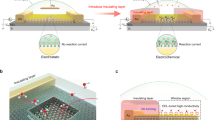
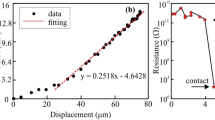
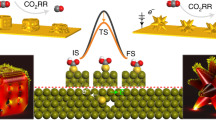
Abstract
On-chip electrocatalytic microdevices (OCEMs) are an emerging electrochemical platform specialized for investigating nanocatalysts at the microscopic level. The OCEM platform allows high-precision electrochemical measurements at the individual nanomaterial level and, more importantly, offers unique perspectives inaccessible with conventional electrochemical methods. This protocol describes the critical concepts, experimental standardization, operational principles and data analysis of OCEMs. Specifically, standard protocols for the measurement of the electrocatalytic hydrogen evolution reaction of individual 2D nanosheets are introduced with data validation, interpretation and benchmarking. A series of factors (e.g., the exposed area of material, the choice of passivation layer and current leakage) that could have effects on the accuracy and reliability of measurement are discussed. In addition, as an example of the high adaptability of OCEMs, the protocol for in situ electrical transport measurement is detailed. We believe that this protocol will promote the general adoption of the OCEM platform and inspire further development in the near future. This protocol requires essential knowledge in chemical synthesis, device fabrication and electrochemistry.
Key points
-
This protocol describes the use of on-chip electrocatalytic microdevices. Example procedures outline the measurement of the electrocatalytic hydrogen evolution reaction of individual 2D nanosheets and in situ electrical transport measurement.
-
Advantages over alternatives include streamlined device structure, convenient benchmark and facial incorporation of in situ techniques.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

















Similar content being viewed by others
Data availability
Some data supporting the findings of this study were previously published (Fig. 2c,d21; Fig. 3b,c12; Fig. 510; Fig. 8a28; and Fig. 17c,d21). The others (Fig. 1d, Fig. 7, Fig. 8b, Fig. 12, Fig. 16 and Fig. 17b,e) are available at https://figshare.com/articles/figure/NP-P220012B_On-chip_Electrocatalytic_Microdevices/22649083 or from the corresponding author upon reasonable request.
References
Jiao, Y., Zheng, Y., Jaroniec, M. & Qiao, S. Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015).
Shindell, D. & Smith, C. J. Climate and air-quality benefits of a realistic phase-out of fossil fuels. Nature 573, 408–411 (2019).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Jin, H. et al. Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 118, 6337–6408 (2018).
Bard, A. J., Fan, F. R. F., Kwak, J. & Lev, O. Scanning electrochemical microscopy. Introduction and principles. Anal. Chem. 61, 132–138 (1989).
Courtney, I. A. & Dahn, J. R. Electrochemical and in situ X‐Ray diffraction studies of the reaction of lithium with tin oxide composites. J. Electrochem. Soc. 144, 2045 (1997).
Iwasita, T. & Nart, F. C. In situ infrared spectroscopy at electrochemical interfaces. Prog. Surf. Sci. 55, 271–340 (1997).
Yang, R. et al. Fabrication of liquid cell for in situ transmission electron microscopy of electrochemical processes. Nat. Protoc. 18, 555–578 (2023).
Yang, H. et al. On-chip electrocatalytic microdevice: an emerging platform for expanding the insight into electrochemical processes. Chem. Soc. Rev. 49, 2916–2936 (2020).
Yu, Y. et al. High phase-purity 1T′-MoS2- and 1T′-MoSe2-layered crystals. Nat. Chem. 10, 638–643 (2018).
Ding, M. et al. An on-chip electrical transport spectroscopy approach for in situ monitoring electrochemical interfaces. Nat. Commun. 6, 7867 (2015).
Pan, Y. et al. Boosting the performance of single-atom catalysts via external electric field polarization. Nat. Commun. 13, 3063 (2022).
Shah, A. H. et al. The role of alkali metal cations and platinum-surface hydroxyl in the alkaline hydrogen evolution reaction. Nat. Catal. 5, 923–933 (2022).
He, Y. et al. Amorphizing noble metal chalcogenide catalysts at the single-layer limit towards hydrogen production. Nat. Catal. 5, 212–221 (2022).
Qi, J. et al. On-chip investigation of electrocatalytic oxygen reduction reaction of 2D materials. Small 18, e2204010 (2022).
Cheng, C. et al. Recent progress on two-dimensional materials. Acta Phys. -Chim. Sin. 37, 2108017–2108010 (2021).
Pomerantseva, E., Bonaccorso, F., Feng, X., Cui, Y. & Gogotsi, Y. Energy storage: the future enabled by nanomaterials. Science 366, eaan8285 (2019).
Sarma, P. V. et al. Electrocatalysis on edge-rich spiral WS2 for hydrogen evolution. ACS Nano 13, 10448–10455 (2019).
Hu, D. et al. Unveiling the layer-dependent catalytic activity of PtSe2 atomic crystals for the hydrogen evolution reaction. Angew. Chem. Int. Ed. Engl. 58, 6977–6981 (2019).
Zhang, J. et al. Unveiling active sites for the hydrogen evolution reaction on monolayer MoS2. Adv. Mater. 29, 1701955 (2017).
Wang, W. et al. Preparation of 2D molybdenum phosphide via surface-confined atomic substitution. Adv. Mater. 34, 2203220 (2022).
Zhu, J. et al. Boundary activated hydrogen evolution reaction on monolayer MoS2. Nat. Commun. 10, 1348 (2019).
Zhang, W. et al. Superior hydrogen evolution reaction performance in 2H-MoS2 to that of 1T phase. Small 15, e1900964 (2019).
Wang, J. et al. Field effect enhanced hydrogen evolution reaction of MoS2 nanosheets. Adv. Mater. 29, 1604464 (2017).
Wu, Y. et al. A two-dimensional MoS2 catalysis transistor by solid-state ion gating manipulation and adjustment (SIGMA). Nano Lett. 19, 7293–7300 (2019).
Zhou, Y. et al. Enhanced performance of in-plane transition metal dichalcogenides monolayers by configuring local atomic structures. Nat. Commun. 11, 2253 (2020).
Tong, X. et al. Dual-regulation of defect sites and vertical conduction by spiral domain for electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. Engl. 61, e202112953 (2022).
He, Y. et al. Self-gating in semiconductor electrocatalysis. Nat. Mater. 18, 1098–1104 (2019).
Liu, X. et al. Activating the electrocatalysis of MoS2 basal plane for hydrogen evolution via atomic defect configurations. Small 18, e2200601 (2022).
Yang, H. et al. Single MoTe2 sheet electrocatalytic microdevice for in situ revealing the activated basal plane sites by vacancies engineering. Nano Res. 14, 4814–4821 (2021).
Ling, N. et al. Active hydrogen evolution on the plasma-treated edges of WTe2. APL Mater. 9, 061108 (2021).
Fu, Q. et al. 2D transition metal dichalcogenides: design, modulation, and challenges in electrocatalysis. Adv. Mater. 33, 1907818 (2021).
Nam, G.-H. et al. In-plane anisotropic properties of 1T′-MoS2 layers. Adv. Mater. 31, 1807764 (2019).
He, Y. et al. Engineering grain boundaries at the 2D limit for the hydrogen evolution reaction. Nat. Commun. 11, 57 (2020).
Engstrom, R. C. & Pharr, C. M. Scanning electrochemical microscopy. Anal. Chem. 61, 1099A–1104A (1989).
Polcari, D., Dauphin-Ducharme, P. & Mauzeroll, J. Scanning electrochemical microscopy: a comprehensive review of experimental parameters from 1989 to 2015. Chem. Rev. 116, 13234–13278 (2016).
Mariano, R. G. et al. Microstructural origin of locally enhanced CO2 electroreduction activity on gold. Nat. Mater. 20, 1000–1006 (2021).
Wang, Y., Skaanvik, S. A., Xiong, X., Wang, S. & Dong, M. Scanning probe microscopy for electrocatalysis. Matter 4, 3483–3514 (2021).
Li, H. et al. Kinetic study of hydrogen evolution reaction over strained MoS2 with sulfur vacancies using scanning electrochemical microscopy. J. Am. Chem. Soc. 138, 5123–5129 (2016).
Zhu, X., Wang, C. & Fu, L. Engineering electrocatalytic microcells for two-dimensional materials. Cell Rep. Phys. Sci. 1, 100190 (2020).
Duan, H. et al. Single-atom-layer catalysis in a MoS2 monolayer activated by long-range ferromagnetism for the hydrogen evolution reaction: beyond single-atom catalysis. Angew. Chem. Int. Ed. Engl. 60, 7251–7258 (2021).
Wang, Z. et al. Reversing interfacial catalysis of ambipolar WSe2 single crystal. Adv. Sci. (Weinh.) 7, 1901382 (2020).
Guo, Y. et al. 2D hybrid superlattice-based on-chip electrocatalytic microdevice for in situ revealing enhanced catalytic activity. ACS Nano 14, 1635–1644 (2020).
Zhou, Y. et al. Unveiling the interfacial effects for enhanced hydrogen evolution reaction on MoS2/WTe2 hybrid structures. Small 15, e1900078 (2019).
Zhang, J. et al. Discovering superior basal plane active two-dimensional catalysts for hydrogen evolution. Mater. Today 25, 28–34 (2019).
Jiang, Z. et al. MoS2 Moiré superlattice for hydrogen evolution reaction. ACS Energy Lett. 4, 2830–2835 (2019).
Zhou, Y. et al. Revealing the contribution of individual factors to hydrogen evolution reaction catalytic activity. Adv. Mater. 30, 1706076 (2018).
Wang, P. et al. Oxygen evolution reaction dynamics monitored by an individual nanosheet-based electronic circuit. Nat. Commun. 8, 645 (2017).
Yang, J. et al. Single atomic vacancy catalysis. ACS Nano 13, 9958–9964 (2019).
You, H. et al. 1T′-MoTe2-based on-chip electrocatalytic microdevice: a platform to unravel oxidation-dependent electrocatalysis. CCS Chem. 1, 396–406 (2019).
Voiry, D. et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 15, 1003–1009 (2016).
Wang, Z. et al. Controllable etching of MoS2 basal planes for enhanced hydrogen evolution through the formation of active edge sites. Nano Energy 49, 634–643 (2018).
Wang, W. et al. Filling the gap between heteroatom doping and edge enrichment of 2D electrocatalysts for enhanced hydrogen evolution. ACS Nano 17, 1287–1297 (2023).
Ding, M. et al. On-chip in situ monitoring of competitive interfacial anionic chemisorption as a descriptor for oxygen reduction kinetics. ACS Cent. Sci. 4, 590–599 (2018).
Xiang, H. et al. Self-gating enhanced carrier transfer in semiconductor electrocatalyst verified in microdevice. Chin. Chem. Lett. 33, 3221–3226 (2022).
Kim, C.-H. & Frisbie, C. D. Field effect modulation of outer-sphere electrochemistry at back-gated, ultrathin ZnO electrodes. J. Am. Chem. Soc. 138, 7220–7223 (2016).
Yan, M. et al. Field-effect tuned adsorption dynamics of VSe2 nanosheets for enhanced hydrogen evolution reaction. Nano Lett. 17, 4109–4115 (2017).
Liu, X. et al. The critical role of electrolyte gating on the hydrogen evolution performance of monolayer MoS2. Nano Lett. 19, 8118–8124 (2019).
Wang, Y., Udyavara, S., Neurock, M. & Frisbie, C. D. Field effect modulation of electrocatalytic hydrogen evolution at back-gated two-dimensional MoS2 electrodes. Nano Lett. 19, 6118–6123 (2019).
Zhu, X. et al. Dual self-built gating boosts the hydrogen evolution reaction. Adv. Mater. 34, 2202479 (2022).
He, Q. et al. In situ probing molecular intercalation in two-dimensional layered semiconductors. Nano Lett. 19, 6819–6826 (2019).
Wang, C. et al. Monolayer atomic crystal molecular superlattices. Nature 555, 231–236 (2018).
Qian, Q. et al. Chiral molecular intercalation superlattices. Nature 606, 902–908 (2022).
Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 9, 9451–9469 (2015).
Wang, Y., Zhang, Z., Mao, Y. & Wang, X. Two-dimensional nonlayered materials for electrocatalysis. Energy Environ. Sci. 13, 3993–4016 (2020).
Cummins, D. R. et al. Efficient hydrogen evolution in transition metal dichalcogenides via a simple one-step hydrazine reaction. Nat. Commun. 7, 11857 (2016).
Ding, M. et al. Nanoelectronic investigation reveals the electrochemical basis of electrical conductivity in Shewanella and Geobacter. ACS Nano 10, 9919–9926 (2016).
Cao, B. et al. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells. Science 373, 1336–1340 (2021).
Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B 210, 235–254 (2017).
Zhang, X. et al. Hidden vacancy benefit in monolayer 2D semiconductors. Adv. Mater. 33, 2007051 (2021).
Li, H. et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 15, 48–53 (2016).
Li, S. et al. Edge-enriched 2D MoS2 thin films grown by chemical vapor deposition for enhanced catalytic performance. ACS Catal. 7, 877–886 (2017).
Shi, J. et al. Controllable growth and transfer of monolayer MoS2 on Au foils and its potential application in hydrogen evolution reaction. ACS Nano 8, 10196–10204 (2014).
Li, G. et al. Ammonium salts: new synergistic additive for chemical vapor deposition growth of MoS2. J. Phys. Chem. Lett. 12, 12384–12390 (2021).
Shinagawa, T., Garcia-Esparza, A. T. & Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 5, 13801 (2015).
Liu, L. et al. Uniform nucleation and epitaxy of bilayer molybdenum disulfide on sapphire. Nature 605, 69–75 (2022).
Bard, A. J., Faulkner, L. R. & White, H. S. Electrochemical Methods: Fundamentals and Applications (John Wiley & Sons, 2022).
Bond, A. M., Oldham, K. B. & Zoski, C. G. Steady-state voltammetry. Anal. Chim. Acta 216, 177–230 (1989).
Chen, R. et al. Use of platinum as the counter electrode to study the activity of nonprecious metal catalysts for the hydrogen evolution reaction. ACS Energy Lett. 2, 1070–1075 (2017).
Zhong, G. et al. Determining the hydronium pKα at platinum surfaces and the effect on pH-dependent hydrogen evolution reaction kinetics. Proc. Natl Acad. Sci. USA 119, e2208187119 (2022).
Acknowledgements
Q.H. is thankful for support through grants (Project 9229079, 9610482 and 7005468) from the City University of Hong Kong and Early Career Scheme Project 21302821 and General Research Fund Project 11314322 from the University Grants Committee of Hong Kong. M.D. acknowledges the support of the Natural Science Foundation of China (Project Nos. 22172075 and 92156024), the Fundamental Research Funds for the Central Universities in China (Project No. 14380273), the Natural Science Foundation of Jiangsu Province (BK20220069) and the Beijing National Laboratory for Molecular Sciences (Project No. BNLMS202107).
Author information
Authors and Affiliations
Contributions
W.W., M.D. and Q.H. developed the protocol. W.W., J.Q. and Z.W. performed the experiments. W.W., J.Q., M.D. and Q.H. envisioned and drafted the manuscript. W.Z., K.B., L.Z. and J.W. helped with experiments and data analysis. C.K., L.W. and Y.P. revised the protocols and the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
: Nature Protocols thanks Youwen Liu, Zheng Liu and Tianyou Zhai for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Ding, M. et al. Nat. Commun. 6, 7867 (2015): https://doi.org/10.1038/ncomms8867
Yu, Y. et al. Nat. Chem. 10, 638–643 (2018): https://doi.org/10.1038/s41557-018-0035-6
He, Y. et al. Nat. Commun. 11, 57 (2020): https://doi.org/10.1038/s41467-019-13631-2
Key data used in this protocol
He, Y. et al. Nat. Mater. 18, 1098–1104 (2019): https://doi.org/10.1038/s41563-019-0426-0
Wang, W. et al. Adv. Mater. 34, 2203220 (2022): https://doi.org/10.1002/adma.202203220
Pan, Y. et al. Nat. Commun. 13, 3063 (2022): https://doi.org/10.1038/s41467-022-30766-x
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Qi, J., Wu, Z. et al. On-chip electrocatalytic microdevices. Nat Protoc 18, 2891–2926 (2023). https://doi.org/10.1038/s41596-023-00866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00866-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.