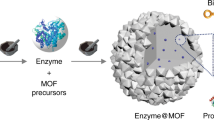
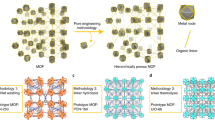
Abstract
Enzymes are natural catalysts with high catalytic activity, substrate specificity and selectivity. Their widespread utilization in industrial applications is limited by their sensitivity to harsh reaction conditions and difficulties relating to their removal and re-use after the reaction is complete. These limitations can be addressed by immobilizing the enzymes in solid porous supports. Covalent organic frameworks (COFs) are ideal candidate carriers because of their good biocompatibility, long-term water stability and large surface area. In post-synthetic immobilization, the enzyme is added to an existing COF; this has had limited success because of enzyme leaching and pore blockage by enzymes that are too large. Direct-immobilization methods—building the COF around the enzyme—allow tailored incorporation of proteins of any size and result in materials with lower levels of leaching and better mass transport of reactants and products. This protocol describes direct-immobilization methods that can be used to fabricate enzyme@COF (@ = engulfing) biocomposites with rationally programmed structures and functions. If COF construction requires harsh reaction conditions, the enzyme can be protected by using a removable metal-organic framework. Alternatively, a direct in situ approach, in which the enzyme and the COF monomers assemble under very mild conditions, can be used. Examples of both approaches are described: enzyme@COF-42-B/43-B capsules (enzymes including catalase, glucose oxidase, etc.) with ZIF-90 or ZPF-2 as protectors, and lipase@NKCOF-98/99 via in situ direct-immobilization methods (synthesis timing: 30–100 min). Example assays for physical and functional characterization of the COF and enzyme@COF materials are also described.
Key points
-
Covalent organic frameworks (COFs) protect enzymes from the harsh reaction conditions required for organic synthetic chemistry. If there is no enzyme leaching and pore blockage, enzyme removal and reuse are possible.
-
Direct in situ assembly of enzyme@COFs works for COFs that form under very mild reaction conditions. In cases in which harsher conditions are required, a sacrificial metal-organic framework can be constructed to protect the enzyme.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The main data discussed in this protocol are available within the figures and the Supplementary Information. Additional data that support the findings of this study can be obtained from the corresponding author on request.
References
Devine, P. N. et al. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2, 409–421 (2018).
Intasian, P. et al. Enzymes, in vivo biocatalysis, and metabolic engineering for enabling a circular economy and sustainability. Chem. Rev. 121, 10367–10451 (2021).
Sheldon, R. A. & Woodley, J. M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 118, 801–838 (2018).
Sheldon, R. A. & van Pelt, S. Enzyme immobilisation in biocatalysis: why, what and how. Chem. Soc. Rev. 42, 6223–6235 (2013).
Liang, W. et al. Metal-organic framework-based enzyme biocomposites. Chem. Rev. 121, 1077–1129 (2021).
Cantone, S. et al. Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 42, 6262–6276 (2013).
Brogan, A. P. S., Bui-Le, L. & Hallett, J. P. Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase. Nat. Chem. 10, 859–865 (2018).
Quin, M. B. & Schmidt-Dannert, C. Engineering of biocatalysts: from evolution to creation. ACS Catal. 1, 1017–1021 (2011).
Sharma, A., Gupta, G., Ahmad, T., Mansoor, S. & Kaur, B. Enzyme engineering: current trends and future perspectives. Food Rev. Int. 37, 121–154 (2019).
Huang, S., Chen, G. & Ouyang, G. Confining enzymes in porous organic frameworks: from synthetic strategy and characterization to healthcare applications. Chem. Soc. Rev. 51, 6824–6863 (2022).
Mohamad, N. R., Marzuki, N. H., Buang, N. A., Huyop, F. & Wahab, R. A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 29, 205–220 (2015).
An, H. et al. Incorporation of biomolecules in metal-organic frameworks for advanced applications. Coord. Chem. Rev. 384, 90–106 (2019).
Hudson, S., Cooney, J. & Magner, E. Proteins in mesoporous silicates. Angew. Chem. Int. Ed. Engl. 47, 8582–8594 (2008).
Fried, D. I., Brieler, F. J. & Fröba, M. Designing inorganic porous materials for enzyme adsorption and applications in biocatalysis. ChemCatChem 5, 862–884 (2013).
Kandambeth, S. et al. Self-templated chemically stable hollow spherical covalent organic framework. Nat. Commun. 6, 6786 (2015).
Sun, Q., Aguila, B., Lan, P. C. & Ma, S. Tuning pore heterogeneity in covalent organic frameworks for enhanced enzyme accessibility and resistance against denaturants. Adv. Mater. 31, e1900008 (2019).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Esrafili, A., Wagner, A., Inamdar, S. & Acharya, A. P. Covalent organic frameworks for biomedical applications. Adv. Healthc. Mater. 10, e2002090 (2021).
Chen, Y. et al. Cell membrane-anchoring covalent organic framework nanosheets for single-laser-triggered synergistic tumor therapy. Chem. Commun. 57, 11685–11688 (2021).
Sun, Q. et al. Covalent organic framework based nanoagent for enhanced mild-temperature photothermal therapy. Biomater. Sci. 9, 7977–7983 (2021).
Zhang, P. et al. Melt polymerization synthesis of a class of robust self-shaped olefin-linked COF foams as high-efficiency separators. Sci. China Chem. 65, 1173–1184 (2022).
Mu, Z. et al. Covalent organic frameworks with record pore apertures. J. Am. Chem. Soc. 144, 5145–5154 (2022).
Xing, C. et al. Enhancing enzyme activity by the modulation of covalent interactions in the confined channels of covalent organic frameworks. Angew. Chem. Int. Ed. Engl. 61, e202201378 (2022).
Wang, C. & Liao, K. Recent advances in emerging metal- and covalent-organic frameworks for enzyme encapsulation. ACS Appl. Mater. Interfaces 13, 56752–56776 (2021).
Sun, Q. et al. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J. Am. Chem. Soc. 140, 984–992 (2018).
Tang, Y. et al. Fabrication of hollow covalent-organic framework microspheres via emulsion-interfacial strategy to enhance laccase immobilization for tetracycline degradation. Chem. Eng. J. 421, 129743 (2021).
Zhao, Z. et al. Engineering olefin-linked covalent organic frameworks for photoenzymatic reduction of CO2. Angew. Chem. Int. Ed. Engl. 61, e202200261 (2022).
Jin, C. et al. Enzyme immobilization in porphyrinic covalent organic frameworks for photoenzymatic asymmetric catalysis. ACS Catal. 12, 8259–8268 (2022).
Zhang, S. et al. Covalent organic frameworks with chirality enriched by biomolecules for efficient chiral separation. Angew. Chem. Int. Ed. Engl. 57, 16754–16759 (2018).
Chao, H. et al. Template-free in situ encapsulation of enzymes in hollow covalent organic framework capsules for the electrochemical analysis of biomarkers. ACS Appl. Mater. Interfaces 14, 20641–20651 (2022).
Huang, N., Wang, P. & Jiang, D. Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 1, 16068 (2016).
Zheng, Y. et al. Green and scalable fabrication of high-performance biocatalysts using covalent organic frameworks as enzyme carriers. Angew. Chem. Int. Ed. Engl. 61, e202208744 (2022).
Liang, K. et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 6, 7240 (2015).
Feng, Y. et al. Antibodies@MOFs: an in vitro protective coating for preparation and storage of biopharmaceuticals. Adv. Mater. 31, e1805148 (2019).
Li, M. et al. Fabricating covalent organic framework capsules with commodious microenvironment for enzymes. J. Am. Chem. Soc. 142, 6675–6681 (2020).
Liang, H., Wang, L., Yang, Y., Song, Y. & Wang, L. A novel biosensor based on multienzyme microcapsules constructed from covalent-organic framework. Biosens. Bioelectron. 193, 113553 (2021).
Chen, G. et al. A convenient and versatile amino-acid-boosted biomimetic strategy for the nondestructive encapsulation of biomacromolecules within metal-organic frameworks. Angew. Chem. Int. Ed. Engl. 58, 1463–1467 (2019).
Wu, Z. et al. Covalent organic networks for in situ entrapment of enzymes with superior robustness and durability. Chem. Eng. J. 450, 138446 (2022).
Maddigan, N. K. et al. Protein surface functionalisation as a general strategy for facilitating biomimetic mineralisation of ZIF-8. Chem. Sci. 9, 4217–4223 (2018).
Chen, G. et al. Modulating the biofunctionality of metal-organic-framework-encapsulated enzymes through controllable embedding patterns. Angew. Chem. Int. Ed. Engl. 59, 2867–2874 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22022808).
Author information
Authors and Affiliations
Contributions
Q.Z. and Y.Z. contributed equally. All authors contributed to the development of the protocol and the design of the experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Peter Crowley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Li, M. et al. J. Am. Chem. Soc. 142, 6675–6681 (2020): https://doi.org/10.1021/jacs.0c00285
Zheng, Y. et al. Angew. Chem. Int. Ed. Engl. 61, e202208744 (2022): https://doi.org/10.1002/anie.202208744
Supplementary information
Supplementary Information
Supplementary Figs. 1–22 and Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Q., Zheng, Y., Zhang, Z. et al. Enzyme immobilization on covalent organic framework supports. Nat Protoc 18, 3080–3125 (2023). https://doi.org/10.1038/s41596-023-00868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00868-x
This article is cited by
-
Dendrimer-induced synthesis of porous organosilica capsules for enzyme encapsulation
Frontiers of Chemical Science and Engineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.