 42, Wiley.
Extended Data Fig. 8 EDS mapping images of meso-i-PtZnCo after ADT.
EDS mapping images of meso-i-PtZnCo after the (a) 5000, (b) 10000, (c) 30000, (d) 50000 CV cycles. Figure adapted with permission from ref. 68, Wiley.
Extended Data Fig. 9 TEM images of Commercial Pt/C after ADT.
TEM images of Commercial Pt/C after the (a) 1, (b) 5000, (c) 10000, (d) 20000 CV cycles. Figure adapted with permission from ref. 68, Wiley.
Supplementary information
Supplementary information
Supplementary Figs. 1–9, Discussion and Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, H., Wang, Y., Sun, L. et al. A general protocol for precise syntheses of ordered mesoporous intermetallic nanoparticles. Nat Protoc 18, 3126–3154 (2023). https://doi.org/10.1038/s41596-023-00872-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00872-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.