Abstract
The production of induced neuronal (iN) cells from human embryonic stem cells (ESCs) and induced pluripotent stem cells by the forced expression of proneural transcription factors is rapid, efficient and reproducible. The ability to generate large numbers of human neurons in such a robust manner enables large-scale studies of human neural differentiation and neuropsychiatric diseases. Surprisingly, similar transcription factor–based approaches for converting mouse ESCs into iN cells have been challenging, primarily because of low cell survival. Here, we provide a detailed approach for the efficient and reproducible generation of functional iN cells from mouse ESC cultures by the genetically induced expression of neurogenin-2. The resulting iN cells display mature pre- and postsynaptic specializations and form synaptic networks. Our method provides the basis for studying neuronal development and enables the direct comparison of cellular phenotypes in mouse and human neurons generated in an equivalent way. The procedure requires 14 d and can be carried out by users with expertise in stem cell culture.
Key points
-
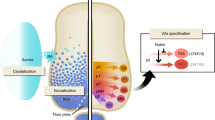
Following lentiviral infection of mouse embryonic stem cells with FUW-M2rtTA and Tet-O-FUW-NGN2-T2A-PURO, neurons are induced with doxycycline and selected with puromycin. The selected cells are co-cultured with mouse primary glia, eliminating the clustering typical of mature mouse NGN2-induced neuronal cells.
-
After 2 weeks of co-culturing, homogeneous cultures of induced neuronal cells exhibit functional neuronal properties, including the ability to form functional synapses that integrate into spontaneously active networks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data for the figures in this study are available at https://doi.org/10.5281/zenodo.7625605.
References
Hanna, J. H., Saha, K. & Jaenisch, R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell 143, 508–525 (2010).
Okita, K. & Yamanaka, S. Induced pluripotent stem cells: opportunities and challenges. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 2198–2207 (2011).
Blanpain, C. et al. Stem cells assessed. Nat. Rev. Mol. Cell Biol. 13, 471–476 (2012).
Marchetto, M. C. & Gage, F. H. Modeling brain disease in a dish: really? Cell Stem Cell 10, 642–645 (2012).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Zhang, Y. et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013).
Mertens, J. et al. Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer’s patients. Cell Stem Cell 28, 1533–1548.e6 (2021).
Pak, C. et al. Cross-platform validation of neurotransmitter release impairments in schizophrenia patient-derived NRXN1-mutant neurons. Proc. Natl Acad. Sci. USA 118, e2025598118 (2021).
Patzke, C. et al. Neuromodulator signaling bidirectionally controls vesicle numbers in human synapses. Cell 179, 498–513.e22 (2019).
Marro, S. G. et al. Neuroligin-4 regulates excitatory synaptic transmission in human neurons. Neuron 103, 617–626.e6 (2019).
Huang, Y. A., Zhou, B., Wernig, M. & Südhof, T. C. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell 168, 427–441.e1 (2017).
Yi, F. et al. Autism-associated SHANK3 haploinsufficiency causes Ih channelopathy in human neurons. Science 352, aaf2669 (2016).
Karow, M. et al. Direct pericyte-to-neuron reprogramming via unfolding of a neural stem cell-like program. Nat. Neurosci. 21, 932–940 (2018).
Biddy, B. A. et al. Single-cell mapping of lineage and identity in direct reprogramming. Nature 564, 219–224 (2018).
Lin, H. C. et al. NGN2 induces diverse neuron types from human pluripotency. Stem Cell Rep. 16, 2118–2127 (2021).
Chanda, S. et al. Generation of induced neuronal cells by the single reprogramming factor ASCL1. Stem Cell Rep. 3, 282–296 (2014).
Treutlein, B. et al. Dissecting direct reprogramming from fibroblast to neuron using single-cell RNA-seq. Nature 534, 391–395 (2016).
Ang, C. E. et al. The novel lncRNA lnc-NR2F1 is pro-neurogenic and mutated in human neurodevelopmental disorders. eLife 8, e41770 (2019).
An, D. et al. Stem cell-derived cranial and spinal motor neurons reveal proteostatic differences between ALS resistant and sensitive motor neurons. eLife 8, e44423 (2019).
Höpfl, G., Gassmann, M. & Desbaillets, I. Differentiating embryonic stem cells into embryoid bodies. Methods Mol. Biol. 254, 79–98 (2004).
Paşca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Bandeira, F., Lent, R. & Herculano-Houzel, S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc. Natl Acad. Sci. USA 106, 14108–14113 (2009).
Clarke, L. E. & Barres, B. A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 14, 311–321 (2013).
Herculano-Houzel, S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 62, 1377–1391 (2014).
Zhang, Z. et al. Optogenetic manipulation of cellular communication using engineered myosin motors. Nat. Cell Biol. 23, 198–208 (2021).
Chanda, S. et al. Direct reprogramming of human neurons identifies MARCKSL1 as a pathogenic mediator of valproic acid-induced teratogenicity. Cell Stem Cell 25, 103–119.e6 (2019).
Aydin, B. et al. Proneural factors Ascl1 and Neurog2 contribute to neuronal subtype identities by establishing distinct chromatin landscapes. Nat. Neurosci. 22, 897–908 (2019).
Iacovino, M. et al. Inducible cassette exchange: a rapid and efficient system enabling conditional gene expression in embryonic stem and primary cells. Stem Cells 29, 1580–1588 (2011).
Czechanski, A. et al. Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nat. Protoc. 9, 559–574 (2014).
Valamehr, B. et al. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc. Natl Acad. Sci. USA 105, 14459–14464 (2008).
Wu, W. et al. Neuronal enhancers are hotspots for DNA single-strand break repair. Nature 593, 440–444 (2021).
Acknowledgements
We thank C. E. Ang, B. Zhou and J. Janas for discussion on the design of, and useful comments on, the experiments and the rest of the Wernig lab for helpful discussion. The project described was supported by U19MH104172 from the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
Y.L. and M.W. conceived the project. Y.L. and M.W. designed and planned the experiments. Y.L. executed the experiments. J.W. contributed to the electrophysiology assay. T.C.S. and M.W. provided critical advice. Y.L. and M.W. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Brady Maher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Zijian, Z. et al. Nat. Cell Biol. 23, 198–208 (2021): https://doi.org/10.1038/s41556-020-00625-2
ChangHui, P. et al. Proc. Natl. Acad. Sci. USA 118, e2025598118 (2021): https://doi.org/10.1073/pnas.2025598118
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Wang, J., Südhof, T.C. et al. Efficient generation of functional neurons from mouse embryonic stem cells via neurogenin-2 expression. Nat Protoc 18, 2954–2974 (2023). https://doi.org/10.1038/s41596-023-00863-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00863-2
This article is cited by
-
The Role of PLAG1 in Mouse Brain Development and Neurogenesis
Molecular Neurobiology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.